The Current Landscape of Antibiotic Use and Antimicrobial Resistance in Japan: Focusing on Common Infections Including Uncomplicated Urinary Tract Infection and Gonorrhea
Abstract
1. Introduction
2. Current Unmet Antibiotic Needs in Japan
2.1. Inappropriate Use of Antibiotics
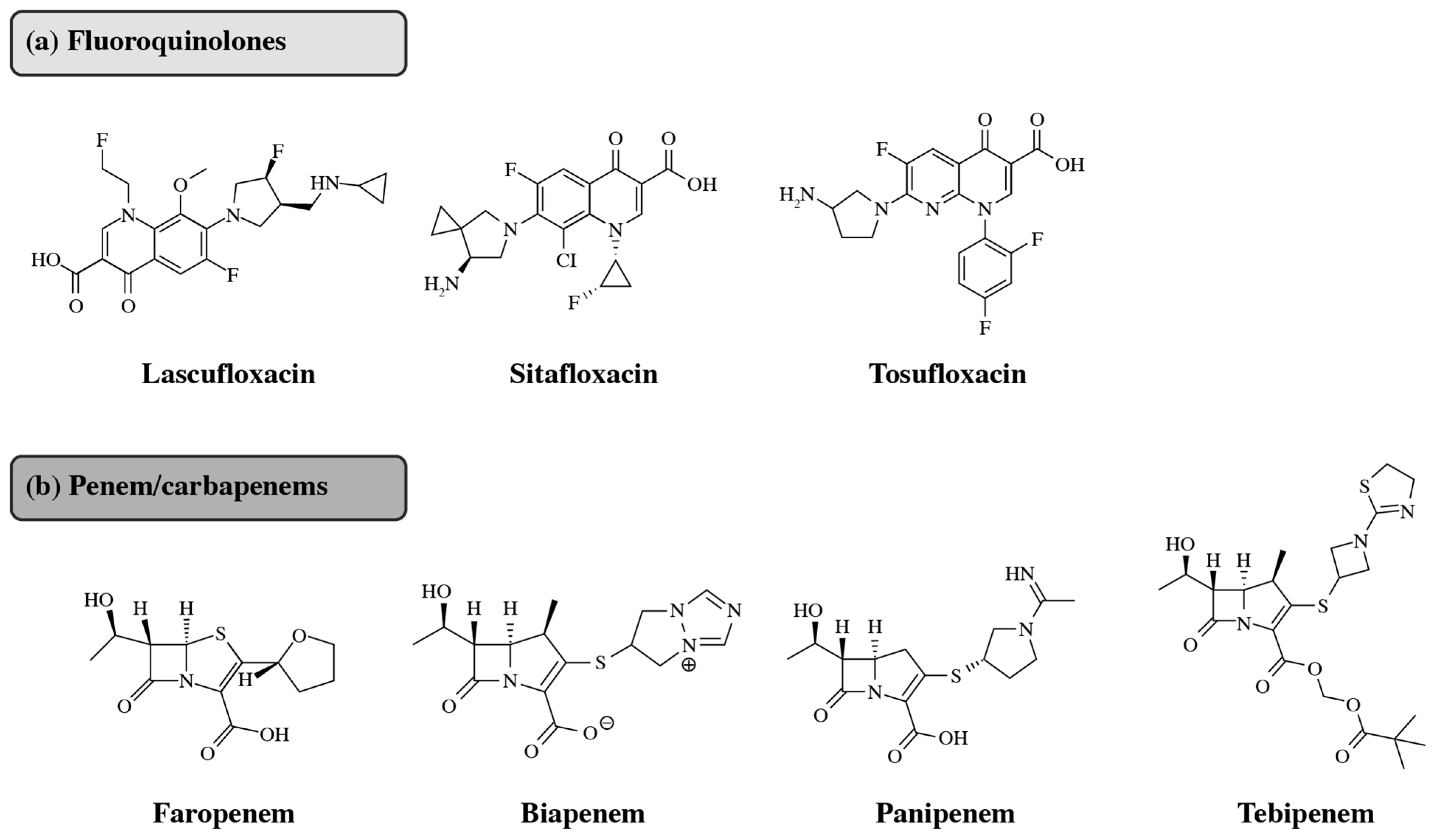
2.2. Novel Antibiotics for Community-Acquired Infections
2.3. The Need for Novel Oral Antibiotic Options
| Antibiotic Class | US Approval Year | EU Approval Year | Japan Approval Year | Administration Forms | Indication | References | |
|---|---|---|---|---|---|---|---|
| Tedizolid | Oxazolidinone | ✓ (2014) | ✓ (2015) | ✓ (2018) | IV, PO | ABSSSI | [84,85,86,87] |
| Oritavancin | Lipoglycopeptide | ✓ (2014) | ✓ (2015) | ✕ | IV | ABSSSI | [88,89] |
| Ceftolozane/ tazobactam | BL/BLI combination | ✓ (2014) | ✓ (2015) | ✓ (2019) | IV | cIAI, cUTI, HABP/VABP | [35,36,37,38] |
| Ceftazidime/ avibactam | BL/BLI combination | ✓ (2015) | ✓ (2016) | ✓ (2024) | IV | cUTI, cIAI, HABP/VABP | [39,90] |
| Rifaximin | Rifamycin | ✓ (2004) | ✕ | ✓ (2016) | PO | Travelers’ diarrhea, overt hepatic encephalopathy, IBS-D | [85,91] |
| Delafloxacin | Fluoroquinolone | ✓ (2017) | ✓ (2019) | ✕ | IV, PO | ABSSSI, CAP | [40,41,92] |
| Ozenoxacin | Quinolone | ✓ (2017) | ✕ | ✓ (2015) | TOP | Impetigo, superficial skin infections, acne | [42,43] |
| Secnidazole | Nitroimidazole | ✓ (2017) | ✕ | ✕ | PO | BV and trichomoniasis | [44] |
| Meropenem/ vaborbactam | BL/BLI combination | ✓ (2017) | ✓ (2018) | ✕ | IV | cUTI, cIAI, HABP/VABP | [39,45] |
| Bedaquiline | Diarylquinoline | ✓ (2012) | ✓ (2014) | ✓ (2018) | PO | Pulmonary MDR-TB | [85,93,94] |
| Fidaxomicin | Macrolide | ✓ (2011) | ✓ (2011) | ✓ (2018) | PO | Infectious enteritis caused by C. difficile | [85,95,96] |
| Plazomicin | Aminoglycoside | ✓ (2018) | ✕ | ✕ | IV | cUTI | [46] |
| Rifamycin | Rifamycin | ✓ (2018) | ✕ | ✕ | PO | Travelers’ diarrhea | [84,97] |
| Eravacycline | Tetracycline | ✓ (2018) | ✓ (2018) | ✕ | IV | cIAI | [47,48] |
| Omadacycline | Tetracycline | ✓ (2018) | ✕ | ✕ | IV, PO | ABSSSI, CABP | [49] |
| Sarecycline | Tetracycline | ✓ (2018) | ✕ | ✕ | PO | Acne | [50] |
| Imipenem/ relebactam | BL/BLI combination | ✓ (2019) | ✓ (2020) | ✓ (2021) | IV | cUTI, cIAI, HABP/VABP | [51,52,98,99] |
| Pretomanid | Nitroimidazole | ✓ (2019) | ✓ (2020) | ✕ | PO | MDR-TB, pulmonary XDR-TB | [53,100] |
| Lefamulin | Pleuromutilin | ✓ (2019) | ✕ | ✕ | IV, PO | CABP | [54] |
| Lascufloxacin | Fluoroquinolone | ✕ | ✕ | ✓ (2019) | IV, PO | Respiratory tract and ENT infections; CABP | [55] |
| Amikacin liposome | Aminoglycoside | ✓ (2018) | ✓ (2020) | ✓ (2021) | Nebulizer dispersion | NTM lung infections caused by MAC | [56,57,58,101] |
| Cefiderocol | BL plus siderophore | ✓ (2019) | ✓ (2020) | ✓ (2023) | IV | cUTI, HABP/VABP; Gram-negative infections with limited treatment options | [59,60,61,62] |
| Durlobactam/ sulbactam | BL/BLI combination | ✓ (2023) | ✕ | ✕ | IV | HABP/VABP (Acinetobacter baumannii- calcoaceticus complex) | [63] |
| Cefepime/ enmetazobactam | BL/BLI combination | ✓ (2024) | ✓ (2024) | ✕ | IV | cUTI, HABP/VABP | [64,65,102] |
| Sulopenem etzadroxil | Carbapenem | ✓ (2024) | ✕ | ✕ | PO | uUTI | [103] |
| Gepotidacin | Triazaacenaphthylene | ✓ (2025) | ✕ | ✕ | PO | uUTI | [103,104] |
| Aztreonam/ avibactam | BL/BLI combination | ✓ (2025) | ✓ (2024) | ✕ | IV | cIAI, cUTI, HABP/VABP | [105,106,107] |
3. Barriers to Diversifying Antibiotics in Japan
4. AMR: Possible Solutions
4.1. National AMR Action Plan
4.2. Resistance-Guided Therapy
4.3. Novel Antibiotics in Development
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Tawfiq, J.A.; Momattin, H.; Al-Ali, A.Y.; Eljaaly, K.; Tirupathi, R.; Haradwala, M.B.; Areti, S.; Alhumaid, S.; Rabaan, A.A.; Al Mutair, A.; et al. Antibiotics in the pipeline: A literature review (2017–2020). Infection 2022, 50, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 25 March 2024).
- Aljeldah, M.M. Antimicrobial resistance and its spread is a global threat. Antibiotics 2022, 11, 1082. [Google Scholar] [CrossRef] [PubMed]
- Marantidis, J.; Sussman, R.D. Unmet needs in complicated urinary tract infections: Challenges, recommendations, and emerging treatment pathways. Infect. Drug Resist. 2023, 16, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Golparian, D.; Eyre, D.W. Antimicrobial resistance in Neisseria gonorrhoeae and treatment of gonorrhea. Methods Mol. Biol. 2019, 1997, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.; Nicolle, L.; Bartoletti, R.; Gales, A.C.; Grigoryan, L.; Huang, H.; Hooton, T.; Lopardo, G.; Naber, K.; Poojary, A.; et al. A global perspective on improving patient care in uncomplicated urinary tract infection: Expert consensus and practical guidance. J. Glob. Antimicrob. Resist. 2022, 28, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Koizumi, R.; Matsunaga, N.; Ohmagari, N. Decline in antimicrobial consumption and stagnation in reducing disease burden due to antimicrobial resistance in Japan. Infect. Dis. Ther. 2023, 12, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Hashimoto, H.; Sonoo, T.; Naraba, H.; Takahashi, Y.; Nakamura, K.; Hatakeyama, S. Gram-negative organisms from patients with community-acquired urinary tract infections and associated risk factors for antimicrobial resistance: A single-center retrospective observational study in Japan. Antibiotics 2020, 9, 438. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022. Available online: https://iris.who.int/bitstream/handle/10665/364996/9789240062702-eng.pdf?sequence=1 (accessed on 25 March 2025).
- Wada, K.; Tsuboi, I.; Takahashi, S.; Yasuda, M.; Miyazaki, J.; Kobayashi, K.; Matsumoto, M.; Hayami, H.; Yamamoto, S.; Kiyota, H.; et al. Third nationwide surveillance of bacterial pathogens in patients with acute uncomplicated cystitis conducted by the Japanese surveillance committee during 2020 and 2021: Antimicrobial susceptibility of Escherichia coli, Klebsiella pneumoniae, and Staphylococcus saprophyticus. J. Infect. Chemother. 2024, 30, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Saito, M.; Sato, J.; Goda, K.; Mitsutake, N.; Kitsuregawa, M.; Nagai, R.; Hatakeyama, S. Indications and classes of outpatient antibiotic prescriptions in Japan: A descriptive study using the national database of electronic health insurance claims, 2012–2015. Int. J. Infect. Dis. 2020, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, T.; Nakane, K.; Yasuda, M.; Maeda, S. Emergence and spread of drug resistant Neisseria gonorrhoeae. J. Urol. 2010, 184, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Derbie, A.; Mekonnen, D.; Woldeamanuel, Y.; Abebe, T. Azithromycin resistant gonococci: A literature review. Antimicrob. Resist. Infect. Control 2020, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yin, Y.; Dai, X.; Chen, S.; Yang, L.; Zhu, B.; Zhong, N.; Cao, W.; Zhang, X.; Wu, Z.; et al. Widespread use of high-sode ceftriaxone therapy for uncomplicated gonorrhea without reported ceftriaxone treatment failure: Results from 5 years of multicenter surveillance data in China. Clin. Infect. Dis. 2020, 70, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hamasuna, R.; Yasuda, M.; Takahashi, S.; Uehara, S.; Kawai, Y.; Miyairi, I.; Arakawa, S.; Kiyota, H. The JAID/JSC guidelines to Clinical Management of Infectious Disease 2017 concerning male urethritis and related disorders. J. Infect. Chemother. 2021, 27, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Golparian, D.; Shimuta, K.; Saika, T.; Hoshina, S.; Iwasaku, K.; Nakayama, S.; Kitawaki, J.; Unemo, M. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 2011, 55, 3538–3545. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Takahashi, S.; Miyazaki, J.; Wada, K.; Kobayashi, K.; Matsumoto, M.; Hayami, H.; Yamamoto, S.; Kiyota, H.; Sato, J.; et al. The third nationwide surveillance of antimicrobial susceptibility against Neisseria gonorrhoeae from male urethritis in Japan, 2016–2017. J. Infect. Chemother. 2023, 29, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Ohwaki, K.; Nakabayashi, T. Relationship between drug lag and factors associated with clinical trials in Japan. J. Clin. Pharm. Ther. 2014, 39, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Brubaker, L. The etiology and management of recurrent urinary tract infections in postmenopausal women. Climacteric 2019, 22, 242–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 11 December 2024).
- Butler, M.S.; Henderson, I.R.; Capon, R.J.; Blaskovich, M.A.T. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 2023, 76, 431–473. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceuticals and Medical Devices Agency. Pharmaceuticals and Medical Devices Agency. Available online: https://www.pmda.go.jp/english/ (accessed on 11 December 2024).
- US Food and Drug Administration. US Food and Drug Administration. Available online: https://www.fda.gov/ (accessed on 11 December 2024).
- European Medicines Agency. European Medicines Agency. Available online: https://www.ema.europa.eu/en/homepage (accessed on 11 December 2024).
- World Health Organization. World Health Organization. Available online: https://www.who.int/ (accessed on 11 December 2024).
- KEGG: Kyoto Encyclopedia of Genes and Genomes. KEGG: Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/kegg/ (accessed on 11 December 2024).
- Brosh-Nissimov, T.; Navon-Venezia, S.; Keller, N.; Amit, S. Risk analysis of antimicrobial resistance in outpatient urinary tract infections of young healthy adults. J. Antimicrob. Chemother. 2019, 74, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Hagiya, H.; Higashionna, T.; Nakano, Y.; Sato, K.; Haruki, Y.; Haruki, M.; Honda, H.; Ogawa, H.; Ueda, K.; et al. Antimicrobial prescription practices for outpatients with uncomplicated cystitis in Japan. Sci. Rep. 2022, 12, 5921. [Google Scholar] [CrossRef] [PubMed]
- JAID/JSC Guideline Committee. The JAID/JSC Guide to Clinical Management of Infectious Diseases 2023. Available online: https://www.kansensho.or.jp/modules/journal/index.php?content_id=11 (accessed on 11 December 2024).
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Kranz, J.; Bartoletti, R.; Bruyere, F.; Cai, T.; Geerlings, S.; Koves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F.M.E.; et al. European Association of Urology guidelines on urological infections: Summary of the 2024 guidelines. Eur. Urol. 2024, 86, 27–41. [Google Scholar] [CrossRef] [PubMed]
- The Government of Japan. National Action Plan on Antimicrobial Resistance (AMR) 2023–2027. Available online: https://www.mhlw.go.jp/content/10900000/001096228.pdf (accessed on 11 December 2024).
- Kusama, Y.; Ishikane, M.; Kihara, T.; Ohmagari, N. Epidemiology of antibiotic treatment for uncomplicated cystitis in adults in Japan. J. Infect. Chemother. 2021, 27, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-lactam-β-lactamase inhibitor combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef] [PubMed]
- Takaya, R.; Mori, N.; Saito, E.; Ohde, S. Cost-effectiveness analysis of CTZ/TAZ for the treatment of ventilated hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia in Japan. BMC Health Serv. Res. 2024, 24, 389. [Google Scholar] [CrossRef] [PubMed]
- Merck. Merck Receives Positive EU CHMP Opinion for ZERBAXA® 3g Dose (Ceftolozane and Tazobactam) for the Treatment of Adults with Hospital-Aquired Pneumonia (HAP), Including Ventilator-Associated Pneumonia (VAP). Available online: https://www.merck.com/news/merck-receives-positive-eu-chmp-opinion-for-zerbaxa-3g-dose-ceftolozane-and-tazobactam-for-the-treatment-of-adults-with-hospital-acquired-pneumonia-hap-including-ventilator-associated-pneum/#:~:text=ZERBAXA%201.5g%20(ceftolozane%201g,%2Dabdominal%20infections%20(cIAI) (accessed on 23 May 2024).
- Khankhel, Z.S.; Dillon, R.J.; Thosar, M.; Bruno, C.; Puzniak, L. Ceftolozane/tazobactam for the treatment of bacteremia: A systematic literature review (SLR). Ann. Clin. Microbiol. Antimicrob. 2022, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Andrei, S.; Valeanu, L.; Chirvasuta, R.; Stefan, M.G. New FDA approved antibacterial drugs: 2015–2017. Discoveries 2018, 6, e81. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Delafloxacin—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000,208611s000lbl.pdf (accessed on 1 June 2024).
- European Medicines Agency. Quofenix—Delafloxacin. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/quofenix#authorisation-details (accessed on 23 May 2024).
- Shi, Z.; Zhang, J.; Tian, L.; Xin, L.; Liang, C.; Ren, X.; Li, M. A comprehensive overview of the antibiotics approved in the last two decades: Retrospects and prospects. Molecules 2023, 28, 1762. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, I.; Canton, R.; Vila, J.; Gargallo-Viola, D.; Zsolt, I.; Garcia-Castillo, M.; Lopez, Y. Microbiological profile of ozenoxacin. Future Microbiol. 2019, 14, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Van Gerwen, O.T.; Legendre, D. Secnidazole: A treatment for trichomoniasis in adolescents and adults. Expert Rev. Anti Infect. Ther. 2022, 20, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Vaborem (Meropenem/Vaborbactam)—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaborem#authorisation-details (accessed on 23 May 2024).
- Kaufman, M.B. Pharmaceutical approval update. Pharm. Ther. 2018, 43, 528–530. [Google Scholar] [PubMed]
- Lee, Y.R.; Burton, C.E. Eravacycline, a newly approved fluorocycline. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, L.S.; Armstrong, T.P.; Kresken, M.; Emery, C.L.; Ying, Y.X.; Sauvonnet, V.; Zambardi, G. Multicenter clinical evaluation of ETEST eravacycline for susceptibility testing of Enterobacteriaceae and Enterococci. J. Clin. Microbiol. 2023, 61, e0165022. [Google Scholar] [CrossRef] [PubMed]
- Markham, A.; Keam, S.J. Omadacycline: First global approval. Drugs 2018, 78, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Deeks, E.D. Sarecycline: First global approval. Drugs 2019, 79, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.A. Imipenem/cilastatin/relebactam: A review in gram-negative bacterial infections. Drugs 2021, 81, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, D.; Matsumoto, S.; Kishi, N.; Ishii, Y.; Mori, M. In vitro antibacterial activity of imipenem/relebactam against clinical isolates in Japan. Microbiol. Spectr. 2022, 10, e0223521. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Pretomanid: First approval. Drugs 2019, 79, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; File, T.M. Lefamulin: A novel semisynthetic pleuromutilin antibiotic for community-acquired bacterial pneumonia. Clin. Infect. Dis. 2020, 71, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Thakare, R.; Singh, S.; Dasgupta, A.; Chopra, S. Lascufloxacin hydrochloride to treat bacterial infection. Drugs Today 2020, 56, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Russo, A.; Peghin, M. Inhaled liposomal antimicrobial delivery in lung infections. Drugs 2020, 80, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Zangiabadian, M.; Malekshahian, D.; Arabpour, E.; Abadi, S.S.D.; Yazarlou, F.; Bostanghadiri, N.; Centis, R.; Aghababa, A.A.; Farahbakhsh, M.; Nasiri, M.J.; et al. Amikacin liposome and Mycobacterium avium complex: A systematic review. PLoS ONE 2022, 17, e0279714. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Amikacin Liposome—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/arikayce-liposomal-product-information_en.pdf (accessed on 1 June 2024).
- El-Lababidi, R.M.; Rizk, J.G. Cefiderocol: A siderophore cephalosporin. Ann. Pharmacother. 2020, 54, 1215–1231. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Cefiderocol—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209445s002lbl.pdf (accessed on 1 June 2024).
- European Medicines Agency. Fetcroja (Cefiderocol)—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/fetcroja-epar-product-information_en.pdf (accessed on 1 June 2024).
- Shionogi. Regarding the Acquisition of Manufacturing and Marketing Approval for the New Siderophore Cephalosporin Antibiotic Fetroja. Available online: https://www.shionogi.com/global/en/news/2023/11/20231130.html (accessed on 23 May 2024).
- Keam, S.J. Sulbactam/durlobactam: First approval. Drugs 2023, 83, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Verdin, P. FDA new drug approvals in Q1 2024. Nat. Rev. Drug Discov. 2024, 23, 331. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Cefepime/Enmetazobactam—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/exblifep-epar-product-information_en.pdf (accessed on 1 June 2024).
- Mohareb, A.M.; Letourneau, A.R.; Sánchez, S.M.; Walensky, R.P.; Hyle, E.P. Addressing antibiotic overuse in the outpatient setting: Lessons from behavioral economics. Mayo Clin. Proc. 2021, 96, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Bologna, E.; Licari, L.C.; Manfredi, C.; Ditonno, F.; Cirillo, L.; Fusco, G.M.; Abate, M.; Passaro, F.; Di Mauro, E.; Crocetto, F.; et al. Carbapenem-resistant Enterobacteriaceae in urinary tract infections: From biological insights to emerging therapeutic alternatives. Medicina 2024, 60, 214. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Thapaliya, D.; Lemonovich, T.L.; Bonomo, R.A. Gepotidacin: A novel, oral, ‘first-in-class’ triazaacenaphthylene antibiotic for the treatment of uncomplicated urinary tract infections and urogenital gonorrhoea. J. Antimicrob. Chemother. 2023, 78, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Cuningham, W.; Perera, S.; Coulter, S.; Wang, Z.; Tong, S.Y.C.; Wozniak, T.M. Repurposing antibiotic resistance surveillance data to support treatment of recurrent infections in a remote setting. Sci. Rep. 2024, 14, 2414. [Google Scholar] [CrossRef] [PubMed]
- Shafrin, J.; Marijam, A.; Joshi, A.V.; Mitrani-Gold, F.S.; Everson, K.; Tuly, R.; Rosenquist, P.; Gillam, M.; Ruiz, M.E. Economic burden of antibiotic-not-susceptible isolates in uncomplicated urinary tract infection: Analysis of a US integrated delivery network database. Antimicrob. Resist. Infect. Control 2022, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, S.; Yasuda, M.; Miyazaki, J.; Wada, K.; Matsumoto, M.; Hayami, H.; Yamamoto, S.; Kiyota, H.; Sato, J.; et al. Fourth national Japanese antimicrobial susceptibility pattern surveillance program: Bacterial isolates from patients with complicated urinary tract infections. J. Infect. Chemother. 2024, 30, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Chlebicki, M.P. Urinary tract infections in adults. Singapore Med. J. 2016, 57, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.K.; Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef] [PubMed]
- Vouloumanou, E.K.; Rafailidis, P.I.; Kazantzi, M.S.; Athanasiou, S.; Falagas, M.E. Early switch to oral versus intravenous antimicrobial treatment for hospitalized patients with acute pyelonephritis: A systematic review of randomized controlled trials. Curr. Med. Res. Opin. 2008, 24, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Antibacterials, Principles of Therapy. Available online: https://bnf.nice.org.uk/treatment-summaries/antibacterials-principles-of-therapy/ (accessed on 5 December 2024).
- Yoshikura, H. Changing demography of genital chlamydia, gonorrhea, genital herpes, condyloma, and syphilis infections in Japan. Jpn. J. Infect. Dis. 2021, 74, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Gonorrhea—About Gonorrhea. Available online: https://www.cdc.gov/gonorrhea/about/index.html (accessed on 23 May 2024).
- Tsevat, D.G.; Wiesenfeld, H.C.; Parks, C.; Peipert, J.F. Sexually transmitted diseases and infertility. Am. J. Obstet. Gynecol. 2017, 216, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Suay-García, B.; Pérez-Gracia, M.T. Future prospects for Neisseria gonorrhoeae treatment. Antibiotics 2018, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Shimuta, K.; Unemo, M.; Nakayama, S.-I.; Morita-Ishihara, T.; Dorin, M.; Kawahata, T.; Ohnishi, M.; Antibiotic-Resistant Gonorrhea Study Group. Antimicrobial Resistance and Molecular Typing of Neisseria gonorrhoeae Isolates in Kyoto and Osaka, Japan, 2010 to 2012: Intensified Surveillance after Identification of the First Strain (H041) with High-Level Ceftriaxone Resistance. Antimicrob. Agents Chemother. 2013, 57, 5225–5232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hogben, M.; Kidd, S.; Burstein, G.R. Expedited partner therapy for sexually transmitted infections. Curr. Opin. Obstet. Gynecol. 2012, 24, 299–304. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.; Avent, M. Oral or intravenous antibiotics? Aust. Prescr. 2020, 43, 45–48. [Google Scholar] [CrossRef] [PubMed]
- García-Castro, M.; Sarabia, F.; Díaz-Morilla, A.; López-Romero, J.M. Approved antibacterial drugs in the last 10 years: From the bench to the clinic. Explor. Drug Sci. 2023, 1, 180–209. [Google Scholar] [CrossRef]
- Pharmaceuticals and Medical Devices Agency. List of Approved Drugs April 2004 to March 2025. Available online: https://www.pmda.go.jp/files/000274881.pdf (accessed on 1 July 2025).
- US Food & Drug Aministration. Sivextro—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/205435s012,205436s007lbl.pdf (accessed on 4 June 2025).
- European Medicines Agency. Sivextro—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/sivextro-epar-product-information_en.pdf (accessed on 4 June 2025).
- European Medicines Agency. Tenkasi—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/tenkasi-epar-product-information_en.pdf (accessed on 4 June 2025).
- US Food and Drug Administration. ORBACTIV—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/206334s006lbl.pdf (accessed on 4 June 2025).
- Kegg Drug Database. New Drug Approvals in Japan. Available online: https://www.kegg.jp/kegg/drug/br08318.html?id=D10779 (accessed on 7 November 2024).
- US Food & Drug Aministration. Xifaxan—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021361s023lbl.pdf (accessed on 4 June 2025).
- Markham, A. Delafloxacin: First global approval. Drugs 2017, 77, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- US Food & Drug Aministration. Sirturo—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/204384s000lbl.pdf (accessed on 4 June 2025).
- European Medicines Agency. Sirturo—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/sirturo-epar-product-information_en.pdf (accessed on 4 June 2025).
- US Food & Drug Aministration. Dificid—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213138lbl.pdf (accessed on 4 June 2025).
- European Medicines Agency. Dificlir—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/dificlir-epar-product-information_en.pdf (accessed on 4 June 2025).
- US Food & Drug Aministration. Aemcolo—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210910s000lbl.pdf (accessed on 4 June 2025).
- Merck. FDA Approves Merck’s RECARBRIO™ (Imipenem, Cilastatin, and Relebactam) for the Treatment of Adults with Complicated Urinary Tract and Complicated Intra-Abdominal Bacterial Infections Where Limited or No Alternative Treatment Options Are Available. Available online: https://www.merck.com/news/fda-approves-mercks-recarbrio-imipenem-cilastatin-and-relebactam-for-the-treatment-of-adults-with-complicated-urinary-tract-and-complicated-intra-abdominal-bacterial-infections-w/ (accessed on 28 November 2024).
- European Medicines Agency. Recarbrio. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/recarbrio (accessed on 28 November 2024).
- European Medicines Agency. Dovprela—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/dovprela-previously-pretomanid-fgk-epar-product-information_en.pdf (accessed on 1 June 2024).
- Insmed. ARIKAYCE (Amikacin Liposome Inhalation Suspension) Approved by Japan’s Ministry of Health, Labour and Welfare for the Treatment of Patients with NTM Lung Disease Caused by MAC Who Did Not Sufficiently Respond to Prior Treatment with MDR. Available online: https://investor.insmed.com/2021-03-23-ARIKAYCE-R-amikacin-liposome-inhalation-suspension-Approved-by-Japans-Ministry-of-Health-Labour-and-Welfare-for-the-Treatment-of-Patients-with-NTM-Lung-Disease-Caused-by-MAC-Who-Did-Not-Sufficiently-Respond-to-Prior-Treatment-with-MDR (accessed on 1 June 2024).
- European Medicines Agency. Exblifep. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/exblifep#authorisation-details (accessed on 28 November 2024).
- US Food & Drug Aministration. FDA Approves New Treatment for Uncomplicated Urinary Tract Infections in Adult Women Who Have Limited or No Alternative Oral Antibiotic Treatment Options. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-new-treatment-uncomplicated-urinary-tract-infections-adult-women-who-have-limited-or-no (accessed on 21 March 2025).
- GSK. Blujepa (Gepotidacin) [Package Insert]. Available online: www.accessdata.fda.gov/drugsatfda_docs/label/2025/218230s000lbl.pdf (accessed on 21 March 2025).
- AbbVie. Emblaveo (Aztreonam and Avibactam)—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/217906Orig1s000lbl.pdf (accessed on 16 May 2025).
- AbbVie. Emblaveo (Aztreonam and Avibactam)—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/emblaveo-epar-product-information_en.pdf (accessed on 16 May 2025).
- Aoki, W.; Uwamino, Y.; Kamoshita, Y.; Inose, R.; Nagata, M.; Hasegawa, N.; Matsushita, H. In vitro activity of aztreonam-avibactam combination against blood culture isolates of Stenotrophomonas maltophilia in Japan before the launch of ceftazidime-avibactam. Microbiol. Spectr. 2025, 13, e0331624. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, H.; Sato, K.; Kajitani, H.; Akaki, T.; Shishido, S. Comparative antimicrobial activities of the newly synthesized quinolone WQ-3034, levofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis and Mycobacterium avium complex. Antimicrob. Agents Chemother. 2000, 44, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Singley, C.M.; Hoover, J.; Nakamura, R.; Echols, R.; Rittenhouse, S.; Tsuji, M.; Yamano, Y. Efficacy of cefiderocol against carbapenem-resistant Gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob. Agents Chemother. 2017, 61, e00700-17. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Tsutani, K. Approval of new drugs 1999–2007: Comparison of the US, the EU and Japan situations. J. Clin. Pharm. Ther. 2010, 35, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Outterson, K.; Rex, J.H.; Jinks, T.; Jackson, P.; Hallinan, J.; Karp, S.; Hung, D.T.; Franceschi, F.; Merkeley, T.; Houchens, C.; et al. Accelerating global innovation to address antibacterial resistance: Introducing CARB-X. Nat. Rev. Drug Discov. 2016, 15, 589–590. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Tripartite Meeting Held Between EMA, FDA and PMDA at EMA, London, on 1–2 September 2016 to Discuss Regulatory Approaches for the Evaluation of Antibacterial Agents. Available online: https://www.ema.europa.eu/en/documents/other/tripartite-meeting-held-between-ema-fda-and-pmda-ema-london-1-2-september-2016-discuss-regulatory-approaches-evaluation-antibacterial-agents_en.pdf (accessed on 21 March 2025).
- European Medicines Agency. Meeting Summary—Second Tripartite Meeting Held Between EMA, PMDA and FDA to Discuss Regulatory Approaches for the Evaluation of Antibacterial Agents. Available online: https://www.ema.europa.eu/en/documents/other/meeting-summary-second-tripartite-meeting-held-between-ema-pmda-and-fda-discuss-regulatory-approaches-evaluation-antibacterial-agents_en.pdf (accessed on 21 March 2025).
- Maruyama, M.; Tsujimura, K.; Kasahara, M.; Yamabe, K. Price comparison of new drugs in Japan, EU and US. Value Health 2017, 20, A655–A656. [Google Scholar] [CrossRef][Green Version]
- Takayama, A.; Narukawa, M. Comparison of new drug accessibility and price between Japan and major European countries. Ther. Innov. Regul. Sci. 2017, 51, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Nagashima, M.; Kawai, N.; Ohmagari, N.; Tateda, K. A narrative review on drug development for the management of antimicrobial- resistant infection crisis in Japan: The past, present, and future. Expert Rev. Anti Infect. Ther. 2022, 20, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Japan Cooperative Clinical Study Group for Co-trimoxazole. Trimethoprim-sulfamethoxazole in the treatment of bacterial infections: Report of clinical trials in Japan. J. Infect. Dis. 1973, 128 (Suppl. 3), S629–S635. [Google Scholar] [CrossRef] [PubMed]
- Mimura, W.; Shinjo, D.; Shoji, K.; Fushimi, K. Prescribed daily-dose-based metrics of oral antibiotic use for hospitalized children in Japan. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e24. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Moller, N.; Muller, A.E.; Paccaud, J.P.; Mouton, J.W. Oral amoxicillin and amoxicillin-clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Kurimoto, Y.; Ezumi, M.; Nakatani, K.; Mizunaga, S.; Yamagishi, Y.; Mikamo, H. In vitro and in vivo antibacterial activity of nitrofurantoin against clinical isolates of E. coli in Japan and evaluation of biological cost of nitrofurantoin resistant strains using a mouse urinary tract infection model. J. Infect. Chemother. 2021, 27, 250–255. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Approves New Treatment for Uncomplicated Urinary Tract Infections. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-uncomplicated-urinary-tract-infections (accessed on 23 May 2024).
- Muraki, Y.; Yagi, T.; Tsuji, Y.; Nishimura, N.; Tanabe, M.; Niwa, T.; Watanabe, T.; Fujimoto, S.; Takayama, K.; Murakami, N.; et al. Japanese antimicrobial consumption surveillance: First report on oral and parenteral antimicrobial consumption in Japan (2009–2013). J. Glob. Antimicrob. Resist. 2016, 7, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, A.; Yahara, K.; Shibayama, K. Trends and patterns of national antimicrobial consumption in Japan from 2004 to 2016. J. Infect. Chemother. 2018, 24, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, S. Research and Development of Quinolones in Daiichi Sankyo Co., Ltd. Infect. Update 2018, 47–57. Available online: https://infectweb.com/app/uploads/en/2018/03/03_SpeFeature.pdf (accessed on 3 July 2025).
- Fischer, J.; Ganellin, C.R. (Eds.) Analogue-based drug discovery; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2006. [Google Scholar]
- Stone, K.C.; Dagan, R.; Arguedas, A.; Leibovitz, E.; Wang, E.; Echols, R.M.; Janjic, N.; Critchley, I.A. Activity of faropenem against middle ear fluid pathogens from children with acute otitis media in Costa Rica and Israel. Antimicrob. Agents Chemother. 2007, 51, 2230–2235. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, C.; Cai, L.; Liu, S.; Zheng, Z.; Li, G.; Chen, J.; Sui, Q. Crystal structure of tebipenem pivoxil. Acta Crystallogr. E Crystallogr. Commun. 2018, 74, 1215–1217. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yin, Y.; Peng, X.; Zheng, H.; Fu, F.; Liu, Z.; Wu, X.; Wu, X.; Zheng, S.; Chen, N.; et al. A randomized, active-controlled, multicentre clinical trial to evaluate the efficacy and safety of oral sitafloxacin versus levofloxacin in Chinese adults with acute uncomplicated or complicated urinary tract infection. Ann. Med. 2021, 53, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kyorin Pharmaceutical. KYORIN and Nanjing Neiwa Faith Signed License Agreement for Lascufloxacin in China. Available online: https://www.kyorin-pharm.co.jp/en/news/KYORIN%20and%20Nanjing%20Neiwa%20Faith%20Signed%20License%20Agreement%20for%20Lascufloxacin%20in%20China.pdf (accessed on 1 June 2024).
- Pharmaceuticals and Medical Devices Agency. Summary of Investigation Results Tosufloxacin Tosilate Hydrate (for Oral Use). Available online: https://www.pmda.go.jp/files/000223964.pdf (accessed on 10 December 2024).
- Griffith, D.C.; Morgan, E.E.; Dudley, M.N.; Loutit, J.S. A Phase 1 study of the safety, tolerability, and pharmacokinetics of biapenem in healthy adult subjects. Antimicrob. Agents Chemother. 2021, 65, e02612-20. [Google Scholar] [CrossRef] [PubMed]
- Goa, K.L.; Noble, S. Panipenem/betamipron. Drugs 2003, 63, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Sitafloxacin: In bacterial infections. Drugs 2011, 71, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Kosai, K.; Yamauchi, S.; Sasaki, D.; Kaku, N.; Uno, N.; Morinaga, Y.; Hasegawa, H.; Miyazaki, T.; Izumikawa, K.; et al. In vitro activity of lascufloxacin against Streptococcus pneumoniae with mutations in the quinolone resistance-determining regions. Antimicrob. Agents Chemother. 2018, 62, e01971-17. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Sasaki, T.; Islam, J.; Tominaga, T.; Nochi, T.; Hara, K.; Tanemura, K. Effects of early-life tosufloxacin tosilate hydrate administration on growth rate, neurobehavior, and gut microbiota at adulthood in male mice. J. Toxicol. Sci. 2023, 48, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Xu, J.; Ischia, J.; Bolton, D. Fluoroquinolone resistance in urinary tract infections: Epidemiology, mechanisms of action and management strategies. BJUI Compass 2024, 5, 5–11. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone and Fluoroquinolone Antibiotics. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products (accessed on 27 November 2024).
- US Food & Drug Aministration. FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics (accessed on 1 July 2025).
- Iacobucci, G. Fluoroquinolone antibiotics: Prescribe only as last resort, says UK regulator. BMJ 2024, 384, q183. [Google Scholar] [CrossRef] [PubMed]
- Ohmagari, N. National action plan on antimicrobial resistance (AMR) 2016–2020 and relevant activities in Japan. Glob. Health Med. 2019, 1, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Daiichi Sankyo Company Ltd. Broad-Spectrum Oral Antibacterial Agent Gracevit® Tablets 50 mg/fine Granules 10% Receives Approval for Manufacturing and Marketing. Available online: https://www.daiichisankyo.com/files/news/pressrelease/pdf/005667/news2008_01_28_107_en.pdf (accessed on 1 June 2024).
- Kobayashi, I.; Matsuzaki, K.; Omika, K.; Hasegawa, M.; Sato, Y. Antibacterial activity of tosufloxacin against major organisms detected from patients with respiratory infections. Jpn. J. Antibiot. 2007, 60, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Baba, H. Faropenem. In Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterials, Antifungal, Antiparasitic, and Antiviral Drugs, 7th ed.; Grayson, M.L., Cosgrove, S., Crowe, S., Hope, W., McCarthy, J., Mills, J., Mouton, J.W., Paterson, D., Eds.; CRC Press: Boca Raton, FL, USA, 2017; Chapter 41. [Google Scholar]
- Daiichii-Sankyo Company Ltd. Reference Data (FY2005 1st Half). Available online: https://www.daiichisankyo.com/files/news/ir/pdf/005171/ser2005_11_07_19_03.pdf (accessed on 10 December 2024).
- Synapse by Patsnap. Panipenem/Betamipron. Available online: https://synapse.patsnap.com/drug/9875dd7137f049ad8b9e5180bf7777f6#approval (accessed on 10 December 2024).
- Jain, A.; Utley, L.; Parr, T.R.; Zabawa, T.; Pucci, M.J. Tebipenem, the first oral carbapenem antibiotic. Expert Rev. Anti Infect. Ther. 2018, 16, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Takahashi, S.; Mitrani-Gold, F.S.; Mulgirigama, A.; Ferrinho, D.A. A systematic scoping review of faropenem and other oral penems: Treatment of Enterobacterales infections, development of resistance and cross-resistance to carbapenems. JAC Antimicrob. Resist. 2022, 4, dlac125. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo Pharma. Sumitomo Dainippon Pharma Announces Partnership with Zuellig Pharma on its Carbapenem Antibiotic Meropenem for Five Countries of South-East Asia and Hong Kong. Available online: https://www.sumitomo-pharma.com/news/20170609-1.html (accessed on 28 November 2024).
- Smith, H.Z.; Hollingshead, C.M.; Kendall, B. Carbapenem-Resistant Enterobacterales; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO global priority pathogen list: A bibliometric analysis of Medline-PubMed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Dax, S.L. Biapenem American Cyanamid Co. IDrugs 1998, 1, 247–255. [Google Scholar] [PubMed]
- Tune, B.M. Nephrotoxicity of beta-lactam antibiotics: Mechanisms and strategies for prevention. Pediatr. Nephrol. 1997, 11, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Takesue, Y.; Nakajima, K.; Ichiki, K.; Ishikawa, K.; Yamada, K.; Tsuchida, T.; Otani, N.; Takahashi, Y.; Ishihara, M.; et al. Correlation between antimicrobial resistance and the wide diverse use of broad-spectrum antibiotics by the Antimicrobial Stewardship Program in Japan. Pharmaceutics 2023, 15, 518. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Available online: https://iris.who.int/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 (accessed on 5 June 2025).
- World Health Organization. Library of AMR National Action Plans. Available online: https://www.who.int/teams/surveillance-prevention-control-AMR/national-action-plan-monitoring-evaluation/library-of-national-action-plans (accessed on 5 June 2025).
- The Government of Japan. National Action Plan on Antimicrobial Resistance (AMR) 2016–2022. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000138942.pdf (accessed on 11 December 2024).
- The White House. United States: National Action Plan for Combating Antibiotic-Resistant Bacteria. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/national-action-plan-for-combating-antibiotic-resistant-bacteria.pdf?sfvrsn=bf707027_1&download=true (accessed on 4 June 2025).
- HM Government. United Kingdom of Great Britain and Northern Ireland: UK Five Year Antimicrobial Resistance Strategy. Available online: https://cdn.who.int/media/docs/default-source/antimicrobial-resistance/amr-spc-npm/nap-library/uk_amr_5_year_national_action_plan-2019-24.pdf?sfvrsn=614851a5_1&download=true (accessed on 4 June 2025).
- Honda, H.; Goto, T.; Uehara, Y.; Takamatsu, A. Promotion of antimicrobial stewardship following issuance of the antimicrobial resistance national action plan in Japan: A systematic review of 2016–2020. Int. J. Antimicrob. Agents 2023, 62, 106829. [Google Scholar] [CrossRef] [PubMed]
- Government of Japan Ministry of Health Labour and Welfare Health Service Bureau Tuberculosis and Infectious Diseases Control Division. Manual of Antimicrobial Stewardship. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000193504.pdf (accessed on 1 April 2024).
- Kusama, Y.; Tsuzuki, S.; Muraki, Y.; Koizumi, R.; Ishikane, M.; Ohmagari, N. The effects of Japan’s National Action Plan on antimicrobial resistance on antimicrobial use. Int. J. Infect. Dis. 2021, 103, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Goto, R.; Inose, R.; Shoji, T.; Akazawa, M.; Muraki, Y. Current status and challenges of health economic evaluations related to antimicrobial stewardship in Japan: A scoping review up to 2023. J. Infect. Chemother. 2025, 31, 102657. [Google Scholar] [CrossRef] [PubMed]
- The Task Force for Combating Antibiotic-Resistant Bacteria. National Action Plan for Combating Antibiotic-Resistant Bacteria: YEAR 5 Report. Available online: https://aspe.hhs.gov/sites/default/files/documents/d5d01eb69710588247eb2aef3a46c118/HHS_ASPE_CARB_Report_Year5.pdf (accessed on 4 June 2025).
- GOV.UK. Fluoroquinolone Antibiotics: New Restrictions and Precautions for Use Due to Very Rare Reports of Disabling and Potentially Long-Lasting or Irreversible Side Effects. Available online: https://www.gov.uk/drug-safety-update/fluoroquinolone-antibiotics-new-restrictions-and-precautions-for-use-due-to-very-rare-reports-of-disabling-and-potentially-long-lasting-or-irreversible-side-effects (accessed on 28 November 2024).
- Hawkey, P.M.; Livermore, D.M. Carbapenem antibiotics for serious infections. BMJ. 2012, 344, e3236. [Google Scholar] [CrossRef] [PubMed]
- Koulenti, D.; Song, A.; Ellingboe, A.; Abdul-Aziz, M.H.; Harris, P.; Gavey, E.; Lipman, J. Infections by multidrug-resistant gram-negative bacteria: What’s new in our arsenal and what’s in the pipeline? Int. J. Antimicrob. Agents 2019, 53, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, D.; Haines, A.S.; Song, Z.; Murphy, A.C.; Hothersall, J.; Stephens, E.R.; Gurney, R.; Cox, R.J.; Crosby, J.; Willis, C.L.; et al. A natural plasmid uniquely encodes two biosynthetic pathways creating a potent anti-MRSA antibiotic. PLoS ONE 2011, 6, e18031. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kenshole, E.; Herisse, M.; Michael, M.; Pidot, S.J. Natural product discovery through microbial genome mining. Curr. Opin. Chem. Biol. 2021, 60, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kozuma, S.; Hirota-Takahata, Y.; Fukuda, D.; Kuraya, N.; Nakajima, M.; Ando, O. Screening and biological activities of pedopeptins, novel inhibitors of LPS produced by soil bacteria. J. Antibiot. 2014, 67, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Wasan, H.; Singh, D.; Reeta, K.H.; Gupta, Y.K. Landscape of push funding in antibiotic research: Current status and way forward. Biology 2023, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- The Joint Programming Initiative on Antimicrobial Resistance. Japan. Available online: https://www.jpiamr.eu/about/jpiamr-members/japan/ (accessed on 19 December 2024).
- Schurer, M.; Patel, R.; van Keep, M.; Horgan, J.; Matthijsse, S.; Madin-Warburton, M. Recent advances in addressing the market failure of new antimicrobials: Learnings from NICE’s subscription-style payment model. Front. Med. Technol. 2023, 5, 1010247. [Google Scholar] [CrossRef] [PubMed]
- Glover, R.E.; Singer, A.; Roberts, A.P.; Kirchhelle, C. Why is the UK subscription model for antibiotics considered successful? Lancet Microbe 2023, 4, e852–e853. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Japan’s AMR Response 2013–2025: Developing, Implementing and Evaluating National AMR Action Plans. Available online: https://iris.who.int/bitstream/handle/10665/380481/9789290620792-eng.pdf?sequence=1 (accessed on 4 June 2025).
- Shrestha, J.; Zahra, F.; Cannady, J.P. Antimicrobial stewardship; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sinawe, H.; Casadesus, D. Urine culture; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Holm, A.; Cordoba, G.; Sørensen, T.M.; Jessen, L.R.; Siersma, V.; Bjerrum, L. Point of care susceptibility testing in primary care—does it lead to a more appropriate prescription of antibiotics in patients with uncomplicated urinary tract infections? Protocol for a randomized controlled trial. BMC Fam. Pract. 2015, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial susceptibility testing: A comprehensive review of currently using methods. Antibiotics 2022, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Shafrin, J.; Marijam, A.; Joshi, A.V.; Mitrani-Gold, F.S.; Everson, K.; Tuly, R.; Rosenquist, P.; Gillam, M.; Ruiz, M.E. Impact of suboptimal or inappropriate treatment on healthcare resource use and cost among patients with uncomplicated urinary tract infection: An analysis of integrated delivery network electronic health records. Antimicrob. Resist. Infect. Control 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Alkhawaldeh, R.; Abu Farha, R.; Abu Hammour, K.; Alefishat, E. Optimizing antimicrobial therapy in urinary tract infections: A focus on urine culture and sensitivity testing. Front. Pharmacol. 2022, 13, 1058669. [Google Scholar] [CrossRef] [PubMed]
- Durukan, D.; Read, T.R.H.; Murray, G.; Doyle, M.; Chow, E.P.F.; Vodstrcil, L.A.; Fairley, C.K.; Aguirre, I.; Mokany, E.; Tan, L.Y.; et al. Resistance-guided antimicrobial therapy using doxycycline–moxifloxacin and doxycycline–2.5 g azithromycin for the treatment of mycoplasma genitalium infection: Efficacy and tolerability. Clin. Infect. Dis. 2019, 71, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Adawiyah, R.A.; Bradshaw, C.S.; Vodstrcil, L.A.; Fairley, C.K.; Zhang, L.; Ong, J.J. Cost-effectiveness of resistance-guided therapy for Mycoplasma genitalium in Australia. Sci. Rep. 2024, 14, 12856. [Google Scholar] [CrossRef] [PubMed]
- Read, T.R.H.; Fairley, C.K.; Murray, G.L.; Jensen, J.S.; Danielewski, J.; Worthington, K.; Doyle, M.; Mokany, E.; Tan, L.; Chow, E.P.F.; et al. Outcomes of resistance-guided sequential treatment of Mycoplasma genitalium infections: A prospective evaluation. Clin. Infect. Dis. 2019, 68, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Klausner, J.D.; Bristow, C.C.; Soge, O.O.; Shahkolahi, A.; Waymer, T.; Bolan, R.K.; Philip, S.S.; Asbel, L.E.; Taylor, S.N.; Mena, L.A.; et al. Resistance-guided treatment of gonorrhea: A prospective clinical study. Clin. Infect. Dis. 2021, 73, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, H.; Fujii, N.; Tajima, T.; Suzuki, S.; Tsuzuki, S.; Matsunaga, N.; Ohmagari, N. Cumulative antibiogram preparation among hospitals participating in the Japan Surveillance for Infection Prevention and Healthcare Epidemiology. J. Infect. Chemother. 2024, 30, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Mohan, R.; Mach, K.E.; Sin, M.L.Y.; Anikst, V.; Buscarini, M.; Wong, P.K.; Gau, V.; Banaei, N.; Liao, J.C. Integrated biosensor assay for rapid uropathogen identification and phenotypic antimicrobial susceptibility testing. Eur. Urol. Focus. 2017, 3, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.R.; Scangarella-Oman, N.E.; Millns, H.; Flight, W.; Gatsi, S.; Jakielaszek, C.; Janmohamed, S.; Lewis, D.A. Efficacy and safety of gepotidacin as treatment of uncomplicated urogenital gonorrhea (EAGLE-1): Design of a randomized, comparator-controlled, Phase 3 study. Infect. Dis. Ther. 2023, 12, 2307–2320. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; McNulty, A.; Avery, A.; Whiley, D.; Tabrizi, S.N.; Hardy, D.; Das, A.F.; Nenninger, A.; Fairley, C.K.; Hocking, J.S.; et al. Solithromycin versus ceftriaxone plus azithromycin for the treatment of uncomplicated genital gonorrhoea (SOLITAIRE-U): A randomised phase 3 non-inferiority trial. Lancet Infect. Dis. 2019, 19, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.W.; Aronin, S.I.; Das, A.F.; Akinapelli, K.; Zelasky, M.T.; Puttagunta, S.; Boucher, H.W. Sulopenem or ciprofloxacin for the treatment of uncomplicated urinary tract infections in women: A Phase 3, randomized trial. Clin. Infect. Dis. 2023, 76, 66–77. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Zoliflodacin in Uncomplicated Gonorrhoea. Available online: https://clinicaltrials.gov/study/NCT03959527?term=NCT03959527&rank=1 (accessed on 6 December 2024).
- ClinicalTrials.gov. Oral Sulopenem-Etzadroxil/Probenecid Versus Ciprofloxacin for Uncomplicated Urinary Tract Infection in Adult Women. Available online: https://clinicaltrials.gov/study/NCT03354598?term=NCT03354598&rank=1 (accessed on 17 December 2024).
- ClinicalTrials.gov. A Study to Investigate the Efficacy and Safety with Gepotidacin in Japanese Female Participants with Uncomplicated Urinary Tract Infection (Acute Cystitis) (EAGLE-J). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05630833 (accessed on 23 May 2024).
- Bradford, P.A.; Miller, A.A.; O’Donnell, J.; Mueller, J.P. Zoliflodacin: An oral spiropyrimidinetrione antibiotic for the treatment of Neisseria gonorrheae, including multi-drug-resistant isolates. ACS Infect. Dis. 2020, 6, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Kurono, Y.; Kudo, F.; Watanabe, A.; Yamatake, T.; Shimada, S.; Suzuki, K. Efficacy, safety, and tissue penetration of solithromycin in Japanese patients with otorhinolaryngological infections. Auris Nasus Larynx 2024, 51, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.; Perry, C.R.; Hooton, T.M.; Scangarella-Oman, N.E.; Millns, H.; Powell, M.; Jarvis, E.; Dennison, J.; Sheets, A.; Butler, D.; et al. Oral gepotidacin versus nitrofurantoin in patients with uncomplicated urinary tract infection (EAGLE-2 and EAGLE-3): Two randomised, controlled, double-blind, double-dummy, Phase 3, non-inferiority trials. Lancet 2024, 403, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.G.; Bax, B.; Chan, P.F.; Osheroff, N. Mechanistic and structural basis for the actions of the antibacterial gepotidacin against Staphylococcus aureus gyrase. ACS Infect. Dis. 2019, 5, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Oviatt, A.A.; Gibson, E.G.; Huang, J.; Mattern, K.; Neuman, K.C.; Chan, P.F.; Osheroff, N. Interactions between gepotidacin and Escherichia coli gyrase and topoisomerase IV: Genetic and biochemical evidence for well-balanced dual-targeting. ACS Infect. Dis. 2024, 10, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.D.; Chan, P.F.; Eggleston, D.S.; Fosberry, A.; Gentry, D.R.; Gorrec, F.; Giordano, I.; Hann, M.M.; Hennessy, A.; Hibbs, M.; et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 2010, 466, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Ingraham, K.; Min, S.; Scangarella-Oman, N.; Rittenhouse, S.; Huang, J. 1249. Genetic evidence that gepotidacin shows well-balanced dual targeting against DNA gyrase and topoisomerase IV in Neisseria gonorrhoeae. Open Forum Infect. Dis. 2020, 7 (Suppl. 1), S642–S643. [Google Scholar] [CrossRef]
- Arends, S.J.R.; Butler, D.; Scangarella-Oman, N.; Castanheira, M.; Mendes, R.E. Antimicrobial activity of gepotidacin tested against Escherichia coli and Staphylococcus saprophyticus isolates causing urinary tract infections in medical centers worldwide (2019 to 2020). Antimicrob. Agents Chemother. 2023, 67, e0152522. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, S.; Golparian, D.; Scangarella-Oman, N.; Unemo, M. In vitro activity of the novel triazaacenaphthylene gepotidacin (GSK2140944) against MDR Neisseria gonorrhoeae. J. Antimicrob. Chemother. 2018, 73, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Koeth, L.M.; DiFranco-Fisher, J.M.; Scangarella-Oman, N.E. Analysis of the effect of urine on the in vitro activity of gepotidacin and levofloxacin against Escherichia coli, Staphylococcus epidermidis, and Staphylococcus saprophyticus. Diagn. Microbiol. Infect. Dis. 2023, 106, 115946. [Google Scholar] [CrossRef] [PubMed]
- GSK. EAGLE-1 Phase III Data Show Potential for Gepotidacin as a New Oral Treatment Option for Uncomplicated Urogenital Gonorrhoea (GC) amid Growing Resistance to Existing Treatments. Available online: www.gsk.com/en-gb/media/press-releases/eagle-1-phase-iii-data-show-potential-for-gepotidacin-as-a-new-oral-treatment-option-for-uncomplicated-gc/ (accessed on 18 April 2024).
- Perry, C.; Hossain, M.; Powell, M.; Raychaudhuri, A.; Scangarella-Oman, N.; Tiffany, C.; Xu, S.; Dumont, E.; Janmohamed, S. Design of two Phase III, randomized, multicenter studies comparing gepotidacin with nitrofurantoin for the treatment of uncomplicated urinary tract infection in female participants. Infect. Dis. Ther. 2022, 11, 2297–2310. [Google Scholar] [CrossRef] [PubMed]
- Buege, M.J.; Brown, J.E.; Aitken, S.L. Solithromycin: A novel ketolide antibiotic. Am. J. Health Syst. Pharm. 2017, 74, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Crabb, D.M.; Duffy, L.B. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob. Agents Chemother. 2009, 53, 2139–2141. [Google Scholar] [CrossRef] [PubMed]
- Woosley, L.N.; Castanheira, M.; Jones, R.N. CEM-101 activity against gram-positive organisms. Antimicrob. Agents Chemother. 2010, 54, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Yamagishi, Y.; Hagihara, M.; Yokoyama, Y.; Suematsu, H.; Asai, N.; Koizumi, Y.; Mikamo, H. Antimicrobial activity of solithromycin and levofloxacin against a murine pneumonia mixed-infection model caused by Streptococcus pneumoniae and anaerobic bacteria. J. Infect. Chemother. 2019, 25, 311–313. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Allergy and Infectious Diseases. NIH Statement on Preliminary Efficacy Results of First-in-Class Gonorrhea Antibiotic Developed Through Public-Private Partnership. Available online: https://www.niaid.nih.gov/news-events/nih-statement-preliminary-efficacy-results-first-class-gonorrhea-antibiotic-developed#:~:text=A%20single%20dose%20of%20a,GARDP)%2C%20the%20study%20sponsor (accessed on 23 May 2024).
- Jacobsson, S.; Golparian, D.; Oxelbark, J.; Kong, F.Y.S.; Da Costa, R.M.A.; Franceschi, F.; Brown, D.; Louie, A.; Drusano, G.; Unemo, M. Pharmacodynamics of zoliflodacin plus doxycycline combination therapy against Neisseria gonorrhoeae in a gonococcal hollow-fiber infection model. Front. Pharmacol. 2023, 14, 1291885. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage therapy for antibiotic-resistant bacterial infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. Where have all the antibiotics gone? Can. J. Infect. Dis. Med. Microbiol. 2006, 17, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Årdal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic development—economic, regulatory and societal challenges. Nat. Rev. Microbiol. 2020, 18, 267–274. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, D.; Handa, Y.; Kayama, Y.; Fujii, K.; Kawamatsu, S.; Kawano, Y.; Vojtek, I.; Powell, D.; Mulgirigama, A.; Gu, Y. The Current Landscape of Antibiotic Use and Antimicrobial Resistance in Japan: Focusing on Common Infections Including Uncomplicated Urinary Tract Infection and Gonorrhea. Antibiotics 2025, 14, 813. https://doi.org/10.3390/antibiotics14080813
Fukuda D, Handa Y, Kayama Y, Fujii K, Kawamatsu S, Kawano Y, Vojtek I, Powell D, Mulgirigama A, Gu Y. The Current Landscape of Antibiotic Use and Antimicrobial Resistance in Japan: Focusing on Common Infections Including Uncomplicated Urinary Tract Infection and Gonorrhea. Antibiotics. 2025; 14(8):813. https://doi.org/10.3390/antibiotics14080813
Chicago/Turabian StyleFukuda, Daisuke, Yutaka Handa, Yoko Kayama, Kenji Fujii, Shinya Kawamatsu, Yoshiaki Kawano, Ivo Vojtek, Danielle Powell, Aruni Mulgirigama, and Yoshiaki Gu. 2025. "The Current Landscape of Antibiotic Use and Antimicrobial Resistance in Japan: Focusing on Common Infections Including Uncomplicated Urinary Tract Infection and Gonorrhea" Antibiotics 14, no. 8: 813. https://doi.org/10.3390/antibiotics14080813
APA StyleFukuda, D., Handa, Y., Kayama, Y., Fujii, K., Kawamatsu, S., Kawano, Y., Vojtek, I., Powell, D., Mulgirigama, A., & Gu, Y. (2025). The Current Landscape of Antibiotic Use and Antimicrobial Resistance in Japan: Focusing on Common Infections Including Uncomplicated Urinary Tract Infection and Gonorrhea. Antibiotics, 14(8), 813. https://doi.org/10.3390/antibiotics14080813






