Bactericidal Activities of Nanoemulsion Containing Piper betle L. Leaf and Hydroxychavicol Against Avian Pathogenic Escherichia coli and Modelling Simulation of Hydroxychavicol Against Bacterial Cell Division Proteins
Abstract
1. Introduction
2. Results
2.1. Formulations of the NEPE and Detection of Hydroxychavicol in NEPE
2.2. Antibacterial Activity of NEPE and Hydroxychavicol Against APEC
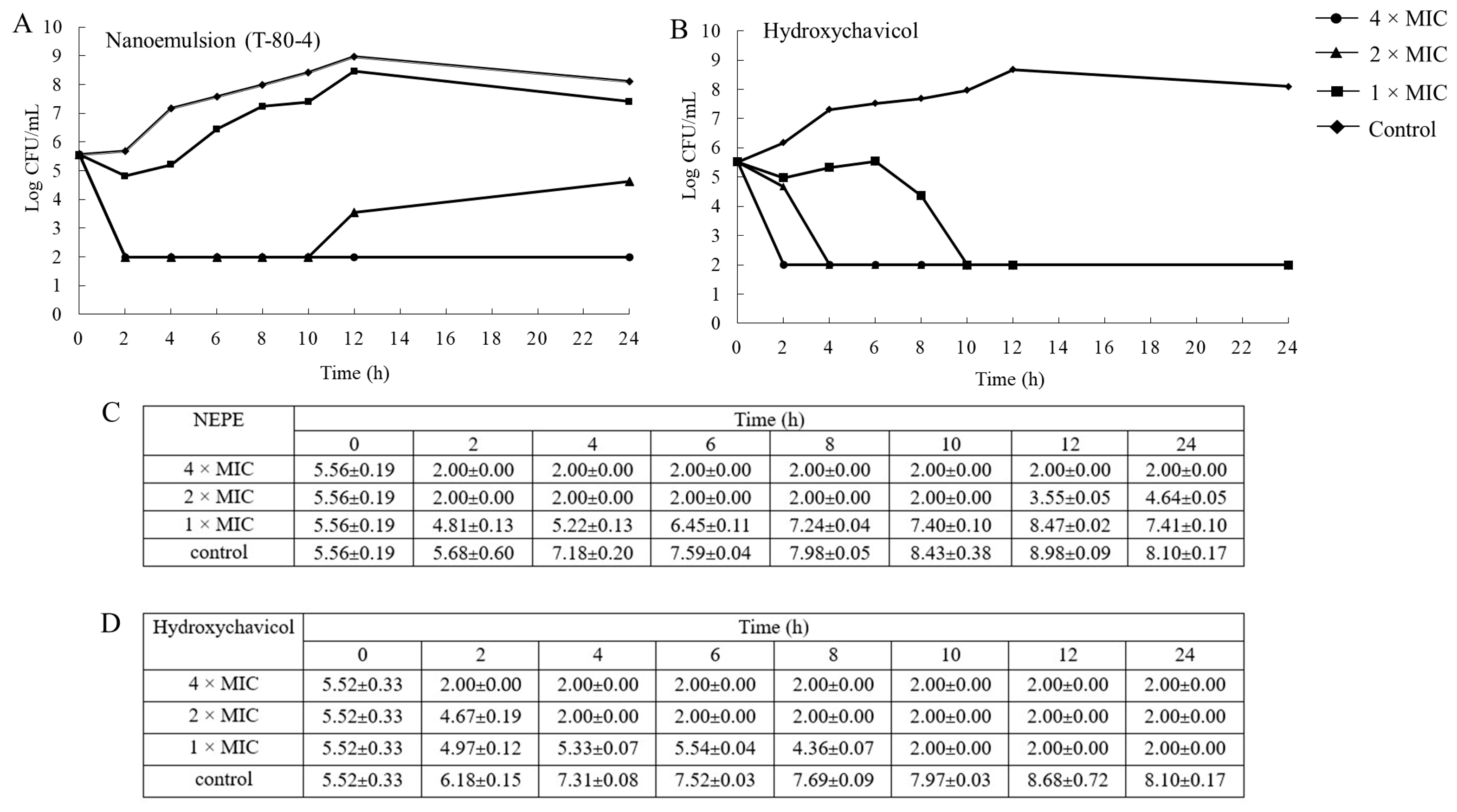
2.3. Time–Kill Study
2.4. Viability of APEC After Treatment with NEPE and Hydroxychavicol as Observed by Confocal Microscopy
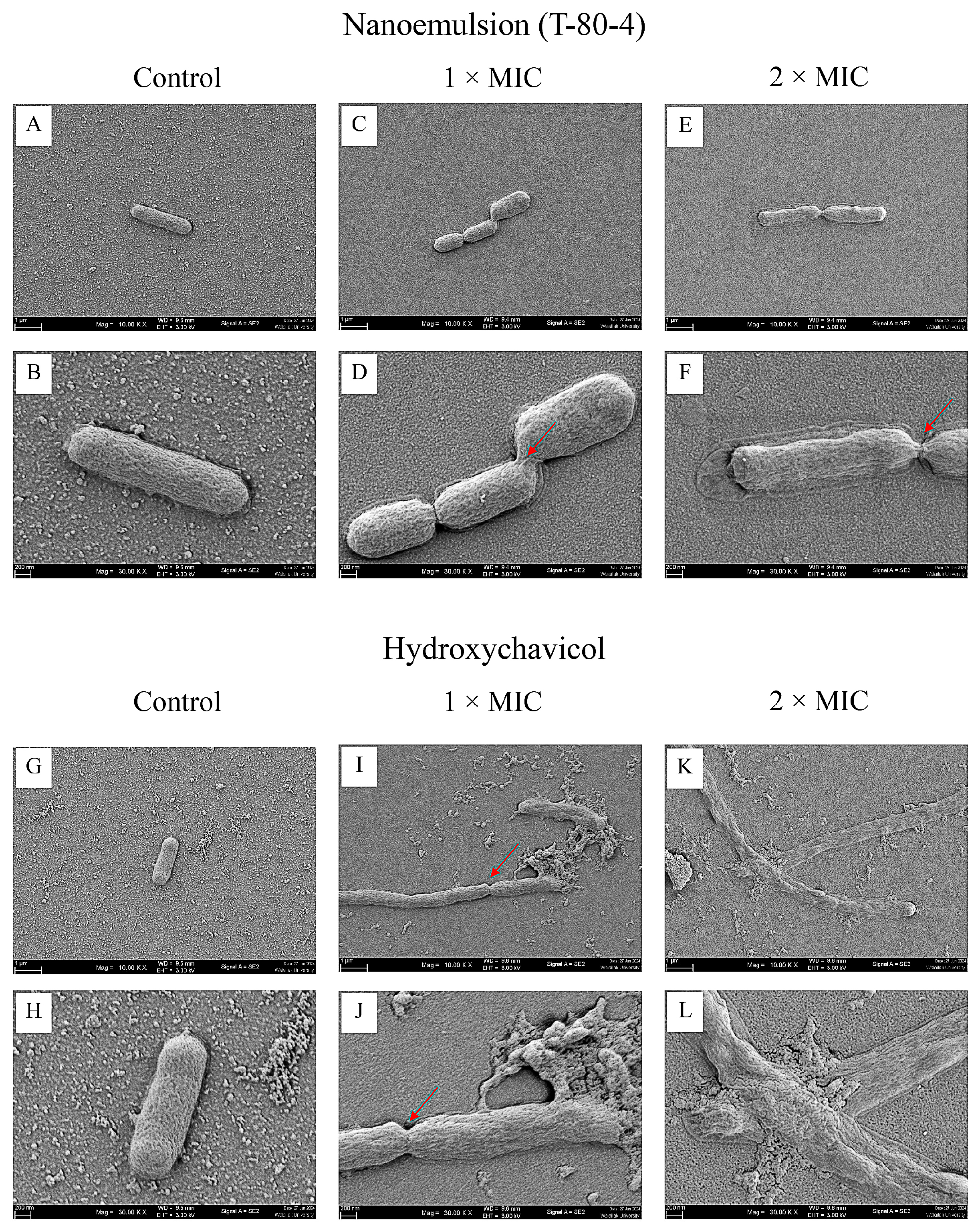
2.5. Morphology of APEC After Treatment with NEPE and Hydroxychavicol by Scanning Electron Microscopy (SEM)
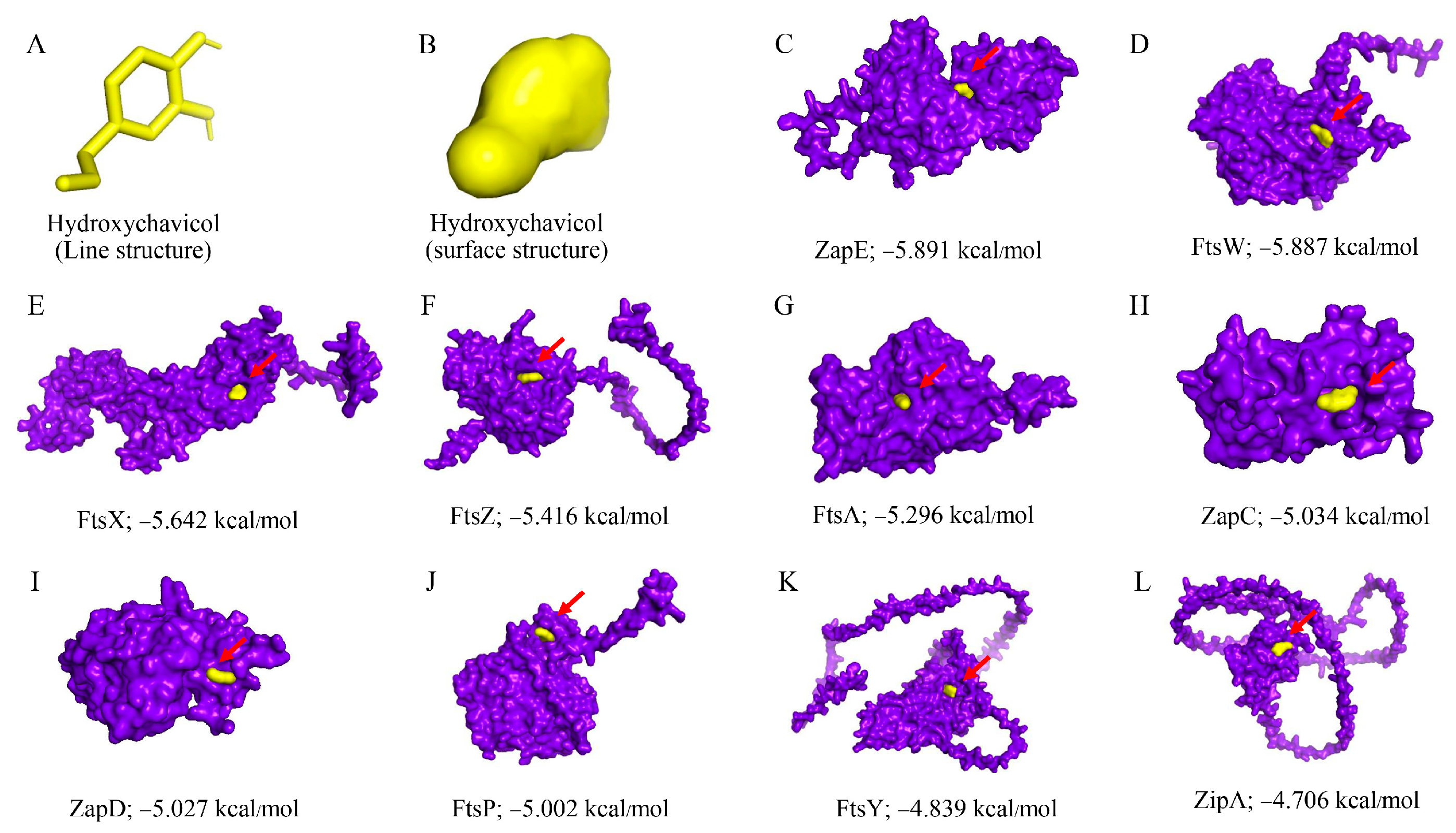
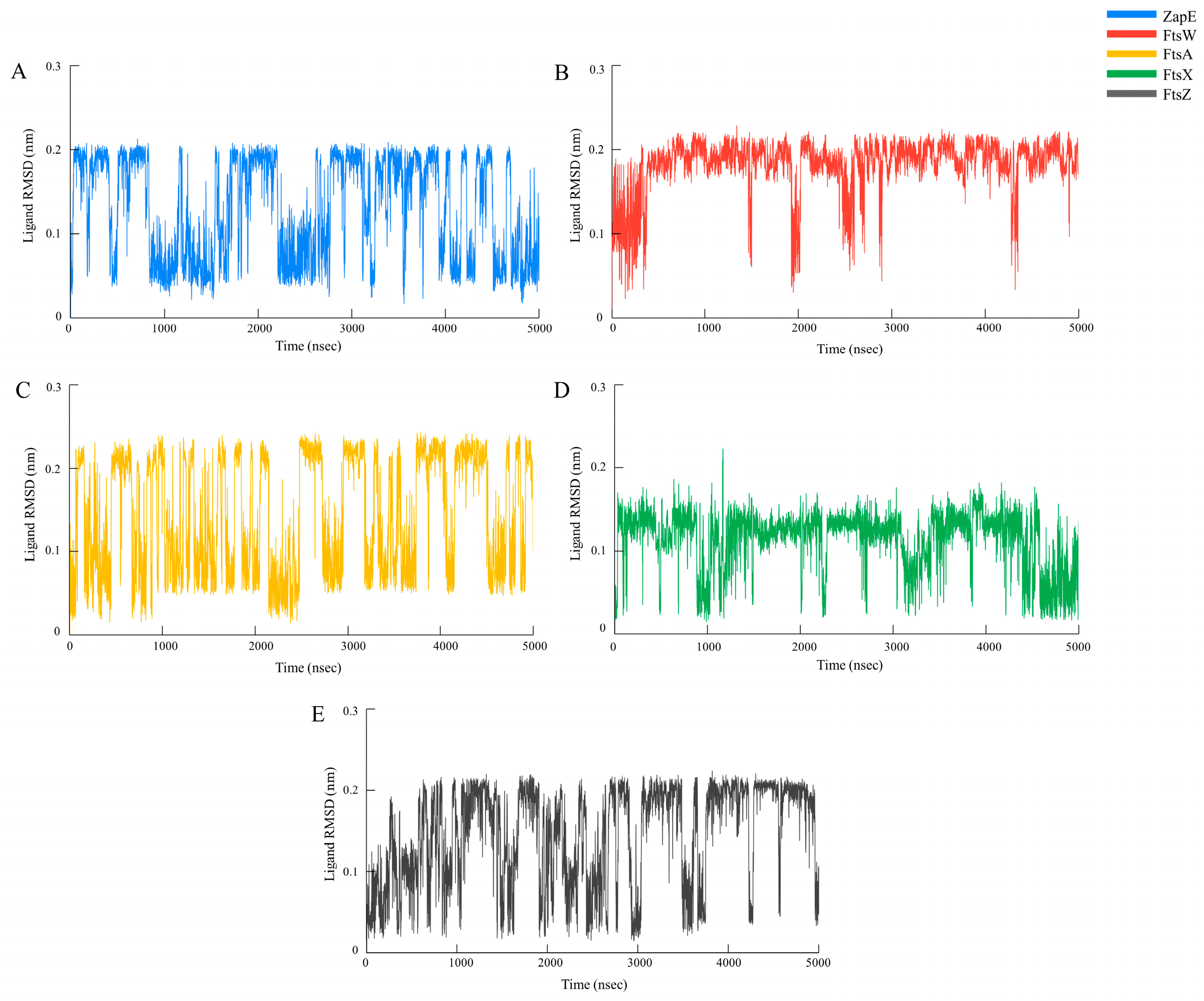
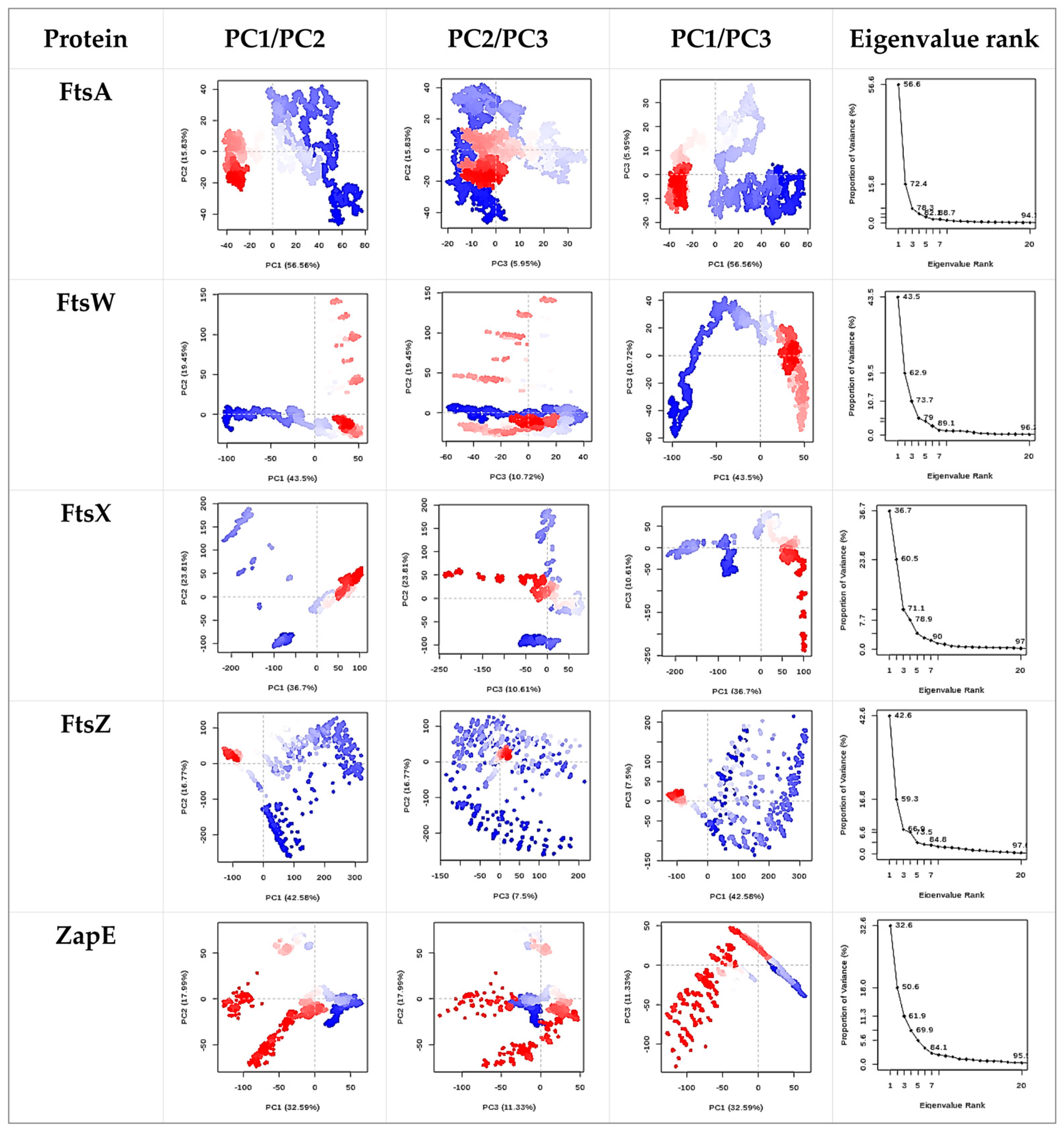
2.6. Molecular Docking and Molecular Dynamics Simulation
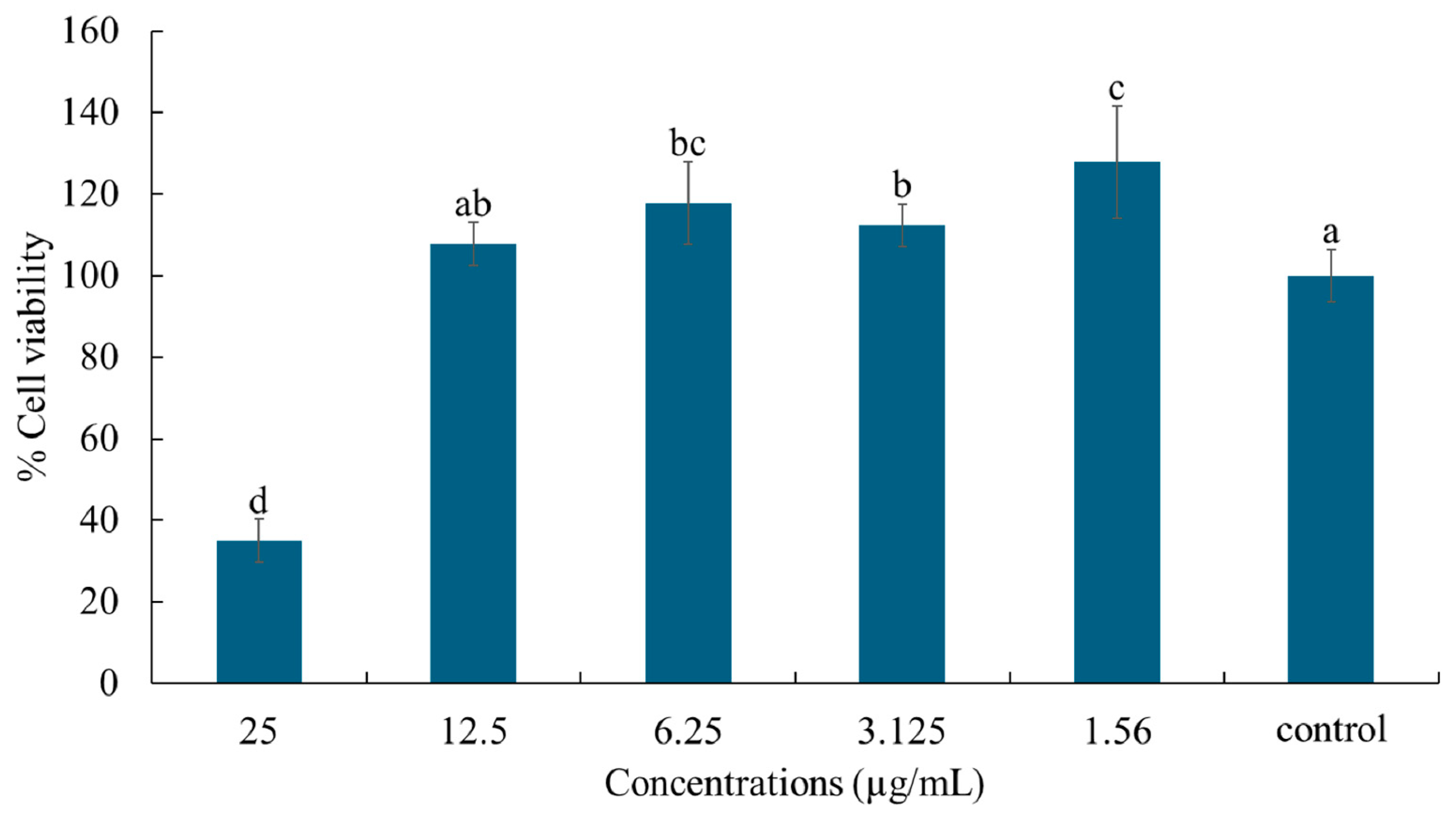
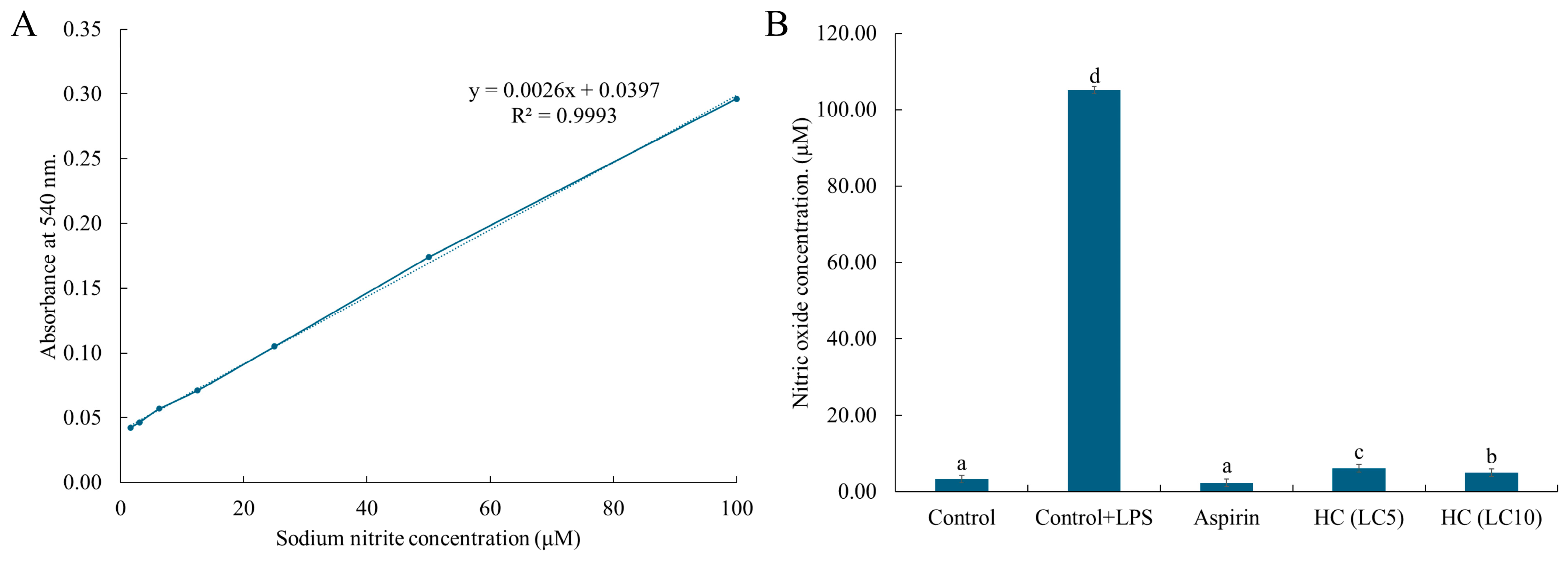
2.7. Cell Viability and Nitric Oxide Production
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Preparation of Plant Extract and Antimicrobial Compounds
4.2.1. Preparation of Ethanol P. betle Extract
4.2.2. Preparation of HDES-Based Nanoemulsion Containing P. betle Leaf
4.3. Detection of Hydroxychavicol in the NEPE
4.4. Determination of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
4.5. Time–Kill Study
4.6. Evaluation of APEC Viability After Treatment with NEPE and Hydroxychavicol Using Confocal Microscopy
4.7. Morphology of APEC After Treatment with NEPE and Hydroxychavicol Assessed Using Scanning Electron Microscopy (SEM)
4.8. Molecular Docking and Molecular Dynamic Simulation
4.9. Cytotoxicity Assay
4.10. Measurement of Nitric Oxide
4.11. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Li, W.; Zhao, Q.; Wu, P.; Huang, X.; Jin, W.; Wang, B.; Li, S.; Liu, W.; Zhang, G. Combined analysis of the microbiome, metabolome and transcriptome of silkie chickens in response to avian pathogenic E. coli (APEC). Microb. Pathog. 2024, 189, 106586. [Google Scholar] [CrossRef]
- Afayibo, D.J.; Zhu, H.; Zhang, B.; Yao, L.; Abdelgawad, H.A.; Tian, M.; Qi, J.; Liu, Y.; Wang, S. Isolation, Molecular Characterization, and Antibiotic Resistance of Avian Pathogenic Escherichia coli in Eastern China. Vet. Sci. 2022, 9, 319. [Google Scholar] [CrossRef]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian pathogenic Escherichia coli (APEC): An overview of virulence and pathogenesis factors, zoonotic potential, and control strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, A.; Canè, C.; Rotondo, N.P.; Cavalluzzi, M.M.; Lentini, G.; Duilio, A. A Comparative Study of the Inhibitory Action of Berberine Derivatives on the Recombinant Protein FtsZ of E. coli. Int. J. Mol. Sci. 2023, 24, 5674. [Google Scholar] [CrossRef]
- Rahman, M.; Wang, P.; Wang, N.; Chen, Y. A key bacterial cytoskeletal cell division protein FtsZ as a novel therapeutic antibacterial drug target. Bosn. J. Basic Med. Sci. 2020, 20, 310. [Google Scholar]
- Casiraghi, A.; Suigo, L.; Valoti, E.; Straniero, V. Targeting bacterial cell division: A binding site-centered approach to the most promising inhibitors of the essential protein FtsZ. Antibiotics 2020, 9, 69. [Google Scholar] [CrossRef]
- Kumar, S.; Juyal, A.; Bisht, S.; Jaiswal, V. Betel leaf (Piper betle): Ethnomedicine to emerging therapeutic frontiers. J. Pharmacogn. Phytochem. 2024, 13, 249–258. [Google Scholar] [CrossRef]
- Nayaka, N.M.D.M.W.; Sasadara, M.M.V.; Sanjaya, D.A.; Yuda, P.E.S.K.; Dewi, N.L.K.A.A.; Cahyaningsih, E.; Hartati, R. Piper betle (L): Recent review of antibacterial and antifungal properties, safety profiles, and commercial applications. Molecules 2021, 26, 2321. [Google Scholar] [CrossRef]
- Lubis, R.R.; Wahyuni, D.D. Antibacterial activity of betle leaf (Piper betle L.) extract on inhibiting Staphylococcus aureus in conjunctivitis patient. Am. J. Clin. Exp. Immunol. 2020, 9, 1. [Google Scholar]
- Gupta, R.K.; Guha, P.; Srivastav, P.P. Phytochemical and biological studies of betel leaf (Piper betle L.): Review on paradigm and its potential benefits in human health. Acta Ecol. Sin. 2023, 43, 721–732. [Google Scholar] [CrossRef]
- Kulnanan, P.; Chuprom, J.; Thomrongsuwannakij, T.; Romyasamit, C.; Sangkanu, S.; Manin, N.; Nissapatom, V.; Pereira, M.; Wirairatana, P.; Mitsuwan, W. Antibacterial, Antibiofilm and Anti-adhesion Activities of Piper betle Leaf Extract Against Avian Pathogenic Escherichia coli. Arch Microbiol. 2021, 204, 49. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Boateng, I.D. A critical review of emerging hydrophobic deep eutectic solvents’ applications in food chemistry: Trends and opportunities. J. Agric. Food Chem. 2022, 70, 11860–11879. [Google Scholar] [CrossRef]
- Kukrić, T.N.; Iličić, R.M.; Jurić, T.M.; Uka, D.B.; Bagi, F.F.; Đurić, S.S.; Popović, B.M. Antifungal efficacy and biofumigation potential of hydrophobic deep eutectic solvents: Postharvest treatment against Monilinia fructicola and Botrytis cinerea. World J. Microbiol. Biotechnol. 2024, 40, 393. [Google Scholar] [CrossRef]
- Silva, J.; Pereira, C.; Mano, F.; Silva, E.; Castro, V.; Sá-Nogueira, I.; Reis, R.; Paiva, A.; Matias, A.; Duarte, A. Therapeutic role of deep eutectic solvents based on menthol and saturated fatty acids on wound healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, Y.; Ding, Z.; Xia, H.; Guo, S. Physicochemical properties and antibacterial activity of hydrophobic deep eutectic solvent-in-water nanoemulsion. J. Mol. Liq. 2021, 338, 116950. [Google Scholar] [CrossRef]
- Li, B.; Shi, M.; Qin, S.; Zeng, C. Antibacterial mechanism of hydrophobic deep eutectic solvents-in-water nanoemulsion: Experimental and molecular dynamic simulation studies. J. Food Meas. Charact. 2025, 19, 1–15. [Google Scholar] [CrossRef]
- Tong, L.; Qi, J.; Wei, L.; Liu, J.; Wang, N.; Dai, S.; Lu, H. Sustainable Treatment of Oil-Based Drilling Cuttings Using a Switchable Fatty-Acids-Based Hydrophobic Deep Eutectic Solvent. Ind. Eng. Chem. Res. 2024, 63, 22034–22042. [Google Scholar] [CrossRef]
- Mak, P.H.; Rehman, M.A.; Kiarie, E.G.; Topp, E.; Diarra, M.S. Production systems and important antimicrobial resistant-pathogenic bacteria in poultry: A review. J. Anim. Sci. Biotechnol. 2022, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Chodkowska, K.A.; Iwiński, H.; Wódz, K.; Nowak, T.; Różański, H. In Vitro Assessment of Antimicrobial Activity of Phytobiotics Composition towards of Avian Pathogenic Escherichia coli (APEC) and Other E. coli Strains Isolated from Broiler Chickens. Antibiotics 2022, 11, 1818. [Google Scholar] [CrossRef] [PubMed]
- Boripun, R.; Paopradit, P.; Prampramote, J.; Narinthorn, R.; Intongead, S.; Sangkanu, S.; Narongrit, T.; Mitsuwan, W. Bactericidal activity of Piper betle L. extract against antibiotic resistant Salmonella spp. isolated from pig farms in Southern Thailand. Vet. Integr. Sci. 2022, 20, 557–569. [Google Scholar] [CrossRef]
- Sungkatavat, P.; Khongkhai, H.; Kanchana, W.; Saengsawarng, P.; Sangkanu, S.; Nissapatorn, V.; de Lourdes Pereira, M.; Ontong, J.C.; Mitsuwan, W. Piper betle extract and its application in bovine teat dipping solution inhibit and eliminate biofilms in bovine mastitis-inducing staphylococci. Vet. World 2023, 16, 2135. [Google Scholar] [CrossRef]
- More, S.K.; Pawar, A.P. Preparation, optimization and preliminary pharmacokinetic study of curcumin encapsulated turmeric oil microemulsion in zebra fish. Eur. J. Pharm. Sci. 2020, 155, 105539. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, T.T.; Nguyen, H.N.; Bui, Q.T.P. Simultaneous Determination of Active Compounds in Piper betle Linn. Leaf Extract and Effect of Extracting Solvents on Bioactivity. Eng. Rep. 2020, 2, e12246. [Google Scholar] [CrossRef]
- Singtongratana, N.; Vadhanasin, S.; Singkhonrat, J. Hydroxychavicol and Eugenol Profiling of Betel Leaves from Piper betle L. Obtained by Liquid-Liquid Extraction and Supercritical Fluid Extraction. Agric. Nat. Resour. 2013, 47, 614–623. [Google Scholar]
- Vargas-Escobar, P.; Quintero-Rincón, P.; Flórez-Acosta, O. Development of a Dermal Nanoemulsion with Antioxidants Derived from Rice Residues Using an HLD Theory Approach. AAPS PharmSciTech 2025, 26, 56. [Google Scholar] [CrossRef]
- Bai, Y.; Luo, X.; Han, Y.; Liu, B.; Zhang, J.; Zhang, M. Facile synthesis of narrow particle size distribution, high solid content, cationic polymer latexes by macroemulsion polymerization-based particle coagulation mechanism. J. Macromol. Sci. 2020, 57, 116–122. [Google Scholar] [CrossRef]
- Ye, D.; Ren, J.; Zhang, Y.; Wang, X.; Guo, J.; Li, B.; Li, Q.; Chen, Y.; Chen, P.; Zhang, B. Lactic acid/tartaric acid-maltitol antibacterial activity against spoilage bacteria and prevention of yellowing and wilting in spinach and oilseed rape. Food Chem. 2025, 471, 142557. [Google Scholar] [CrossRef]
- Carsono, N.; Tumilaar, S.G.; Kurnia, D.; Latipudin, D.; Satari, M.H. A review of bioactive compounds and antioxidant activity properties of piper species. Molecules 2022, 27, 6774. [Google Scholar] [CrossRef]
- Singh, D.; Narayanamoorthy, S.; Gamre, S.; Majumdar, A.G.; Goswami, M.; Gami, U.; Cherian, S.; Subramanian, M. Hydroxychavicol, a key ingredient of Piper betle induces bacterial cell death by DNA damage and inhibition of cell division. Free Radic. Biol. Med. 2018, 120, 62–71. [Google Scholar] [CrossRef]
- Roy, A.; Anbarasu, A. Unveiling Berberine analogues as potential inhibitors of Escherichia coli FtsZ through machine learning molecular docking and molecular dynamics approach. Sci. Rep. 2025, 15, 14668. [Google Scholar] [CrossRef]
- Bray, S.A.; Senapathi, T.; Barnett, C.B.; Grüning, B.A. Intuitive, reproducible high-throughput molecular dynamics in Galaxy: A tutorial. J. Cheminform. 2020, 12, 54. [Google Scholar] [CrossRef]
- Eawsakul, K.; Klangbud, W.K.; Saengsawang, P.; Ongtanasup, T.; Ratchasong, K.; Boripun, R.; Nissapatorn, V.; Pereira, M.d.L.; Turni, C.; Makkliang, F. Bactericidal and antibiofilm activities of Piper betle extract against Burkholderia pseudomallei: In vitro and in silico approaches. Biofouling 2025, 41, 36–51. [Google Scholar] [CrossRef]
- Witte, A.; Brand, E.; Mayrhofer, P.; Narendja, F.; Lubitz, W. Mutations in cell division proteins FtsZ and FtsA inhibit φX174 protein-E-mediated lysis of Escherichia coli. Arch. Microbiol. 1998, 170, 259–268. [Google Scholar] [CrossRef]
- Srihirun, S.; Mathithiphark, S.; Phruksaniyom, C.; Kongphanich, P.; Inthanop, W.; Sriwantana, T.; Tancharoen, S.; Sibmooh, N.; Vivithanaporn, P. Hydroxychavicol Inhibits In Vitro Osteoclastogenesis via the Suppression of NF-kappaB Signaling Pathway. Biomol. Ther. 2024, 32, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Bani, S. Hydroxychavicol inhibits immune responses to mitigate cognitive dysfunction in rats. J. Neuroimmunol. 2010, 226, 48–58. [Google Scholar] [CrossRef]
- Ou, J.; Zhong, F.; Huang, P.; Zhang, Y.; Xie, S.; Wu, P.; Li, J.; Qiu, H.; Wang, C.; Huang, Y. Hydroxychavicol derivatives from Piper betle Linn. as natural PDE4 inhibitors with anti-inflammatory effects. Bioorg. Chem. 2025, 157, 108294. [Google Scholar] [CrossRef] [PubMed]
- Thomrongsuwannakij, T.; Narinthorn, R.; Mahawan, T.; Blackall, P.J. Molecular and phenotypic characterization of avian pathogenic Escherichia coli isolated from commercial broilers and native chickens. Poult. Sci. 2022, 101, 101527. [Google Scholar] [CrossRef] [PubMed]
- Sonphakdi, T.; Tani, A.; Payaka, A.; Ungcharoenwiwat, P. Antibacterial and toxicity studies of phytochemicals from Piper betle leaf extract. J. King Saud Univ. Sci. 2024, 36, 103430. [Google Scholar] [CrossRef]
- Sivaranjani, M.; McCarthy, M.C.; Sniatynski, M.K.; Wu, L.; Dillon, J.-A.R.; Rubin, J.E.; White, A.P. Biofilm formation and antimicrobial susceptibility of E. coli associated with colibacillosis outbreaks in broiler chickens from Saskatchewan. Front. Microbiol. 2022, 13, 841516. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Deng, X.; Tan, M.; Yu, C.; Zhang, M.; Sun, Y.; Jiang, N. In vitro antibiofilm activity of resveratrol against avian pathogenic Escherichia coli. BMC Vet. Res. 2021, 17, 249. [Google Scholar] [CrossRef]
- Margolin, W. FtsZ and the division of prokaryotic cells and organelles. Nat. Rev. Mol. Cell Biol. 2005, 6, 862–871. [Google Scholar] [CrossRef]
- Roach, E.J.; Uehara, T.; Daigle, D.M.; Six, D.A.; Khursigara, C.M. The next-generation beta-lactamase inhibitor taniborbactam restores the morphological effects of cefepime in KPC-producing Escherichia coli. Microbiol. Spectr. 2021, 9, e0091821. [Google Scholar] [CrossRef]
- Sahlan, M.; Mahira, K.F.; Pratami, D.K.; Rizal, R.; Ansari, M.J.; Al-Anazi, K.M.; Farah, M.A. The cytotoxic and anti-inflammatory potential of Tetragonula sapiens propolis from Sulawesi on raw 264.7 cell lines. J. King Saud Univ.-Sci. 2021, 33, 101314. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef] [PubMed]
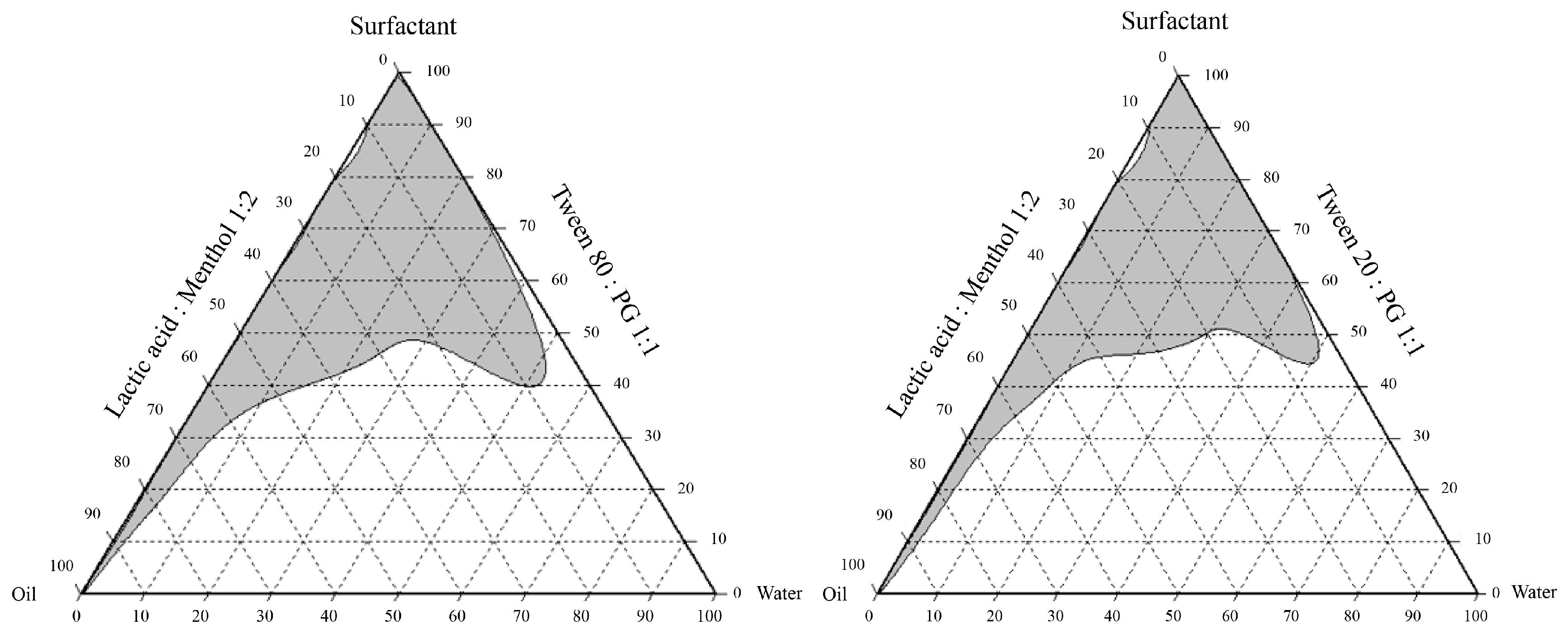
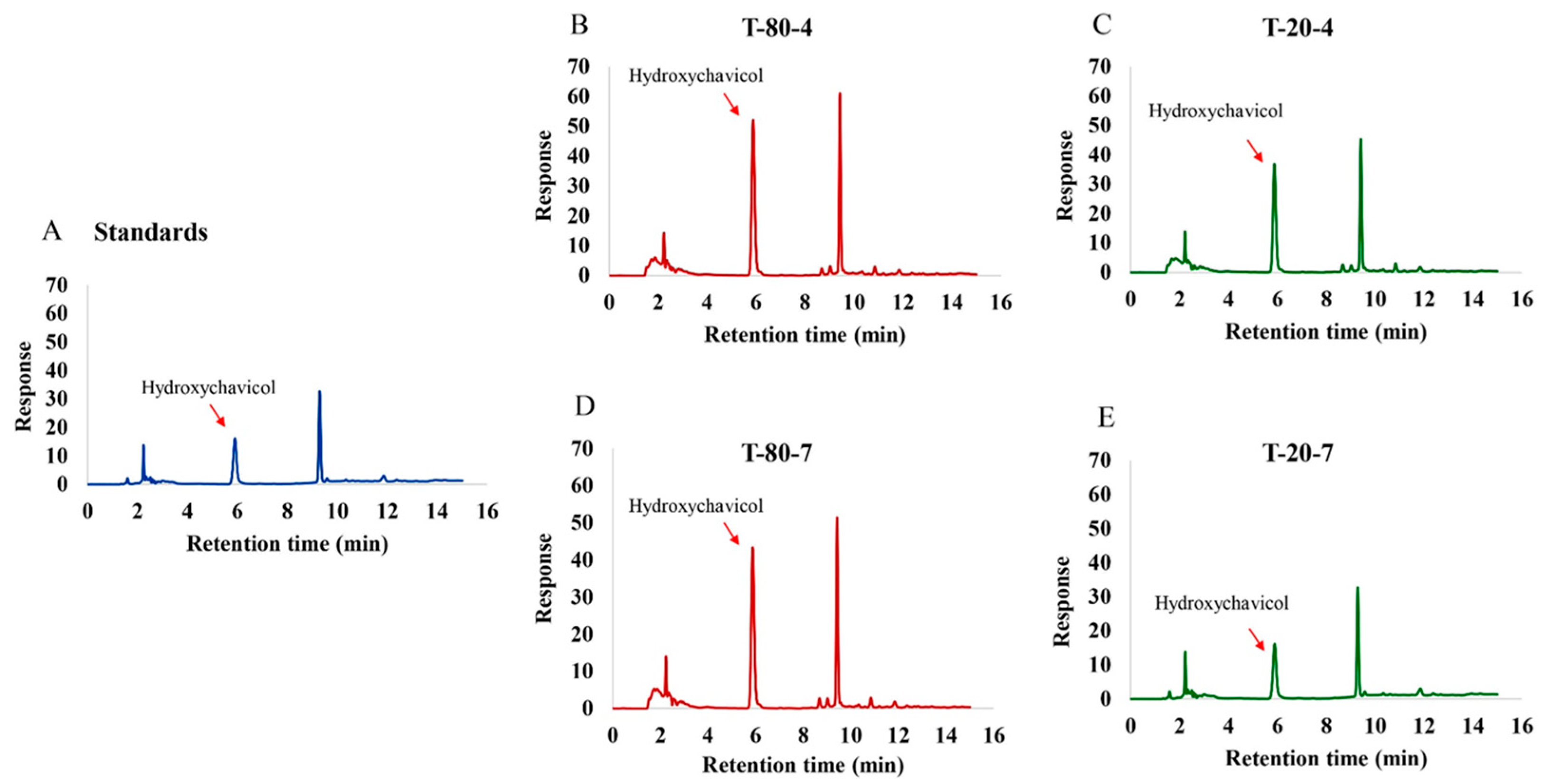



| Formulations of NEPE | Ingredients (g) | |||
|---|---|---|---|---|
| Lactic Acid: Menthol (1:2 Mole Ratio) | Surfactant Mixture | Water | P. betle Powder | |
| T-80-4 | 30 | 60 a | 10 | 10 |
| T-80-7 | 40 | 50 a | 10 | 10 |
| T-20-4 | 30 | 60 b | 10 | 10 |
| T-20-7 | 40 | 50 b | 10 | 10 |
| Formulations of NEPE | Characteristics | |||
|---|---|---|---|---|
| Hydroxychavicol Detected (mg/mL) | Particle Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | |
| T-80-4 | 4.24 ± 0.01 a | 162.60 ± 4.255 a | 0.46 ± 0.01 a | −45.53 ± 0.17 a |
| T-80-7 | 2.62 ± 0.01 b | 353.30 ± 26.535 a | 0.31 ± 0.04 ab | −47.63 ± 0.09 b |
| T-20-4 | 2.20 ± 0.01 c | 642.33 ± 121.64 a | 0.62 ± 0.08 c | −23.97 ± 0.81 c |
| T-20-7 | 1.88 ± 0.01 d | 24,693.33 ± 899.79 b | 0.61 ± 0.09 bc | −16.03 ± 0.53 d |
| Isolates | MIC/MBC | ||||||
|---|---|---|---|---|---|---|---|
| NEPE a | Ethanol P. betle Extract b | Hydroxychavicol b | Gentamicin c | ||||
| T-80-4 | T-80-7 | T-20-4 | T-20-7 | ||||
| CHUL7 | 0.06/0.125 | 0.06/0.125 | 0.125/0.25 | 0.06/0.125 | 1.0/1.0 | 0.25/0.25 | 4/4 |
| CHUL8 | 0.06/0.125 | 0.06/0.25 | 0.125/0.25 | 0.06/0.25 | 1.0/1.0 | 0.25/0.25 | 1/1 |
| CHUL9 | 0.06/0.25 | 0.125/0.125 | 0.125/0.25 | 0.125/0.125 | 1.0/1.0 | 0.25/0.25 | 0.5/1 |
| CHUL10 | 0.06/0.25 | 0.125/0.125 | 0.125/0.25 | 0.125/0.125 | 1.0/1.0 | 0.25/0.25 | 1/2 |
| CHUL13 | 0.125/0.25 | 0.125/0.25 | 0.125/0.25 | 0.125/0.125 | 1.0/1.0 | 0.25/0.25 | 0.5/1 |
| CHUL47 | 0.25/0.25 | 0.125/0.125 | 0.25/0.25 | 0.125/0.125 | 1.0/1.0 | 0.25/0.25 | 0.5/2 |
| CHUL49 | 0.25/0.25 | 0.125/0.25 | 0.125/0.25 | 0.125/0.125 | 1.0/1.0 | 0.25/0.25 | 0.5/2 |
| CHUL50 | 0.125/0.125 | 0.125/0.125 | 0.125/0.25 | 0.125/0.25 | 1.0/1.0 | 0.25/0.25 | 1/2 |
| CHUL53 | 0.125/0.25 | 0.125/0.125 | 0.125/0.125 | 0.125/0.125 | 1.0/1.0 | 0.25/0.25 | 1/2 |
| CHUL57 | 0.125/0.125 | 0.125/0.125 | 0.06/0.125 | 0.125/0.125 | 1.0/1.0 | 0.25/0.25 | 0.5/1 |
| ATCC25922 | 0.125/0.125 | 0.125/0.125 | 0.125/0.125 | 0.125/0.125 | 0.5/1.0 | 0.25/0.25 | 0.5/1 |
| Protein | Binding Energy | |
|---|---|---|
| Hydroxychavicol | Cefepime | |
| ZapE | −5.891 | −6.887 |
| FtsW | −5.887 | −6.575 |
| FtsX | −5.642 | −6.334 |
| FtsZ | −5.416 | −8.057 |
| FtsA | −5.296 | −6.897 |
| ZapC | −5.034 | −6.373 |
| ZapD | −5.027 | −6.116 |
| FtsP | −5.002 | −6.920 |
| FtsY | −4.839 | −5.676 |
| ZipA | −4.706 | −5.823 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratchasong, K.; Saengsawang, P.; Yusakul, G.; Makkliang, F.; Lakhanapuram, H.K.; Wintachai, P.; Thomrongsuwannakij, T.; Nwabor, O.F.; Punyapornwithaya, V.; Romyasamit, C.; et al. Bactericidal Activities of Nanoemulsion Containing Piper betle L. Leaf and Hydroxychavicol Against Avian Pathogenic Escherichia coli and Modelling Simulation of Hydroxychavicol Against Bacterial Cell Division Proteins. Antibiotics 2025, 14, 788. https://doi.org/10.3390/antibiotics14080788
Ratchasong K, Saengsawang P, Yusakul G, Makkliang F, Lakhanapuram HK, Wintachai P, Thomrongsuwannakij T, Nwabor OF, Punyapornwithaya V, Romyasamit C, et al. Bactericidal Activities of Nanoemulsion Containing Piper betle L. Leaf and Hydroxychavicol Against Avian Pathogenic Escherichia coli and Modelling Simulation of Hydroxychavicol Against Bacterial Cell Division Proteins. Antibiotics. 2025; 14(8):788. https://doi.org/10.3390/antibiotics14080788
Chicago/Turabian StyleRatchasong, Kunchaphorn, Phirabhat Saengsawang, Gorawit Yusakul, Fonthip Makkliang, Hemanth Kumar Lakhanapuram, Phitchayapak Wintachai, Thotsapol Thomrongsuwannakij, Ozioma Forstinus Nwabor, Veerasak Punyapornwithaya, Chonticha Romyasamit, and et al. 2025. "Bactericidal Activities of Nanoemulsion Containing Piper betle L. Leaf and Hydroxychavicol Against Avian Pathogenic Escherichia coli and Modelling Simulation of Hydroxychavicol Against Bacterial Cell Division Proteins" Antibiotics 14, no. 8: 788. https://doi.org/10.3390/antibiotics14080788
APA StyleRatchasong, K., Saengsawang, P., Yusakul, G., Makkliang, F., Lakhanapuram, H. K., Wintachai, P., Thomrongsuwannakij, T., Nwabor, O. F., Punyapornwithaya, V., Romyasamit, C., & Mitsuwan, W. (2025). Bactericidal Activities of Nanoemulsion Containing Piper betle L. Leaf and Hydroxychavicol Against Avian Pathogenic Escherichia coli and Modelling Simulation of Hydroxychavicol Against Bacterial Cell Division Proteins. Antibiotics, 14(8), 788. https://doi.org/10.3390/antibiotics14080788









