Staphylococcus aureus in Bovine Mastitis: A Narrative Review of Prevalence, Antimicrobial Resistance, and Advances in Detection Strategies
Abstract
1. Introduction
2. Literature Search Strategy
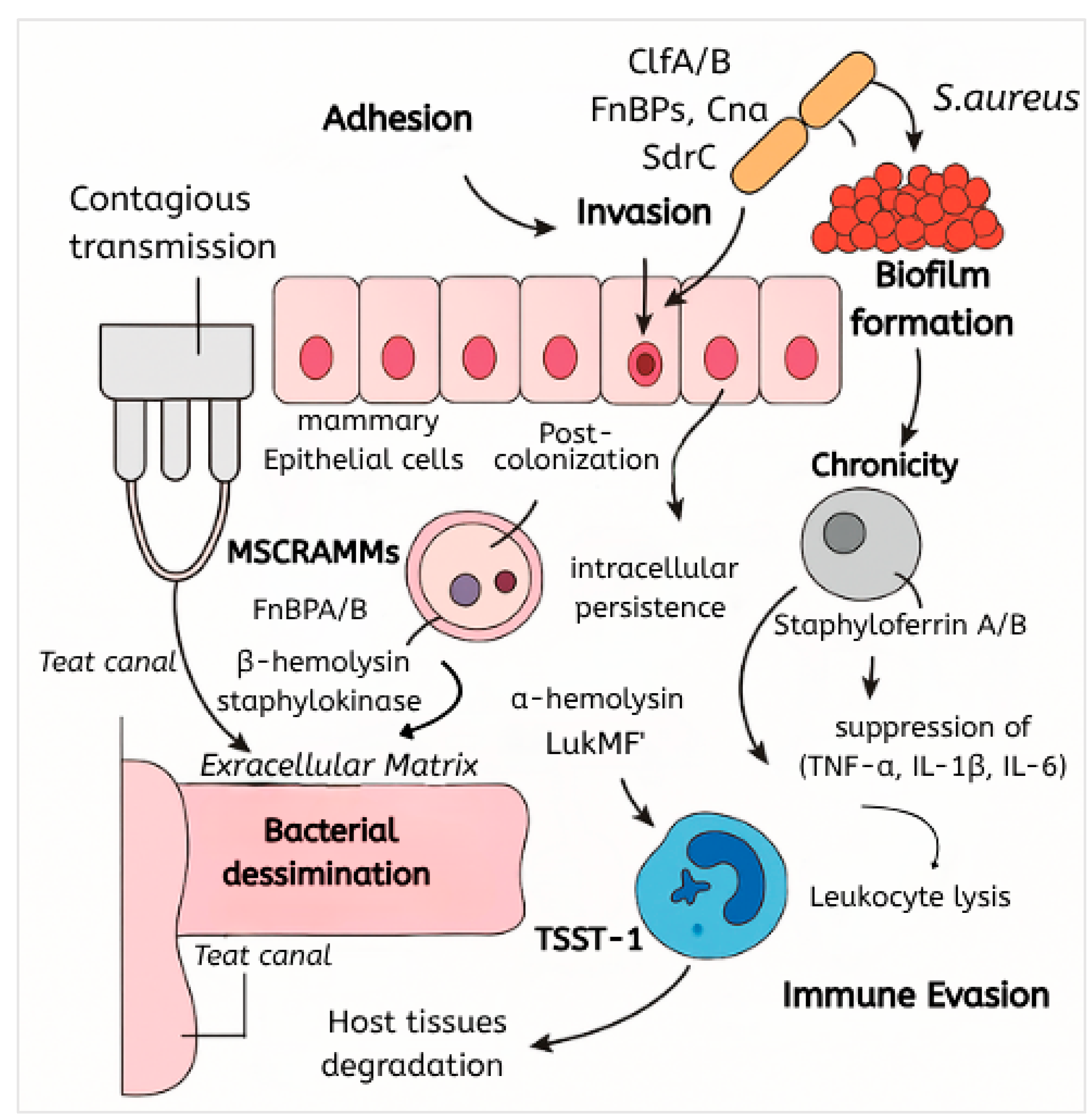
3. Pathogenesis and Virulence Factors of S. aureus in Bovine Mastitis
3.1. Overview of Pathogenesis
3.2. Adhesion and Colonization Mechanisms
3.3. Toxin Production and Tissue Damage
3.4. Biofilm Formation and Persistence
3.5. Intracellular Invasion of S. aureus in Bovine Mastitis
3.6. Immune Evasion Strategies
4. Prevalence of S. aureus in Bovine Mastitis
4.1. Geographic Distribution
4.2. Clinical vs. Subclinical Mastitis
4.3. Herd-Level Risk Factors and Management Practices
5. Resistance Profiles and Commonly Reported Antimicrobials
5.1. Resistance Rates
5.2. Detection of Specific Resistance Genes
| Continent | Country | mecA (%) | blaZ (%) | tetK (%) |
|---|---|---|---|---|
| Africa | Egypt | 100 | NR | NR |
| South Africa | NR | NR | NR | |
| Asia | Pakistan | 17.02 | 55.31 | 46.80 |
| Pakistan | 21 | NR | NR | |
| Pakistan | 44.82 | NR | NR | |
| South Korea | 6.1 | NR | NR | |
| South Korea | 10.7 | NR | NR | |
| Bangladesh | 21.42 | NR | NR | |
| Thailand | 0 | NR | NR | |
| Thailand | 0 | NR | NR | |
| China | 3.09 | NR | NR | |
| Europe | Germany | 0.8 | NR | NR |
| Ireland | NR | ~61.8 | NR | |
| North America | USA (Vermont) | 0 | 2.6–4.8 | NR |
| USA (Maine) | 0 | NR | NR | |
| Canada | 0 | NR | NR | |
| South America | Brazil | 0 | 100 | NR |
| Oceania | New Zealand | NR | 23 | NR |
- -
- mecA prevalence peaks in Egypt (100%) and Pakistan (17–44.8%), while Thailand, USA, Canada, and Brazil report 0%.
- -
- blaZ is highest in Brazil (100%), followed by Pakistan (55.3%) and New Zealand (23%).
- -
- tetK is only reported in Pakistan (46.8%); no other regions provide data.
- -
- Critical gaps exist for Africa (outside Egypt) and South America (outside Brazil). Phenotypic resistance often lacks genetic validation.
- -
- NR: not reported
5.3. Prevalence and Characteristics of MRSA
5.4. Comparative and Cumulative Resistance Patterns
6. Detection and Identification Methods for S. aureus in Bovine Mastitis
6.1. Conventional Culture and Biochemical Identification
6.2. Molecular Detection Methods
6.3. Automated and Commercial Diagnostic Systems
6.4. Comparative Performance and Validation
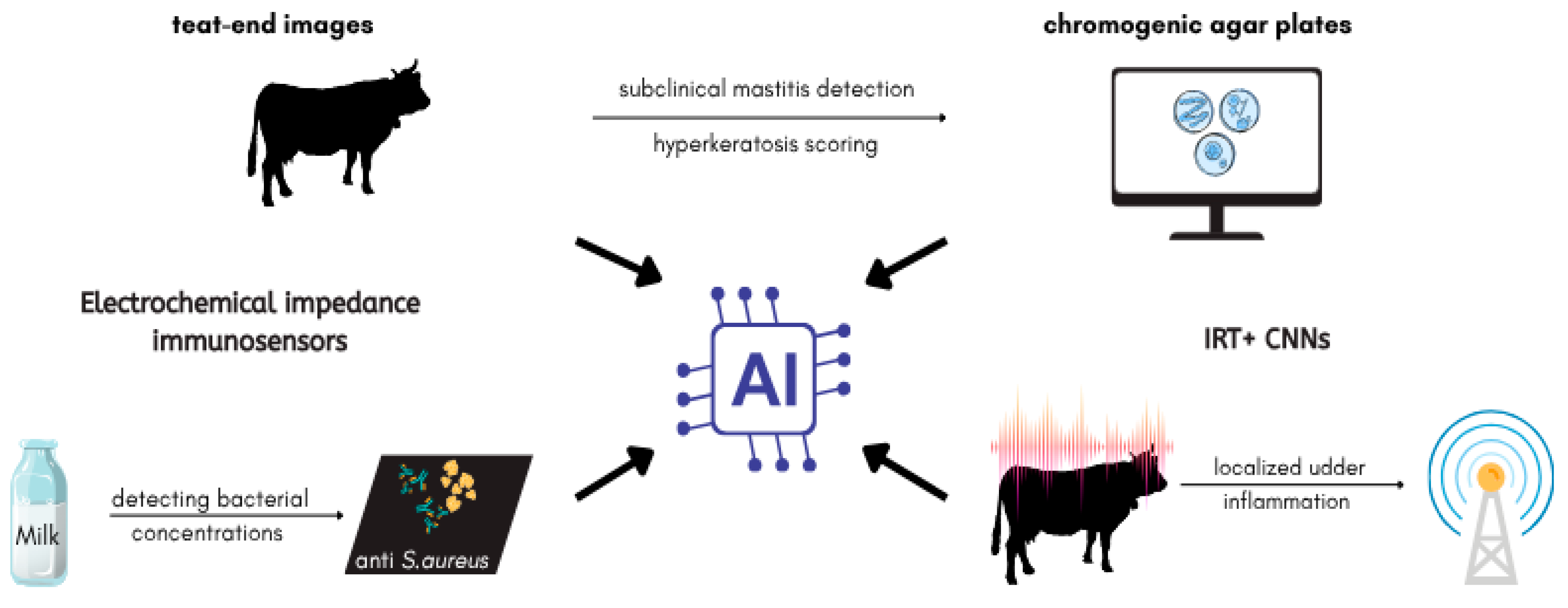
7. Application of Artificial Intelligence in the Diagnosis and Management of S. aureus-Associated Bovine Mastitis
7.1. AI-Assisted Detection of S. aureus
7.2. Predictive Modeling for Mastitis Risk and Herd Monitoring
7.3. AI-Enhanced Treatment and AMR Management
7.4. Challenges and Future Directions
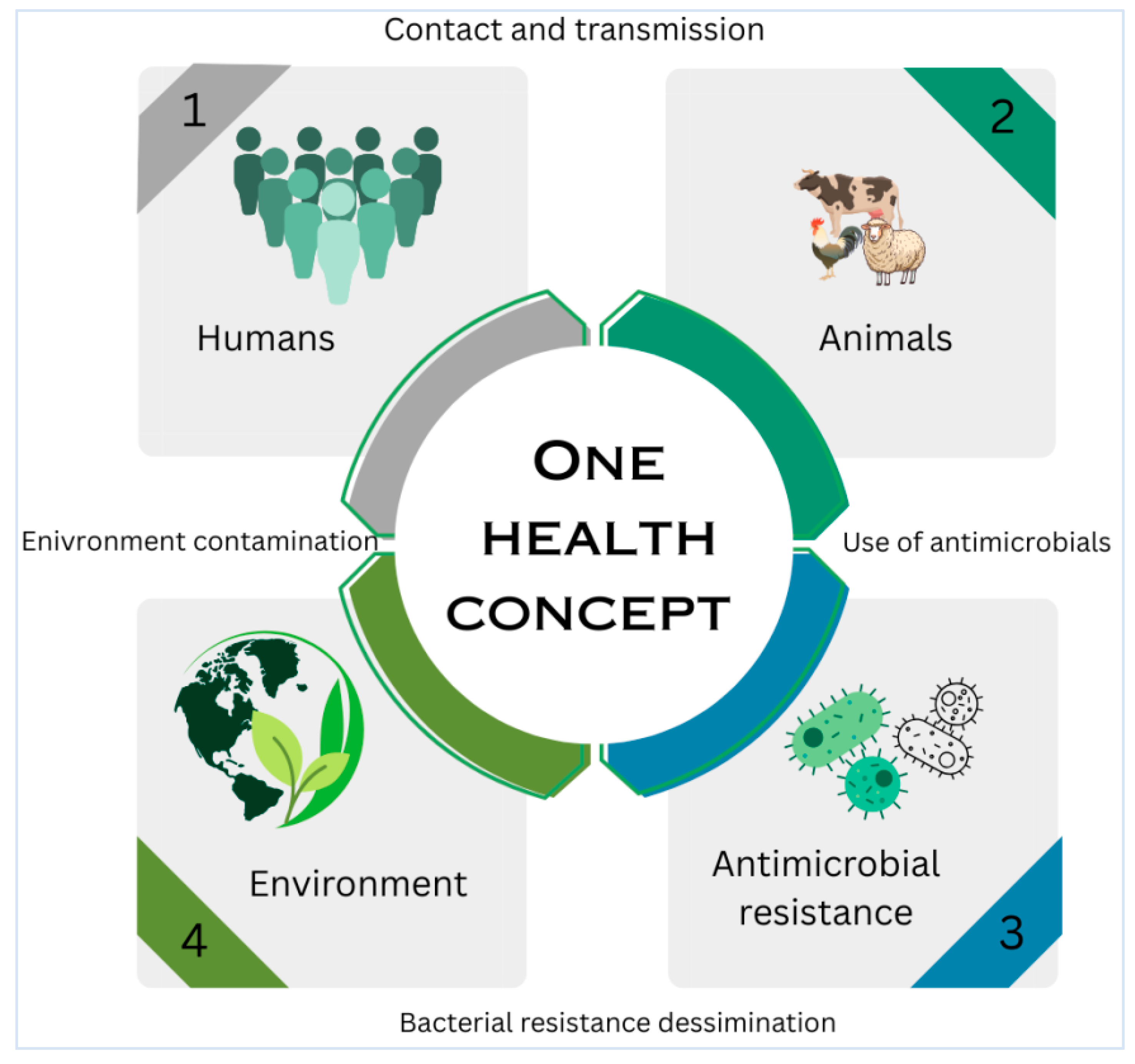
8. Public Health Implications of S. aureus from Bovine Mastitis
8.1. Zoonotic Transmission and Occupational Exposure
8.2. Foodborne Transmission and Enterotoxin-Related Risks
8.3. MRSA Strains and Human Health Threats
8.4. One Health Interventions and Surveillance
9. Prevention and Prophylaxis of S. aureus in Bovine Mastitis
9.1. Hygienic and Management Practices
9.2. Vaccination and Immunoprophylaxis
9.3. Antimicrobial Prophylaxis and Dry Cow Therapy
9.4. Selective Treatment Protocols
9.5. Resistance Concerns Linked to Preventive Antimicrobial Use
9.6. Herd-Level Outcomes and Program Efficacy
10. Future Directions
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| AI | Artificial Intelligence |
| BTM | Bulk Tank Milk |
| CFU/mL | Colony Forming Units per Milliliter |
| CMT | California Mastitis Test |
| CM | Clinical Mastitis |
| CNN | Convolutional Neural Network |
| DCT | Dry Cow Therapy |
| EF-Tu | Elongation Factor Thermo-unstable |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IRT | Infrared Thermography |
| ITS | Internal Teat Sealant |
| LFD | Lateral Flow Dipstick |
| LFIA | Lateral Flow Immunoassay |
| MALDI-TOF MS | Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry |
| MDR | Multidrug Resistance |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| MSCRAMMs | Microbial Surface Components Recognizing Adhesive Matrix Molecules |
| MSSA | Methicillin-Susceptible Staphylococcus aureus |
| NAS | Non-aureus Staphylococci |
| PCR | Polymerase Chain Reaction |
| PIA | Polysaccharide Intercellular Adhesin |
| qPCR | Quantitative Polymerase Chain Reaction |
| RAA | Recombinase-Aided Amplification |
| RF | Random Forest (Machine Learning Algorithm) |
| SCC | Somatic Cell Count |
| SCCmec | Staphylococcal Cassette Chromosome mec |
| SDCT | Selective Dry Cow Therapy |
| SHAP | SHapley Additive exPlanations (Explainable AI Technique) |
| SNP | Single Nucleotide Polymorphism |
| ST | Sequence Type (used in molecular typing) |
| TSST-1 | Toxic Shock Syndrome Toxin-1 |
| WGS | Whole-Genome Sequencing |
| XAI | Explainable Artificial Intelligence |
| XGBoost | Extreme Gradient Boosting (Machine Learning Algorithm) |
References
- Dyson, R.; Charman, N.; Hodge, A.; Rowe, S.M.; Taylor, L.F. A survey of mastitis pathogens including antimicrobial susceptibility in southeastern Australian dairy herds. J. Dairy Sci. 2022, 105, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, C.; Horsman, S.; Wood, C.; Clark, R.; Price, R.; Henning, J.; Grewar, J.D.; Wood, B.J.; Ranjbar, S.; McGowan, M.R.; et al. Bacterial culture and susceptibility test results for clinical mastitis samples from Australia’s subtropical dairy region. J. Dairy Sci. 2024, 107, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, Z.; Cabrera, V.E.; Hogeveen, H.; Ruegg, P.L. Economic impact of subclinical mastitis treatment in early lactation using intramammary nisin. J. Dairy Sci. 2024, 107, 4634–4645. [Google Scholar] [CrossRef]
- Zaghen, F.; Sora, V.M.; Zanirato, G.; Zecconi, A. From One Heath to One Sustainability: The Role of Contagious Mastitis Pathogens in Decreasing the Dairy Herd Sustainability. Pathogens 2024, 13, 914. [Google Scholar] [CrossRef]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Velázquez-Ordoñez, V.; Delgadillo-Ruiz, L.; Zaragoza-Bastida, A. Bovine mastitis, a worldwide impact disease: Prevalence, antimicrobial resistance, and viable alternative approaches. Vet. Anim. Sci. 2023, 21, 100306. [Google Scholar] [CrossRef]
- Saeed, S.I.; Kamaruzzaman, N.F.; Gahamanyi, N.; Nguyen, T.T.H.; Hossain, D.; Kahwa, I. Confronting the complexities of antimicrobial management for Staphylococcus aureus causing bovine mastitis: An innovative paradigm. Ir. Vet. J. 2024, 77, 4. [Google Scholar] [CrossRef]
- Exel, C.E.; Halasa, T.; Koop, G.; Steeneveld, W.; Lam, T.J.G.M.; Benedictus, L.; Gussmann, M. A stochastic modelling approach to determine the effect of diverse Staphylococcus aureus strains on the economic and epidemiological outcomes of mastitis intervention strategies in dairy cattle. Prev. Vet. Med. 2022, 199, 105566. [Google Scholar] [CrossRef]
- Fenta, M.D.; Tafere, F.A.; Mebratu, A.S.; Malede, B.A. Quarter-wise proportion and beta-lactam resistance rate of bovine mastitis associated- Staphylococcus aureus among infectious episodes in Ethiopia: Systematic review and meta-Analysis. Heliyon 2023, 9, e18180. [Google Scholar] [CrossRef] [PubMed]
- Tora, E.T.; Bekele, N.B.; Suresh Kumar, R.S. Bacterial profile of bovine mastitis in Ethiopia: A systematic review and meta-analysis. PeerJ 2022, 10, e13253. [Google Scholar] [CrossRef]
- Fesseha, H.; Mathewos, M.; Aliye, S.; Wolde, A. Study on Prevalence of Bovine Mastitis and Associated Risk Factors in Dairy Farms of Modjo Town and Suburbs, Central Oromia, Ethiopia. Vet. Med. Res. Rep. 2021, 12, 271–283. [Google Scholar] [CrossRef]
- Wang, K.; Cha, J.; Liu, K.; Deng, J.; Yang, B.; Xu, H.; Wang, J.; Zhang, L.; Gu, X.; Huang, C.; et al. The prevalence of bovine mastitis-associated Staphylococcus aureus in China and its antimicrobial resistance rate: A meta-analysis. Front. Vet. Sci. 2022, 9, 1006676. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, I.N.; Latorre-Fernández, J.; Reuben, R.C.; Trabelsi, I.; González-Azcona, C.; Arfaoui, A.; Usman, Y.; Lozano, C.; Zarazaga, M.; Torres, C. Beyond the Wild MRSA: Genetic Features and Phylogenomic Review of mecC-Mediated Methicillin Resistance in Non-aureus Staphylococci and Mammaliicocci. Microorganisms 2023, 12, 66. [Google Scholar] [CrossRef]
- Dendani Chadi, Z.; Dib, L.; Zeroual, F.; Benakhla, A. Usefulness of molecular typing methods for epidemiological and evolutionary studies of Staphylococcus aureus isolated from bovine intramammary infections. Saudi J. Biol. Sci. 2022, 29, 103338. [Google Scholar] [CrossRef]
- Kurban, D.; Roy, J.-P.; Kabera, F.; Fréchette, A.; Um, M.M.; Albaaj, A.; Rowe, S.; Godden, S.; Adkins, P.R.F.; Middleton, J.R.; et al. Diagnosing Intramammary Infection: Meta-Analysis and Mapping Review on Frequency and Udder Health Relevance of Microorganism Species Isolated from Bovine Milk Samples. Animals 2022, 12, 3288. [Google Scholar] [CrossRef]
- Mues, L.; Kemper, N.; Blumenberg, J.A. Occurrence and diagnostic of intermittent shedding of Staphylococcus aureus in bovine mammary infection. Front. Vet. Sci. 2025, 12, 1523698. [Google Scholar] [CrossRef]
- Rowe, S.; House, J.K.; Pooley, H.; Bullen, S.; Humphris, M.; Ingenhoff, L.; Norris, J.M.; Zadoks, R.N. Evaluation of point-of-care tests for identification of pathogens to inform clinical mastitis treatment decisions in pasture- and confinement-managed dairy cows in Australia. J. Dairy Sci. 2024, 107, 8271–8285. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Barkema, H.W.; Nobrega, D.B.; Xu, C.; Han, B.; Zhang, C.; Yang, J.; Li, X.; Gao, J. Virulence of Bacteria Causing Mastitis in Dairy Cows: A Literature Review. Microorganisms 2025, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; de Oliveira Mendes, T.A.; Fitzgerald, J.R.; de Oliveira Barros Ribon, A. Diversity and pathogenesis of Staphylococcus aureus from bovine mastitis: Current understanding and future perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef]
- Kerro Dego, O.; Vidlund, J. Staphylococcal mastitis in dairy cows. Front. Vet. Sci. 2024, 11, 1356259. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, X.; Zhang, Y.; Wang, F.; Zhang, F.; Jia, Y.; Wu, D.; Tohti, K.; Cheng, M.; Zhu, J. Anti-Staphylococcus aureus Single-Chain Fragment Variables Play a Protective Anti-Inflammatory Role In Vitro and In Vivo. Vaccines 2021, 9, 1300. [Google Scholar] [CrossRef]
- Carlson, S.K.; Erickson, D.L.; Wilson, E. Staphylococcus aureus metal acquisition in the mastitic mammary gland. Microb. Pathog. 2020, 144, 104179. [Google Scholar] [CrossRef] [PubMed]
- Sabino, Y.N.V.; Cotter, P.D.; Mantovani, H.C. Anti-virulence compounds against Staphylococcus aureus associated with bovine mastitis: A new therapeutic option? Microbiol. Res. 2023, 271, 127345. [Google Scholar] [CrossRef] [PubMed]
- Touati, A.; Ibrahim, N.A.; Idres, T. Disarming Staphylococcus aureus: Review of Strategies Combating This Resilient Pathogen by Targeting Its Virulence. Pathogens 2025, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Debruyn, E.; Ghumman, N.Z.; Peng, J.; Tiwari, H.K.; Gogoi-Tiwari, J. Alternative approaches for bovine mastitis treatment: A critical review of emerging strategies, their effectiveness and limitations. Res. Vet. Sci. 2025, 185, 105557. [Google Scholar] [CrossRef]
- Pérez, V.K.C.; da Costa, G.M.; Guimarães, A.S.; Heinemann, M.B.; Lage, A.P.; Dorneles, E.M.S. Relationship between virulence factors and antimicrobial resistance in Staphylococcus aureus from bovine mastitis. J. Glob. Antimicrob. Resist. 2020, 22, 792–802. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.-S.; El Nahhas, N.; Mabrok, M.A. Methicillin-Resistant Staphylococcus aureus (MRSA): One Health Perspective Approach to the Bacterium Epidemiology, Virulence Factors, Antibiotic-Resistance, and Zoonotic Impact. Infect. Drug Resist. 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect. Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef]
- Akhtar, M.; Naqvi, S.U.-A.-S.; Liu, Q.; Pan, H.; Ma, Z.; Kong, N.; Chen, Y.; Shi, D.; Kulyar, M.F.-A.; Khan, J.A.; et al. Short Chain Fatty Acids (SCFAs) Are the Potential Immunomodulatory Metabolites in Controlling Staphylococcus aureus-Mediated Mastitis. Nutrients 2022, 14, 3687. [Google Scholar] [CrossRef]
- Pedersen, R.R.; Krömker, V.; Bjarnsholt, T.; Dahl-Pedersen, K.; Buhl, R.; Jørgensen, E. Biofilm Research in Bovine Mastitis. Front. Vet. Sci. 2021, 8, 656810. [Google Scholar] [CrossRef]
- Vargová, M.; Zigo, F.; Výrostková, J.; Farkašová, Z.; Rehan, I.F. Biofilm-Producing Ability of Staphylococcus aureus Obtained from Surfaces and Milk of Mastitic Cows. Vet. Sci. 2023, 10, 386. [Google Scholar] [CrossRef]
- Fidelis, C.E.; Orsi, A.M.; Freu, G.; Gonçalves, J.L.; Santos, M.V.D. Biofilm Formation and Antimicrobial Resistance of Staphylococcus aureus and Streptococcus uberis Isolates from Bovine Mastitis. Vet. Sci. 2024, 11, 170. [Google Scholar] [CrossRef]
- Cáceres, M.E.; Ledesma, M.M.; Lombarte Serrat, A.; Vay, C.; Sordelli, D.O.; Giacomodonato, M.N.; Buzzola, F.R. Growth conditions affect biofilms of Staphylococcus aureus producing mastitis: Contribution of MALDI-TOF-MS to strain characterization. Curr. Res. Microb. Sci. 2021, 2, 100073. [Google Scholar] [CrossRef]
- Song, M.; Tang, Q.; Ding, Y.; Tan, P.; Zhang, Y.; Wang, T.; Zhou, C.; Xu, S.; Lyu, M.; Bai, Y.; et al. Staphylococcus aureus and biofilms: Transmission, threats, and promising strategies in animal husbandry. J. Anim. Sci. Biotechnol. 2024, 15, 44. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Liu, Q.; Zhao, Y. Virulence factors in biofilm formation and therapeutic strategies for Staphylococcus aureus: A review. Anim. Zoonoses, 2024; in press. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, X.; Feng, X.; Shang, X.; Liu, Q.; Zhang, N.; Yang, H. Molecular characteristics of Staphylococcus aureus strains isolated from subclinical mastitis of water buffaloes in Guangdong Province, China. Front. Vet. Sci. 2023, 10, 1177302. [Google Scholar] [CrossRef]
- Xu, D.; Hu, G.; Luo, J.; Cheng, J.; Wu, D.; Cheng, L.; Huang, X.; Fu, S.; Liu, J. Staphylococcus aureus induces mitophagy to promote its survival within bovine mammary epithelial cells. Vet. Microbiol. 2023, 280, 109697. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, H.; Li, F.; Zhang, Y.; Yang, J.; Shen, Y.; Hu, N.; Zou, Q.; Qin, L.; Zeng, H.; et al. Staphylococcus aureus induces mitophagy via the HDAC11/IL10 pathway to sustain intracellular survival. J. Transl. Med. 2025, 23, 156. [Google Scholar] [CrossRef]
- Esemu, S.N.; Nya’Nying, S.F.; Ndip, L.M.; Bessong, P.O.; Tanih, N.F.; Smith, S.I.; Ndip, R.N. Isolation and characterization of methicillin-resistant Staphylococcus aureus from bovine mastitis in North West Cameroon: Public health implications. BMC Res. Notes 2024, 17, 389. [Google Scholar] [CrossRef]
- Eidaroos, N.H.; Algammal, A.M.; Mohamaden, W.I.; Alenzi, A.M.; Alghamdi, S.; Kabrah, A.; El-Mahallawy, H.S.; Eid, H.M.; Algwad, A.A.; Asfor, S.A.; et al. Virulence traits, agr typing, multidrug resistance patterns, and biofilm ability of MDR Staphylococcus aureus recovered from clinical and subclinical mastitis in dairy cows. BMC Microbiol. 2025, 25, 155. [Google Scholar] [CrossRef]
- Gad, W.A.; Osman, S.A.; Abd El-Razik, K.A.; Soror, A.H.; Fouad, E.A. A novel diagnostic technique for diagnosis of Staphylococcus aureus subclinical mastitis using gold nanoparticle-based ELISA. Open Vet. J. 2024, 14, 3388–3396. [Google Scholar] [CrossRef]
- Getahun, D.D.; Tarekegn, H.T.; Azene, B.T.; Abebe, L.T.; Belete, M.A.; Tessema, T.S. Virulence genes and antibiotic resistance profiling of staphylococcus species isolated from mastitic dairy cows in and around Bahir dar, Ethiopia. BMC Microbiol. 2025, 25, 210. [Google Scholar] [CrossRef]
- Keinprecht, H.; Irimaso, E.; Rosel, A.C.; Stessl, B.; Ntakirutimana, C.; Marek, L.; Fischer, O.W.; Szostak, M.P.; Zöchbauer, J.; Wittek, T.; et al. Diversity of Staphylococcus aureus associated with mastitis from dairy cows in Rwanda. J. Glob. Antimicrob. Resist. 2024, 36, 326–335. [Google Scholar] [CrossRef]
- Jawad, A.; Dagman, S.N.; Yaseen, M.M.; Al-Karagoly, H. Phylogenetic analysis of Staphylococcus aureus enterotoxin A gene in Iraqi breed cows with bovine mastitis: Implications for disease management. Open Vet. J. 2024, 14, 1644–1657. [Google Scholar] [CrossRef]
- Islam, M.M.; Hossain, M.I.; Islam, M.S.; Azam, M.G.; Sultana, S. Prevalence, antibiotic resistance patterns, and virulence factors of Staphylococcus aureus isolates associated with bovine mastitis in northern Bangladesh. Heliyon 2025, 11, e42107. [Google Scholar] [CrossRef]
- Zhang, N.; Hou, L.; Li, D.; Lan, W.; Zhao, Y.; Sun, X. Establishment and Application of Duplex Recombinase-Aided Amplification Combined with Lateral Flow Dipsticks for Rapid and Simultaneous Visual Detection of Klebsiella pneumoniae and Staphylococcus aureus in Milk. Foods 2025, 14, 573. [Google Scholar] [CrossRef]
- Hemati, Z.; Abdolmohammadi Khiav, L.; Zahmatkesh, A. Virulence genes and antibiotic resistance profiles of Staphylococcus aureus isolated from bovine mastitis milk samples. Iran. J. Vet. Res. 2023, 24, 258–264. [Google Scholar] [CrossRef]
- Shahzad, M.A.; Yousaf, A.; Ahsan, A.; Irshad, H.; Riaz, A.; Khan, A.; Ullah, I.; Sattar, S.; Bostan, N.; Javed, S. Virulence and resistance profiling of Staphylococcus aureus isolated from subclinical bovine mastitis in the Pakistani Pothohar region. Sci. Rep. 2024, 14, 14569. [Google Scholar] [CrossRef]
- Haq, I.U.; Kamal, M.; Swelum, A.A.; Khan, S.; los Ríos-Escalante, P.R.D.; Usman, T. Alarming multidrug resistance in Staphylococcus aureus isolated from raw milk of cows with subclinical mastitis: Antibiotic resistance patterns and occurrence of selected resistance genes. PLoS ONE 2024, 19, e0301200. [Google Scholar] [CrossRef]
- Shah, A.U.; Ali Khan, J.; Avais, M.; Zaman, S.H.; Munir, Z.; Abbas, S.; Tariq, M.; ur Rahman, M.; Tariq, F.; Nawaz, S.; et al. Prevalence and chemotherapy of Staphylococcus aureus mastitis in dairy cattle. PLoS ONE 2025, 20, e0315480. [Google Scholar] [CrossRef]
- Camsing, A.; Phetburom, N.; Chopjitt, P.; Pumhirunroj, B.; Patikae, P.; Watwiengkam, N.; Yongkiettrakul, S.; Kerdsin, A.; Boueroy, P. Occurrence of antimicrobial-resistant bovine mastitis bacteria in Sakon Nakhon, Thailand. Vet. World 2024, 17, 1202–1209. [Google Scholar] [CrossRef]
- Son, H.M.; Duc, H.M. Prevalence and Phage-Based Biocontrol of Methicillin-Resistant Staphylococcus aureus Isolated from Raw Milk of Cows with Subclinical Mastitis in Vietnam. Antibiotics 2024, 13, 638. [Google Scholar] [CrossRef]
- Naranjo-Lucena, A.; Becker, P.; Madigan, G.; Cupial, R.; Byrne, B.; Johnson, A. Longitudinal Patterns in the Isolation and Antimicrobial Resistance of Bovine Mastitis-Causing Bacteria in Ireland. Antibiotics 2025, 14, 243. [Google Scholar] [CrossRef]
- Fusar Poli, S.; Locatelli, C.; Monistero, V.; Freu, G.; Cremonesi, P.; Castiglioni, B.; Lecchi, C.; Longheu, C.M.; Tola, S.; Guaraglia, A.; et al. Staphylococcus aureus and methicillin-resistant staphylococci and mammaliicocci in the bulk tank milk of dairy cows from a livestock-dense area in northern Italy. Res. Vet. Sci. 2025, 182, 105482. [Google Scholar] [CrossRef]
- Dobrut, A.; Siemińska, I.; Sroka-Oleksiak, A.; Drożdż, K.; Sobońska, J.; Mroczkowska, U.; Brzychczy-Włoch, M. Molecular and phenotypic identification of bacterial species isolated from cows with mastitis from three regions of Poland. BMC Vet. Res. 2024, 20, 193. [Google Scholar] [CrossRef]
- Zalewska, M.; Brzozowska, P.; Rzewuska, M.; Kawecka-Grochocka, E.; Urbańska, D.M.; Sakowski, T.; Bagnicka, E. The quality and technological parameters of milk obtained from dairy cows with subclinical mastitis. J. Dairy Sci. 2025, 108, 1285–1300. [Google Scholar] [CrossRef]
- Hutu, I.; Lungu, B.C.; Spataru, I.I.; Torda, I.; Iancu, T.; Barrow, P.A.; Mircu, C. Microbiological and Molecular Investigation of Antimicrobial Resistance in Staphylococcus aureus Isolates from Western Romanian Dairy Farms: An Epidemiological Approach. Animals 2024, 14, 2266. [Google Scholar] [CrossRef]
- Aguirre-Sánchez, J.R.; Castro-del Campo, N.; Medrano-Félix, J.A.; Martínez-Torres, A.O.; Chaidez, C.; Querol-Audi, J.; Castro-del Campo, N. Genomic insights of S. aureus associated with bovine mastitis in a high livestock activity region of Mexico. J. Vet. Sci. 2024, 25, e42. [Google Scholar] [CrossRef]
- Moreno, J.; Diana, L.; Martínez, M.; Iribarnegaray, V.; Puentes, R. Comprehensive analysis of antimicrobial resistance, biofilm formation and virulence factors of staphylococci isolated from bovine mastitis. Heliyon 2025, 11, e42749. [Google Scholar] [CrossRef]
- Leon, M.; Rubin, J.; Raverty, S.; Ghosh, K. Frequency and antimicrobial susceptibility of Staphylococcus aureus isolated from clinical bovine mastitis cases in British Columbia, Canada. J. Vet. Diagn. Investig. 2024, 37, 10406387241306096. [Google Scholar] [CrossRef]
- Singh, A.; Somula, H.; Wieland, M. A retrospective cohort study investigating the association of postcalving intramammary infection and milk yield, somatic cell count, clinical mastitis, and culling risk in first-lactation dairy cows. J. Dairy Sci. 2025, 108, 4234–4247. [Google Scholar] [CrossRef]
- Neri, T.A.N.; Park, H.; Kang, S.; Baek, S.H.; Nam, I.S. Comparative Antimicrobial Resistance and Prevalence of Methicillin Resistance in Coagulase-Positive Staphylococci from Conventional and Organic Dairy Farms in South Korea. Antibiotics 2024, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Eleodoro, J.I.; Muraga, L.; Vanot, R.L.; Fagnani, R. Identification and antimicrobial susceptibility of milk isolates from cows with subclinical mastitis in the northwest of Paraná State, Brazil. Vet. Ital. 2023, 59, 71–81. [Google Scholar] [CrossRef]
- Bonsaglia, E.C.R.; Rossi, B.F.; Possebon, F.S.; Silva, N.C.C.; Gonçalves, J.L.; Castilho, I.G.; Fernandes Junior, A.; dos Santos, M.V.; Rall, V.L.M. In Vitro Adhesion and Invasion Rates of Staphylococcus aureus Isolated from Mastitic Cows Are Modulated by the agr System and MSCRAMM Genes. Vet. Sci. 2025, 12, 270. [Google Scholar] [CrossRef]
- Sharifi, A.; Mahmoudi, P.; Sobhani, K. The prevalence of adhesion and biofilm genes in Staphylococcus aureus isolates from bovine mastitis: A comprehensive meta-analysis. Vet. Med. Sci. 2024, 10, e31378. [Google Scholar] [CrossRef]
- Rocha, G.D.; de Simoni Gouveia, J.J.; da Costa, M.M.; Soares, R.A.N.; Gouveia, G.V. Resistance and virulence in Staphylococcus aureus by whole-genome sequencing: A comparative approach in blaZ-positive isolates. Braz. J. Microbiol. 2024, 55, 955–967. [Google Scholar] [CrossRef]
- Na, S.; Intanon, M.; Srithanasuwan, A.; Chaisri, W.; Suriyasathaporn, W. Evidence of vancomycin-resistant Staphylococcus aureus, multidrug-resistant S. aureus, and Enterococcus faecium-causing mastitis in Thailand and Cambodia. Vet. World 2025, 18, 202–209. [Google Scholar] [CrossRef]
- Tartor, Y.H.; Enany, M.E.; Ismail, N.I.; El-Demerdash, A.S.; Eidaroos, N.H.; Algendy, R.M.; Mahmmod, Y.; Elsohaby, I. Vancomycin-resistant Staphylococcus aureus endangers Egyptian dairy herds. Sci. Rep. 2024, 14, 30606. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, Y.; Bai, R.; Pei, X.; Xu, H.; Zhu, K.; Wu, C. Antimicrobial resistance profiles of common mastitis pathogens on large Chinese dairy farms. JDS Commun. 2023, 5, 185–189. [Google Scholar] [CrossRef]
- Kang, H.J.; You, J.-Y.; Kim, S.H.; Moon, J.-S.; Kim, H.-Y.; Kim, J.-M.; Lee, Y.J.; Kang, H.-M. Characteristics of methicillin-resistant Staphylococcus aureus isolates from bovine mastitis milk in South Korea: Molecular characteristics, biofilm, virulence, and antimicrobial resistance. Microbiol. Spectr. 2024, 12, e01197-24. [Google Scholar] [CrossRef]
- Karell, J.; Petzl, W.; Gangl, A.; Huber-Schlenstedt, R.; Sorge, U.S. Changes in antimicrobial resistance of Staphylococcus aureus in bovine quarter milk samples from southern Germany between 2012 and 2022. J. Dairy Sci. 2024, 107, 3802–3812. [Google Scholar] [CrossRef]
- Nesaraj, J.; Grinberg, A.; Laven, R.; Chanyi, R.; Altermann, E.; Bandi, C.; Biggs, P.J. The Host Adaptation of Staphylococcus aureus to Farmed Ruminants in New Zealand, With Special Reference to Clonal Complex 1. Environ. Microbiol. Rep. 2025, 17, e70087. [Google Scholar] [CrossRef] [PubMed]
- Aouadhi, C.; Jouini, A.; Maaroufi, K.; Maaroufi, A. Antibacterial Effect of Eight Essential Oils against Bacteria Implicated in Bovine Mastitis and Characterization of Primary Action Mode of Thymus capitatus Essential Oil. Antibiotics 2024, 13, 237. [Google Scholar] [CrossRef]
- Roadcap, E.; Lichtenwalner, A.; Kennedy-Wade, B.; Adjapong, G.; Chakrawarti, A.; De Sant’Anna, F.M.; Barlow, J.W. Whole genome sequencing identifies exotoxin and antimicrobial resistance profiles of Staphylococcus aureus from Maine dairy farms. BMC Vet. Res. 2025, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Chakrawarti, A.; Casey, C.L.; Burk, A.; Mugabi, R.; Ochoa, A.; Barlow, J.W. An observational study demonstrates human-adapted Staphylococcus aureus strains have a higher frequency of antibiotic resistance compared to cattle-adapted strains isolated from dairy farms making farmstead cheese. BMC Vet. Res. 2024, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, D.; Jeon, H.; Somasundaram, P.; Soundrarajan, N.; Park, C. Bactericidal activities and biochemical features of 16 antimicrobial peptides against bovine-mastitis causative pathogens. Vet. Res. 2024, 55, 150. [Google Scholar] [CrossRef]
- Rahman, M.R.T.; Guay, L.-D.; Fliss, I.; Biron, E. Structure–Activity Study of the Antimicrobial Lipopeptide Humimycin A and Screening Against Multidrug-Resistant Staphylococcus aureus. Antibiotics 2025, 14, 385. [Google Scholar] [CrossRef]
- Michira, L.; Kagira, J.; Maina, N.; Waititu, K.; Kiboi, D.; Ongera, E.; Ngotho, M. Prevalence of subclinical mastitis, associated risk factors and antimicrobial susceptibility pattern of bacteria isolated from milk of dairy cattle in Kajiado Central sub-county, Kenya. Vet. Med. Sci. 2023, 9, 2885–2892. [Google Scholar] [CrossRef]
- Shahid, M.; Hussain, R.; Nawaz, Z.; Aslam, B.; Ahmad, M.Z.; Siddique, A.B.; Ahsan, H.; Fatima, A.; Khan, I.; Mustafa, B.; et al. Occurrence of Virulence Genes among Methicillin-Resistant Staphylococcus aureus Isolated from Subclinical Bovine Mastitis. ACS Omega 2023, 8, 38111–38117. [Google Scholar] [CrossRef]
- Peña-Mosca, F.; Dean, C.; Machado, V.; Fernandes, L.; Pinedo, P.; Doster, E.; Heins, B.; Sharpe, K.; Ray, T.; Feijoo, V.; et al. Investigation of intramammary infections in primiparous cows during early lactation on organic dairy farms. J. Dairy Sci. 2023, 106, 9377–9392. [Google Scholar] [CrossRef]
- Wodaje, A.; Belete, M.A.; Menkir, A.S.; Zegeye, Z.B.; Yihunie, F.B. Detection of Virulence Genes and Antimicrobial Susceptibility Profiles of Staphylococcus aureus Isolates From Bovine Mastitis in Chagni, Northwestern Ethiopia. Vet. Med. Int. 2025, 2025, 6473601. [Google Scholar] [CrossRef]
- Abd El-Razik, K.A.; Arafa, A.A.; Fouad, E.A.; Soror, A.H.; Abdalhamed, A.M.; Elgioushy, M. Phenotypic and genotypic characterization of erythromycin-resistant Staphylococcus aureus isolated from bovine subclinical mastitis in Egypt. Vet. World 2023, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Klassen, A.; Dittmar, K.; Schulz, J.; Einax, E.; Donat, K. Estimation of the performance of two real-time polymerase chain reaction assays for detection of Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus dysgalactiae in pooled milk samples in a field study. J. Dairy Sci. 2023, 106, 9228–9243. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; El-Tarabili, R.M.; Bahnass, M.M.; Alshahrani, M.A.; Saif, A.; Alwutayd, K.M.; Safhi, F.A.; Mansour, A.T.; Alblwi, N.A.N.; Ghoneim, M.M.; et al. Partnering essential oils with antibiotics: Proven therapies against bovine Staphylococcus aureus mastitis. Front. Cell. Infect. Microbiol. 2023, 13, 1265027. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Mi, S.; Dari, G.; Zhang, Z.; Chen, S.; Yu, Y. Ferroptosis-Related Genes as Molecular Markers in Bovine Mammary Epithelial Cells Challenged with Staphylococcus aureus. Int. J. Mol. Sci. 2025, 26, 2506. [Google Scholar] [CrossRef]
- Doghri, I.; Jacques, M.; Nichols, S.; Roy, J.-P.; Dufour, S. Visualization of Staphylococcus aureus in the bovine mammary gland by fluorescence in situ hybridization. Res. Vet. Sci. 2025, 189, 105634. [Google Scholar] [CrossRef]
- Penati, M.; Ulloa, F.; Locatelli, C.; Monistero, V.; Pavesi, L.F.; Santandrea, F.; Piccinini, R.; Moroni, P.; Bronzo, V.; Addis, M.F. An update on non-aureus staphylococci and mammaliicocci in cow milk: Unveiling the presence of Staphylococcus borealis and Staphylococcus rostri by MALDI-TOF MS. Vet. Res. Commun. 2024, 48, 2555–2561. [Google Scholar] [CrossRef]
- Lopes, T.; Fidelis, C.E.; Silva, A.T.F.; Mota, R.A.; Rall, V.L.M.; dos Santos, M.V.; Gonçalves, J.L. MALDI–TOF bacterial subtyping for rapid detection of biomarkers in Staphylococcus aureus from subclinical bovine mastitis. J. Appl. Microbiol. 2023, 134, lxad249. [Google Scholar] [CrossRef]
- Tamminen, L.-M.; Dahlberg, J. Evaluation of an automatic image classifier for analysis of bacterial growth on a multiple-agar plate developed for bovine mastitis. PLoS ONE 2025, 20, e0318698. [Google Scholar] [CrossRef]
- Fonseca, M.; Kurban, D.; Roy, J.-P.; Santschi, D.E.; Molgat, E.; Dufour, S. Usefulness of differential somatic cell count for udder health monitoring: Association of differential somatic cell count and somatic cell score with quarter-level milk yield and milk components. J. Dairy Sci. 2025, 108, 3900–3916. [Google Scholar] [CrossRef]
- Kajdanek, A.; Kluska, M.; Matusiak, R.; Kazimierczak, J.; Dastych, J. A Rapid and Inexpensive PCR Test for Mastitis Diagnosis Based on NGS Data. Pathogens 2024, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Soares, A.C.; Popolin-Neto, M.; Paulovich, F.V.; Oliveira, O.N.; Mattoso, L.H.C. Detection of Staphylococcus aureus in milk samples using impedance spectroscopy and data processing with information visualization techniques and multidimensional calibration space. Sens. Actuators Rep. 2022, 4, 100083. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, X.; He, Z.; Feng, Y.; Liu, G. Accurate detection of dairy cow mastitis with deep learning technology: A new and comprehensive detection method based on infrared thermal images. Anim. Int. J. Anim. Biosci. 2022, 16, 100646. [Google Scholar] [CrossRef] [PubMed]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows. Sensors 2018, 18, 862. [Google Scholar] [CrossRef]
- Porter, I.R.; Wieland, M.; Basran, P.S. Feasibility of the use of deep learning classification of teat-end condition in Holstein cattle. J. Dairy Sci. 2021, 104, 4529–4536. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.L.N.; Martins, C.M.d.M.R.; Porto, L.F.; Nobrega, D.B.; dos Santos, M.V. Accuracy of an AI-based automated plate reading mobile application for the identification of clinical mastitis-causing pathogens in chromogenic culture media. Sci. Rep. 2024, 14, 1208. [Google Scholar] [CrossRef]
- Kotlarz, K.; Mielczarek, M.; Biecek, P.; Wojdak-Maksymiec, K.; Suchocki, T.; Topolski, P.; Jagusiak, W.; Szyda, J. An Explainable Deep Learning Classifier of Bovine Mastitis Based on Whole-Genome Sequence Data—Circumventing the p >> n Problem. Int. J. Mol. Sci. 2024, 25, 4715. [Google Scholar] [CrossRef]
- Krolitzki, E.; Schwaminger, S.P.; Pagel, M.; Ostertag, F.; Hinrichs, J.; Berensmeier, S. Current practices with commercial scale bovine lactoferrin production and alternative approaches. Int. Dairy J. 2022, 126, 105263. [Google Scholar] [CrossRef]
- Bobbo, T.; Matera, R.; Pedota, G.; Manunza, A.; Cotticelli, A.; Neglia, G.; Biffani, S. Exploiting machine learning methods with monthly routine milk recording data and climatic information to predict subclinical mastitis in Italian Mediterranean buffaloes. J. Dairy Sci. 2023, 106, 1942–1952. [Google Scholar] [CrossRef]
- Mitsunaga, T.M.; Nery Garcia, B.L.; Pereira, L.B.R.; Costa, Y.C.B.; da Silva, R.F.; Delbem, A.C.B.; dos Santos, M.V. Current Trends in Artificial Intelligence and Bovine Mastitis Research: A Bibliometric Review Approach. Anim. Open Access J. 2024, 14, 2023. [Google Scholar] [CrossRef]
- Esener, N.; Maciel-Guerra, A.; Giebel, K.; Lea, D.; Green, M.J.; Bradley, A.J.; Dottorini, T. Mass spectrometry and machine learning for the accurate diagnosis of benzylpenicillin and multidrug resistance of Staphylococcus aureus in bovine mastitis. PLOS Comput. Biol. 2021, 17, e1009108. [Google Scholar] [CrossRef]
- Beck, K.L.; Haiminen, N.; Agarwal, A.; Carrieri, A.P.; Madgwick, M.; Kelly, J.; Pylro, V.; Kawas, B.; Wiedmann, M.; Ganda, E. Development and evaluation of statistical and artificial intelligence approaches with microbial shotgun metagenomics data as an untargeted screening tool for use in food production. mSystems 2024, 9, e00840-24. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Kemp, B. Digital Twins in Livestock Farming. Animals 2021, 11, 1008. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.M.; Zhang, E.; Godden, S.M.; Vasquez, A.K.; Nydam, D.V. Comparison of a machine learning model with a conventional rule-based selective dry cow therapy algorithm for detection of intramammary infections. J. Dairy Sci. 2025, 108, 760–772. [Google Scholar] [CrossRef]
- Michielon, A.; Litta, P.; Bonelli, F.; Don, G.; Farisè, S.; Giannuzzi, D.; Milanesi, M.; Pietrucci, D.; Vezzoli, A.; Cecchinato, A.; et al. Mind the Step: An Artificial Intelligence-Based Monitoring Platform for Animal Welfare. Sensors 2024, 24, 8042. [Google Scholar] [CrossRef]
- Voogt, A.M.; Schrijver, R.S.; Temürhan, M.; Bongers, J.H.; Sijm, D.T.H.M. Opportunities for Regulatory Authorities to Assess Animal-Based Measures at the Slaughterhouse Using Sensor Technology and Artificial Intelligence: A Review. Anim. Open Access J. 2023, 13, 3028. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Dhand, N.K.; Wang, Z.; Hu, K.; Thomson, P.C.; House, J.K.; Khatkar, M.S. Review of applications of deep learning in veterinary diagnostics and animal health. Front. Vet. Sci. 2025, 12, 1511522. [Google Scholar] [CrossRef]
- van der Voort, M.; Jensen, D.; Kamphuis, C.; Athanasiadis, I.N.; Vries, A.D.; Hogeveen, H. Invited review: Toward a common language in data-driven mastitis detection research. J. Dairy Sci. 2021, 104, 10449–10461. [Google Scholar] [CrossRef]
- Majumder, S.; Sackey, T.; Viau, C.; Park, S.; Xia, J.; Ronholm, J.; George, S. Genomic and phenotypic profiling of Staphylococcus aureus isolates from bovine mastitis for antibiotic resistance and intestinal infectivity. BMC Microbiol. 2023, 23, 43. [Google Scholar] [CrossRef]
- Mzee, T.; Kumburu, H.; Kazimoto, T.; Leekitcharoenphon, P.; van Zwetselaar, M.; Masalu, R.; Mlaganile, T.; Sonda, T.; Wadugu, B.; Mushi, I.; et al. Molecular Characterization of Staphylococcus aureus Isolated from Raw Milk and Humans in Eastern Tanzania: Genetic Diversity and Inter-Host Transmission. Microorganisms 2023, 11, 1505. [Google Scholar] [CrossRef]
- Rasool, Z.; Noreen, H.; Anjum, A.; Rizvi, A.; Rabaan, A.A.; Halwani, M.A.; Sabour, A.A.; Aljeldah, M.; Shammari, B.R.A.; Alhajri, S.M.; et al. Genotypic and Phenotypic Characterization of Erythromycin-Resistant Staphylococcus aureus Isolated from Bovine Mastitis and Humans in Close Contact. Trop. Med. Infect. Dis. 2022, 8, 26. [Google Scholar] [CrossRef]
- Rodríguez, M.F.; Gomez, A.P.; Parra-Giraldo, C.M.; Ceballos-Garzon, A. Proteomics and Genetic Approaches Elucidate the Circulation of Low Variability Staphylococcus aureus Strains on Colombian Dairy Farms. Microb. Ecol. 2023, 86, 2320–2329. [Google Scholar] [CrossRef]
- Exel, C.E.; Gerritsen, K.; Spaninks, M.; Duim, B.; Koop, G.; Benedictus, L. Association of Staphylococcus aureus genotypes with milk or colonization of extramammary sites in Dutch dairy cattle indicates strain variation in reservoirs for intramammary infections. Res. Vet. Sci. 2023, 154, 138–144. [Google Scholar] [CrossRef]
- Fazoli, K.G.Z.; Rey, L.M.R.; Rúbio, K.A.J.; Garcia Souza, M.A.; Oliveira, H.M.d.S.; Ribeiro, D.C.; Pereira, K.R.d.J.D.; Kawamo, D.M.; Gomes, T.K.A.; da Silva, I.B.; et al. Resistance Profile of Bovine Mastitis Isolates, Presence of the mecA Gene and Identification of ESBL Producing Strains from Small Rural Dairy Properties. Anim. Open Access J. 2023, 13, 1147. [Google Scholar] [CrossRef]
- Abebe, R.; Markos, A.; Abera, M.; Mekbib, B. Incidence rate, risk factors, and bacterial causes of clinical mastitis on dairy farms in Hawassa City, southern Ethiopia. Sci. Rep. 2023, 13, 10945. [Google Scholar] [CrossRef]
- Singh, I.; Roshan, M.; Vats, A.; Behera, M.; Gautam, D.; Rajput, S.; Rana, C.; De, S. Evaluation of Virulence, Antimicrobial Resistance and Biofilm Forming Potential of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Bovine Suspected with Mastitis. Curr. Microbiol. 2023, 80, 198. [Google Scholar] [CrossRef]
- Sivakumar, R.; Pranav, P.S.; Annamanedi, M.; Chandrapriya, S.; Isloor, S.; Rajendhran, J.; Hegde, N.R. Genome sequencing and comparative genomic analysis of bovine mastitis-associated Staphylococcus aureus strains from India. BMC Genom. 2023, 24, 44. [Google Scholar] [CrossRef]
- Guo, W.; Qiu, M.; Pu, Z.; Long, N.; Yang, M.; Ren, K.; Ning, R.; Zhang, S.; Peng, F.; Sun, F.; et al. Geraniol-a potential alternative to antibiotics for bovine mastitis treatment without disturbing the host microbial community or causing drug residues and resistance. Front. Cell. Infect. Microbiol. 2023, 13, 1126409. [Google Scholar] [CrossRef]
- Crespi, E.; Pereyra, A.M.; Puigdevall, T.; Rumi, M.V.; Testorelli, M.F.; Caggiano, N.; Gulone, L.; Mollerach, M.; Gentilini, E.R.; Srednik, M.E. Antimicrobial resistance studies in staphylococci and streptococci isolated from cows with mastitis in Argentina. J. Vet. Sci. 2021, 23, e12. [Google Scholar] [CrossRef]
- Yang, F.; Shi, W.; Meng, N.; Zhao, Y.; Ding, X.; Li, Q. Antimicrobial resistance and virulence profiles of staphylococci isolated from clinical bovine mastitis. Front. Microbiol. 2023, 14, 1190790. [Google Scholar] [CrossRef]
- Tsutamoto, S.; Iwasaki, Y.; Shinohara, A.; Imamiya, R.; Samukawa, K.; Kawada-Matsuo, M.; Komatsuzawa, H.; Yamada, Y.; Mandokoro, K.; Iwao, H.; et al. Triterpenoid saponin from Panax ginseng increases the sensitivity of methicillin-resistant Staphylococcus aureus to β-lactam and aminoglycoside antibiotics. Microbiol. Spectr. 2024, 12, e03227-23. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khezri, A.; Nørstebø, H.; Ahmad, R. A culture-, amplification-independent, and rapid method for identification of pathogens and antibiotic resistance profile in bovine mastitis milk. Front. Microbiol. 2023, 13, 1104701. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, J. A Novel Strategy to Identify Endolysins with Lytic Activity against Methicillin-Resistant Staphylococcus aureus. Int. J. Mol. Sci. 2023, 24, 5772. [Google Scholar] [CrossRef] [PubMed]
- Kaczorek-Łukowska, E.; Małaczewska, J.; Sowińska, P.; Szymańska, M.; Wójcik, E.A.; Siwicki, A.K. Staphylococcus aureus from Subclinical Cases of Mastitis in Dairy Cattle in Poland, What Are They Hiding? Antibiotic Resistance and Virulence Profile. Pathogens 2022, 11, 1404. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, D.; Zhang, Y.; Hu, Y.; Lu, J.; Zeng, Z.; Zeng, D. Preparation and Antimicrobial Activity of a Film-Forming Polyhexamethylene Biguanide Teat Disinfectant. Int. J. Mol. Sci. 2023, 24, 17444. [Google Scholar] [CrossRef]
- Wierzbicki, M.; Kot, M.; Lange, A.; Kalińska, A.; Gołębiewski, M.; Jaworski, S. Evaluation of the Antimicrobial, Cytotoxic, and Physical Properties of Selected Nano-Complexes in Bovine Udder Inflammatory Pathogen Control. Nanotechnol. Sci. Appl. 2024, 17, 77–94. [Google Scholar] [CrossRef]
- Gazzola, A.; Zucali, M.; Addis, M.F.; Bava, L.; Morandi, S.; Pisanu, S.; Pagnozzi, D.; Passera, A.; Brasca, M.; Piccinini, R. Assessment of Lactococcus cremoris preparations for the pre- and post-milking teat disinfection. BMC Vet. Res. 2024, 20, 432. [Google Scholar] [CrossRef]
- Bechtold, V.; Petzl, W.; Huber-Schlenstedt, R.; Sorge, U.S. Distribution of Bovine Mastitis Pathogens in Quarter Milk Samples from Bavaria, Southern Germany, between 2014 and 2023—A Retrospective Study. Animals 2024, 14, 2504. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Vidlund, J.; Gelalcha, B.D.; Gillespie, B.E.; Agga, G.E.; Schneider, L.; Swanson, S.M.; Frady, K.D.; Kerro Dego, O. Efficacy of novel staphylococcal surface associated protein vaccines against Staphylococcus aureus and non-aureus staphylococcal mastitis in dairy cows. Vaccine 2024, 42, 1247–1258. [Google Scholar] [CrossRef]
- Zengin, H.; Alkaç, Z.K.; Vezir, Y.; Tanyıldızı, S.; Korkak, F.A.; Hark, B.D.; Dagoglu, G. Evaluation of the efficacy of a new inactivated vaccine against Staphylococcus aureus, Echerchia coli and Mycoplasma bovis mastitis in cows. Vet. Ital. 2024, 60, 1–11. [Google Scholar] [CrossRef]
- Rainard, P.; Gilbert, F.B.; Germon, P.; Foucras, G. Invited review: A critical appraisal of mastitis vaccines for dairy cows. J. Dairy Sci. 2021, 104, 10427–10448. [Google Scholar] [CrossRef]
- Côté-Gravel, J.; Malouin, F. Symposium review: Features of Staphylococcus aureus mastitis pathogenesis that guide vaccine development strategies*. J. Dairy Sci. 2019, 102, 4727–4740. [Google Scholar] [CrossRef] [PubMed]
- Clabby, C.; Valldecabres, A.; Dillon, P.; O’Sullivan, K.; Arkins, S.; Flynn, J.; McCarthy, S.; Boloña, P.S. The association between somatic cell count and selective dry cow therapy, milking routine, and dry cow management practices in early-lactation cows from 21 commercial grazing dairy herds. J. Dairy Sci. 2024, 107, 7106–7120. [Google Scholar] [CrossRef] [PubMed]
- Clabby, C.; McParland, S.; Dillon, P.; Arkins, S.; Flynn, J.; Murphy, J.; Boloña, P.S. Internal teat sealants alone or in combination with antibiotics at dry-off—The effect on udder health in dairy cows in five commercial herds. Animal 2022, 16, 100449. [Google Scholar] [CrossRef]
- Izhar, M.Z.; Nawaz, M.; Yaqub, T.; Avais, M.; Anjum, A.A. In vitro characterization of probiotic potential of Lactobacillus plantarum CM49 against selected cattle mastitogens. BMC Microbiol. 2024, 24, 310. [Google Scholar] [CrossRef]
- Stafford, E.; Kot, M. Optimal reduced-mixing for an SIS infectious-disease model. J. Biol. Dyn. 2022, 16, 746–765. [Google Scholar] [CrossRef]
- Waller, K.P.; Myrenås, M.; Börjesson, S.; Kim, H.; Widerström, M.; Monsen, T.; Sandholt, A.K.S.; Östlund, E.; Cha, W. Genotypic characterization of Staphylococcus chromogenes and Staphylococcus simulans from Swedish cases of bovine subclinical mastitis. J. Dairy Sci. 2023, 106, 7991–8004. [Google Scholar] [CrossRef]
- Gharaibeh, M.H.; Abu-Qatouseh, L.F. First molecular characterization of capsule expression and antibiotic susceptibility profile of Staphylococcus aureus isolates from bovine mastitis in Jordan. Vet. World 2022, 15, 2269–2274. [Google Scholar] [CrossRef]
- Hoque, M.N.; Talukder, A.K.; Saha, O.; Hasan, M.M.; Sultana, M.; Rahman, A.A.; Das, Z.C. Antibiogram and virulence profiling reveals multidrug resistant Staphylococcus aureus as the predominant aetiology of subclinical mastitis in riverine buffaloes. Vet. Med. Sci. 2022, 8, 2631–2645. [Google Scholar] [CrossRef]
- Maisano, A.M.; Luini, M.; Gazzola, A.; Sala, L.; Vezzoli, F.; Bertocchi, L.; Lorenzi, V.; Cremonesi, P.; Castiglioni, B.; Bergagna, S.; et al. Staphylococcus aureus adlb gene is associated with high prevalence of intramammary infection in dairy herds of northern Italy: A cross-sectional study. J. Dairy Sci. 2023, 106, 3421–3435. [Google Scholar] [CrossRef]
- Miyazawa, R.; Shimoda, S.; Matsuda, K.; Tobe, R.; Ando, T.; Yoneyama, H. Characterization of Staphylococcus aureus Isolates from Bovine Mastitis and Bulk Tank Milk: First Isolation of Methicillin-Susceptible Staphylococcus aureus in Japan. Microorganisms 2022, 10, 2117. [Google Scholar] [CrossRef]
- Singha, S.; Koop, G.; Rahman, M.M.; Ceciliani, F.; Addis, M.F.; Howlader, M.M.R.; Hossain, M.K.; Piccinini, R.; Locatelli, C.; Persson, Y.; et al. Pathogen group-specific risk factors for intramammary infection in water buffalo. PLoS ONE 2024, 19, e0299929. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, F.; Rahmani, H.K.; Bidarian, B.; Khoramian, B. Isolation and evaluation of the efficacy of bacteriophages against multidrug-resistant (MDR), methicillin-resistant (MRSA) and biofilm-producing strains of Staphylococcus aureus recovered from bovine mastitis. BMC Vet. Res. 2022, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Sikora, K.; Nowacki, A.; Szweda, P.; Woziwodzka, A.; Bartoszewska, S.; Piosik, J.; Dmochowska, B. Antimicrobial, Cytotoxic and Mutagenic Activity of Gemini QAS Derivatives of 1,4:3,6-Dianhydro-l-iditol. Molecules 2022, 27, 757. [Google Scholar] [CrossRef]
- Toledo-Silva, B.; Beuckelaere, L.; De Visscher, A.; Geeroms, C.; Meyer, E.; Piepers, S.; Thiry, D.; Haesebrouck, F.; De Vliegher, S. Novel Quantitative Assay to Describe In Vitro Bovine Mastitis Bacterial Pathogen Inhibition by Non-aureus Staphylococci. Pathogens 2022, 11, 264. [Google Scholar] [CrossRef]
- Neelam; Jain, V.K.; Singh, M.; Joshi, V.G.; Chhabra, R.; Singh, K.; Rana, Y.S. Virulence and antimicrobial resistance gene profiles of Staphylococcus aureus associated with clinical mastitis in cattle. PLoS ONE 2022, 17, e0264762. [Google Scholar] [CrossRef]
- de los Santos, R.; González-Revello, Á.; Majul, L.; Umpiérrez, A.; Aldrovandi, A.; Gil, A.; Hirigoyen, D.; Zunino, P. Subclinical bovine mastitis associated with Staphylococcus spp. in eleven Uruguayan dairy farms. J. Infect. Dev. Ctries. 2022, 16, 630–637. [Google Scholar] [CrossRef]
- Ferronato, G.; Simonetto, A.; Gilioli, G.; Zecconi, A. Modeling Mastitis Risk Management Effects on Dairy Milk Yield and Global Warming Potential. Anim. Open Access J. 2024, 15, 50. [Google Scholar] [CrossRef]
- Brito, L.F.; Bedere, N.; Douhard, F.; Oliveira, H.R.; Arnal, M.; Peñagaricano, F.; Schinckel, A.P.; Baes, C.F.; Miglior, F. Review: Genetic selection of high-yielding dairy cattle toward sustainable farming systems in a rapidly changing world. Animal 2021, 15, 100292. [Google Scholar] [CrossRef]

| Stage of Intracellular Pathogenesis | Recent Mechanistic Findings |
|---|---|
| Adhesion and entry | Invasion is triggered when fibronectin-binding proteins A/B (FnBPs) on the bacterial surface bridge soluble fibronectin to host α5β1-integrins, activating focal-adhesion kinase and actin remodeling that “zipper” S. aureus into BMECs. High-prevalence mastitis lineages (e.g., CC97, CC151) show enriched fnbA/B expression and an enhanced internalization capacity [18]. |
| Early intracellular phase | Once inside endocytic vacuoles, S. aureus inhibits lysosomal acidification and downregulates mTORC1 nutrient signaling, reducing amino acid import and milk protein synthesis, thereby weakening host tissue functions [36]. |
| Subversion of host quality-control pathways | Two complementary studies have shown that bovine mastitis isolates induce mitophagy to eliminate mitochondria-derived reactive oxygen species (ROS) that would otherwise kill the bacteria. One group linked this effect to the upregulation of the PINK1/Parkin mitophagy pathway in bovine mammary epithelial cells (BMECs) [36]. Another independent study demonstrated a similar mechanism in macrophages, involving the HDAC11/IL-10 signaling axis, which promoted the intracellular survival of S. aureus [37]. |
| Persistence & antibiotic tolerance | Nutrient limitation and oxidative stress inside cells select for small-colony variants (SCVs) with slowed metabolism, low membrane potential, and high tolerance to β-lactams and aminoglycosides, explaining frequent treatment failures [18] |
| Escape or reseeding | Periodic host-cell lysis or exocytosis releases bacteria back into milk ducts, reseeding the udder and neighboring quarters, which clarifies why culling or dry-cow therapy is often required for eradication [36] |
| Country | Total Samples Analyzed | Sample Type | Prevalence of S. aureus (%) | Type of Mastitis | Reference |
|---|---|---|---|---|---|
| Africa | |||||
| Cameroon | 300 | Individual cow milk | 67.0 | Clinical and Subclinical | [38] |
| Egypt | 352 | Individual cow milk | 44.4 | Clinical and Subclinical | [39] |
| Egypt | 200 | Pooled milk | 0.39 | Subclinical | [40] |
| Ethiopia | 600 | Quarter milk | 0.19 | Clinical and Subclinical | [41] |
| Rwanda | 1080 | Quarter milk | 12.5 | Not specified | [42] |
| Asia | |||||
| Iraq | 50 | Individual cow milk | 66.0 | Clinical and Subclinical | [43] |
| Bangladesh | 120 | Individual cow milk | 46.66 | Clinical | [44] |
| China | 57 | Raw milk | 7.0 | Not Specified | [45] |
| Iran | 200 | Individual cow milk | 12 | Not specified | [46] |
| Pakistan | 173 | CMT-positive milk | 40.0 | Subclinical | [47] |
| Pakistan | 310 | Raw milk | 30.32 | Subclinical | [48] |
| Pakistan | 200 | Individual cow milk | 42.5 | Clinical and Subclinical | [49] |
| Thailand | 84 | Lactating cow milk | 4.76 | Clinical and Subclinical | [50] |
| Vietnam | 400 | Raw milk | 12.0 | Clinical and Subclinical | [51] |
| Europe | |||||
| Ireland | 7.833 | Milk samples from herds | 21.37–25.59 (annual) | Clinical | [52] |
| Italy | 88 | Bulk tank milk | 32.95% (herd-level) | Not Specified | [53] |
| Italy | 120 herds (BTM samples) | Bulk tank milk | 59.16 (herd-level) | Clinical and Subclinical | [4] |
| Poland | 100 | Milk | 22.0 | Clinical and Subclinical | [54] |
| Poland | 462 | Composite udder milk | 7.79 | Clinical and Subclinical | [55] |
| Romania | 325 | CMT-positive milk | 46.1 | Clinical and Subclinical | [56] |
| Americas | |||||
| Mexico | 50 | Individual cow (CMT) | 42 (cow-level) | Subclinical | [57] |
| Uruguay | 191 | Herd isolates | 81.1 | Clinical and Subclinical | [58] |
| Canada | 8.957 | Clinical milk samples | 15.0 | Clinical | [59] |
| USA | 5.703 | Individual cow milk | 3.3 | Clinical and Subclinical | [60] |
| Technique | Sensitivity | Specificity | Cost | Turnaround Time | Field Applicability | Key Insights |
|---|---|---|---|---|---|---|
| Culture | Moderate (50–89.7%; ↓ in mixed infections) | Moderate–High (challenges in CNS differentiation) | $ Low | Days (24–72 h) | ★★☆☆☆ (Lab-dependent) | Standard method; sensitivity improves after competitive pathogen elimination. Limitations for non-culturable strains |
| PCR | High (near 100%; ↑ with pre-incubation) | High (species-specific) | $$$ High | Hours (4–24 h) | ★★☆☆☆ (Lab equipment) | Detects virulence/resistance genes (nuc, mecA); requires enrichment for low loads. Higher cost limits field use. |
| qPCR | Very High (≥95%) | Very High (≥99%) | $$$ High | Hours (2–6 h) | ★★☆☆☆ (Equipment needed) | Probe-based assays achieve 100% specificity; ideal for bulk milk screening. Cost-prohibitive in resource-limited settings. |
| RAA-LFD | Very High (60 fg DNA; 1.78 × 103 CFU/mL) | Very High (no cross-reactivity) | $ Low | Minutes (45–85 min) | ★★★★★ (Portable, dipstick) | Rapid, equipment-free detection; ideal for field use. Detects S. aureus in spiked milk after 6-hr enrichment. |
| MALDI-TOF | High (score > 2.0) | High (species ID) | $$$ High (instrument) | Hours (post-culture) | ★★☆☆☆ (Lab-based) | Rapid ID post-culture is ineffective for detecting AMR. Limited by database accuracy. |
| AI-Based Systems | Variable (>85% for S. aureus; ↓ for rare pathogens) | Very High (91.9–99.1%) | $$ Moderate | Days (24–48 h) | ★★★☆☆ (On-farm/Lab) | Automated colony ID (e.g., Bacticam); struggles with mixed infections and transport delays. WGS offers high accuracy but costly/non-portable. |
| Strategy Category | Specific Approach | Key Findings/Efficacy |
|---|---|---|
| Hygiene Practices | Pre-/post-milking teat disinfection (e.g., PHMB, iodine, LAB-based dips) |
|
| Environmental management (bedding, housing, cubicle hygiene) |
| |
| Milking protocols (gloves, aseptic techniques, machine sanitation) |
| |
| Vaccination | Whole-cell/bacterin vaccines (e.g., S. aureus surface proteins, toxoids) |
|
| Multivalent vaccines (e.g., S. aureus + E. coli + M. bovis) |
| |
| Novel approaches (live-attenuated mutants, DNA/nanogel vaccines) |
| |
| Dry Cow Therapy (DCT) | Selective DCT (antibiotics + ITS for infected cows; ITS alone for low-SCC cows) |
|
| Adjuncts: Internal teat sealants (ITS) |
| |
| Non-antibiotic alternatives (probiotics, nanoparticles) |
| |
| AI-Driven Interventions | Algorithm-based DCT decision support |
|
| PCR pooling for herd surveillance (e.g., 10-cow pools) |
| |
| Optimal mixing/segregation modeling (e.g., “bang-bang” strategy) |
| |
| Bayesian latent class models for diagnostics |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touaitia, R.; Ibrahim, N.A.; Touati, A.; Idres, T. Staphylococcus aureus in Bovine Mastitis: A Narrative Review of Prevalence, Antimicrobial Resistance, and Advances in Detection Strategies. Antibiotics 2025, 14, 810. https://doi.org/10.3390/antibiotics14080810
Touaitia R, Ibrahim NA, Touati A, Idres T. Staphylococcus aureus in Bovine Mastitis: A Narrative Review of Prevalence, Antimicrobial Resistance, and Advances in Detection Strategies. Antibiotics. 2025; 14(8):810. https://doi.org/10.3390/antibiotics14080810
Chicago/Turabian StyleTouaitia, Rahima, Nasir Adam Ibrahim, Abdelaziz Touati, and Takfarinas Idres. 2025. "Staphylococcus aureus in Bovine Mastitis: A Narrative Review of Prevalence, Antimicrobial Resistance, and Advances in Detection Strategies" Antibiotics 14, no. 8: 810. https://doi.org/10.3390/antibiotics14080810
APA StyleTouaitia, R., Ibrahim, N. A., Touati, A., & Idres, T. (2025). Staphylococcus aureus in Bovine Mastitis: A Narrative Review of Prevalence, Antimicrobial Resistance, and Advances in Detection Strategies. Antibiotics, 14(8), 810. https://doi.org/10.3390/antibiotics14080810








