Profiles of Sensitivity to Antibiotics and Heavy Metals in Strains of Pseudomonas mendocina Isolates from Leachate Pond
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of Pseudomonas
2.2. Antibiotic Sensitivity Profile in Pseudomonas mendocina Isolates
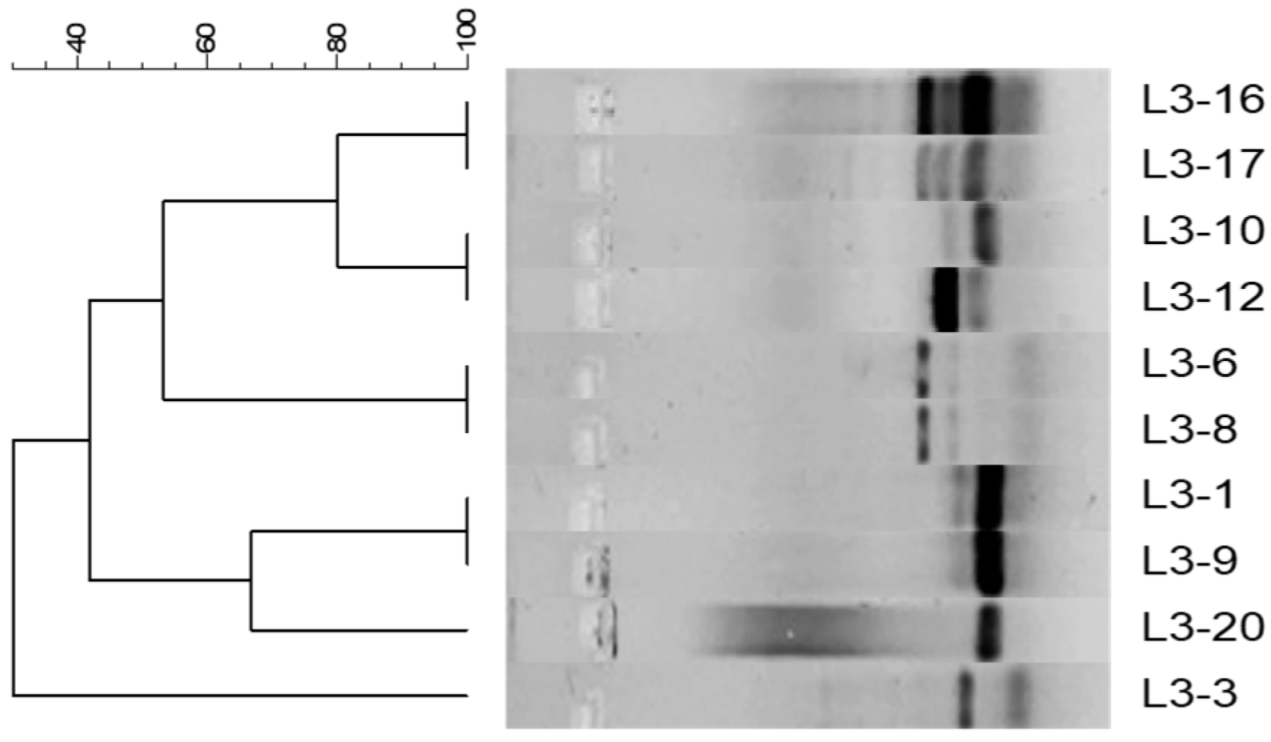
2.3. Genetic Relationship Between Pseudomonas mendocina Isolates
2.4. Conjugation Assays in Pseudomonas mendocina Isolates with Decreased Antibiotic Sensitivity
2.5. Heavy Metal Susceptibility Profiles of Pseudomonas mendocina Isolates
3. Discussion
4. Materials and Methods
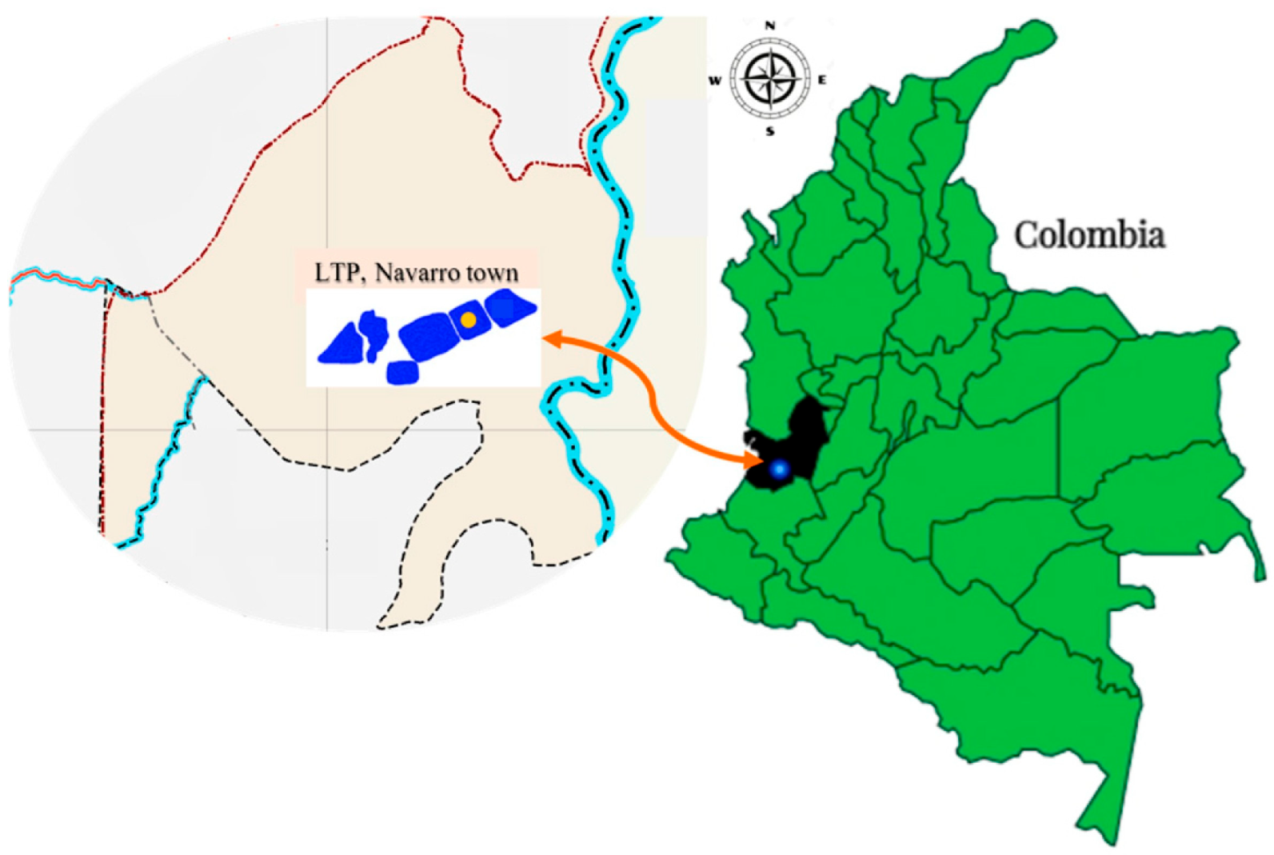
4.1. Sampling and Collection Site of Leachate Sample
4.2. Isolation and Identification of Pseudomonas
4.3. Determination of Antibiotic Susceptibility Profiles in P. mendocina Isolates
4.4. Sensitivity Profile of P. mendocina Isolates to Heavy Metals
4.5. DNA Extraction of P. mendocina Isolates
4.6. Amplification by PCR of Repetitive Extragenic Palindromic Sequences (Rep-PCR)
4.7. Horizontal Gene Transfer via Conjugation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruiz-Roldán, L.; de Toro, M.; Sáenz, Y. The Whole Genome Analysis of Environmental Pseudomonas mendocina Strains: Virulence Mechanisms and Phylogeny. Genes 2021, 12, 115. [Google Scholar] [CrossRef]
- Palleroni, N.J.; Doudoroff, M.; Stanier, R.Y.; Solánes, R.E.; Mandel, M. Taxonomy of the aerobic pseudomonads: The properties of the Pseudomonas stutzeri group. J. Gen. Microbiol. 1970, 60, 215–231. [Google Scholar] [CrossRef]
- Ioannou, P.; Vougiouklakis, G. A Systematic Review of Human Infections by Pseudomonas mendocina. Trop. Med. Infect. Dis. 2020, 5, 71. [Google Scholar] [CrossRef]
- Rapsinski, G.J.; Makadia, J.; Bhanot, N.; Min, Z. Pseudomonas mendocina native valve infective endocarditis: A case report. J. Med. Case Rep. 2016, 10, 275. [Google Scholar] [CrossRef]
- Aragone, M.R.; Maurizi, D.M.; Clara, L.O.; Navarro Estrada, J.L.; Ascione, A. Pseudomonas mendocina, an environmental bacterium isolated from a patient with human infective endocarditis. J. Clin. Microbiol. 1992, 30, 1583–1584. [Google Scholar] [CrossRef]
- Johansen, H.K.; Kjeldsen, K.; Høiby, N. Pseudomonas mendocina as a cause of chronic infective endocarditis in patients with situs inversus. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2001, 7, 650–652. [Google Scholar] [CrossRef]
- Mert, A.; Yilmaz, M.; Ozaras, R.; Kocak, F.; Dagsali, S. Native valve endocarditis due to Pseudomonas mendocina in a patient with mental retardation and a review of literature. Scand. J. Infect. Dis. 2007, 39, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Suel, P.; Martin, P.; Berthelot, G.; Robaday, S.; Etienne, M.; Chibani, A. Une endocardite à Pseudomonas mendocina [A case of Pseudomonas mendocina endocarditis]. Med. Mal. Infect. 2011, 41, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Thamizhselvi, S.; Pooja, A.; Prajna, L.; Rameshkumar, G.; Prajna, N.V.; Karpagam, R. Incidence, Clinical Profile, and Management of Keratitis Caused by Uncommon Species of Pseudomonas at a Tertiary Eye Care Center. Cornea 2023, 42, 359–364. [Google Scholar] [CrossRef]
- Huang, C.R.; Lien, C.Y.; Tsai, W.C.; Lai, W.A.; Hsu, C.W.; Tsai, N.W.; Chang, C.C.; Lu, C.H.; Chien, C.C.; Chang, W.N. The clinical characteristics of adult bacterial meningitis caused by non-Pseudomonas (Ps.) aeruginosa Pseudomonas species: A clinical comparison with Ps. aeruginosa meningitis. Kaohsiung J. Med. Sci. 2018, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.; Maisuradze, N.; Maglakelidze, D.; Kalra, T.; McFarlane, I.M. Pseudomonas mendocina Urinary Tract Infection: A Case Report and Literature Review. Cureus 2022, 14, e23583. [Google Scholar] [CrossRef]
- Nseir, W.; Taha, H.; Abid, A.; Khateeb, J. Pseudomonas mendocina sepsis in a healthy man. Isr. Med. Assoc. J. IMAJ 2011, 13, 375–376. [Google Scholar]
- Gani, M.; Rao, S.; Miller, M.; Scoular, S. Pseudomonas mendocina Bacteremia: A Case Study and Review of Literature. Am. J. Case Rep. 2019, 20, 453–458. [Google Scholar] [CrossRef]
- Goldberg, M.E.; Blyth, M.; Swiatlo, E. Pseudomonas mendocina Bacteremia in a Hemodialysis Patient With a Central Venous Catheter. Cureus 2020, 12, e10853. [Google Scholar] [CrossRef] [PubMed]
- Ezeokoli, E.U.; Polat, M.U.; Ogundipe, O.; Szela, J. A Case of Pseudomonas mendocina Bacteremia in an Elderly Man With Bilateral Leg Lesions. Cureus 2021, 13, e17777. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.Y.; Lai, C.H.; Fung, C.P.; Wang, J.H. Pseudomonas mendocina spondylodiscitis: A case report and literature review. Scand. J. Infect. Dis. 2005, 37, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Jerónimo, T.M.; Guedes, A.M.; Stieglmair, S.; Guerreiro, R.; Laranjo, C.; Bernardo, I.; Neves, P.L. Pseudomonas mendocina: The first case of peritonitis on peritoneal dialysis. Nefrol. Publicación Of. Soc. Española Nefrol. 2017, 37, 647–649. [Google Scholar] [CrossRef]
- Bhatt, V.D.; Ahir, V.B.; Koringa, P.G.; Jakhesara, S.J.; Rank, D.N.; Nauriyal, D.S.; Kunjadia, A.P.; Joshi, C.G. Milk microbiome signatures of subclinical mastitis-affected cattle analysed by shotgun sequencing. J. Appl. Microbiol. 2012, 112, 639–650. [Google Scholar] [CrossRef]
- Lee, S.A.; Wrona, L.J.; Cahoon, A.B.; Crigler, J.; Eiteman, M.A.; Altman, E. Isolation and Characterization of Bacteria That Use Furans as the Sole Carbon Source. Appl. Biochem. Biotechnol. 2016, 178, 76–90. [Google Scholar] [CrossRef]
- Roy, D.; Azaïs, A.; Benkaraache, S.; Drogui, P.; Tyagi, R. Composting leachate: Characterization, treatment, and future perspectives. Rev. Environ. Sci. Biotechnol. 2018, 17, 323–349. [Google Scholar] [CrossRef]
- Del Moro, G.; Prieto-Rodríguez, L.; De Sanctis, M.; Di Iaconi, C.; Malato, S.; Mascolo, G. Landfill leachate treatment: Comparison of standalone electrochemical degradation and combined with a novel biofilter. Chem. Eng. J. 2016, 288, 87–98. [Google Scholar] [CrossRef]
- Morales, J.P. Análisis Hidrológico Del Lixiviado Generado En El Relleno Sanitario Del Cantón Gonzalo Pizarro, Ecuador. Rev. Cienc. UNEMI 2022, 15, 24–33. [Google Scholar]
- Moody, C.M.; Townsend, T.G. A comparison of landfill leachates based on waste composition. Waste Manag. 2017, 63, 267–274. [Google Scholar] [CrossRef]
- Wu, D.; Huang, Z.; Yang, K.; Graham, D.; Xie, B. Relationships between antibiotics and antibiotic resistance gene levels in municipal solid waste leachates in Shanghai, China. Environ. Sci. Technol. 2015, 49, 4122–4128. [Google Scholar] [CrossRef]
- You, X.; Wu, D.; Wei, H.; Xie, B.; Lu, J. Fluoroquinolones and β-lactam antibiotics and antibiotic resistance genes in autumn leachates of seven major municipal solid waste landfills in China. Environ. Int. 2018, 113, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Threedeach, S.; Chiemchaisri, W.; Watanabe, T.; Chiemchaisri, C.; Honda, R.; Yamamoto, K. Antibiotic resistance of Escherichia coli in leachates from municipal solid waste landfills: Comparison between semi-aerobic and anaerobic operations. Bioresour. Technol. 2012, 113, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yan, C.; Shen, J.; Wei, R.; Gao, Y.; Miao, A.; Xiao, L.; Yang, L. Characterization of Aerobic Denitrifying Bacterium Pseudomonas mendocina Strain GL6 and Its Potential Application in Wastewater Treatment Plant Effluent. Int. J. Environ. Res. Public Health 2019, 16, 364. [Google Scholar] [CrossRef]
- Kunhikannan, S.; Thomas, C.J.; Sumana, M.N.; Franks, A.E.; Kumar, S.; Nagarathna, S.; Petrovski, S.; Shindler, A.E. Exploring the antibiogram of soil isolates from an indian hospital precinct: Link to antibiotic usage. BMC Res. Notes 2023, 16, 173. [Google Scholar] [CrossRef]
- Mondragón-Quiguanas, A.; Villaquirán-Muriel, M.Á.; Rivera, S.P.; Rosero-García, D.; Aranaga, C.; Correa, A.; Falco, A. β-Lactam-Resistant Enterobacterales Isolated from Landfill Leachates. Pathogens 2022, 11, 1077. [Google Scholar] [CrossRef]
- Falco, A.; Villaquirán-Muriel, M.Á.; Gallo Pérez, J.D.; Mondragón-Quiguanas, A.; Aranaga, C.; Correa, A. Identification of Vibrio metschnikovii and Vibrio injensis Isolated from Leachate Ponds: Characterization of Their Antibiotic Resistance and Virulence-Associated Genes. Antibiotics 2023, 12, 1571. [Google Scholar] [CrossRef]
- Toba, F.; Falco, A.; Aranaga, C.; Alonso, G. Caracterización de bacterias aisladas en un reservorio de agua en Venezuela. In Una Aproximación a la Multirresistencia Bacteriana en Ambientes Naturales; Editorial Universidad Santiago de Cali: Cali, Colombia, 2018; pp. 93–120. [Google Scholar]
- Su, Y.; Wang, J.; Huang, Z.; Xie, B. On-site removal of antibiotics and antibiotic resistance genes from leachate by aged refuse bioreactor: Effects of microbial community and operational parameters. Chemosphere 2017, 178, 486–495. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Sazykin, I.S.; Sazykina, M.A. The influence of heavy metals, polyaromatic hydrocarbons, and polychlorinated biphenyls pollution on the development of antibiotic resistance in soils. Environ. Sci. Pollut. Res. Int. 2018, 25, 9283–9292. [Google Scholar] [CrossRef]
- Chong, T.M.; Yin, W.F.; Mondy, S.; Grandclément, C.; Dessaux, Y.; Chan, K.G. Heavy-metal resistance of a France vineyard soil bacterium, Pseudomonas mendocina strain S5.2, revealed by whole-genome sequencing. J. Bacteriol. 2012, 194, 6366. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shen, Q.; Liu, F.; Ma, J.; Xu, G.; Wang, Y.; Wu, M. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai, China. J. Hazard. Mater. 2012, 235–236, 178–185. [Google Scholar] [CrossRef]
- A Health Perspective on the Role of the Environment in One Health; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. Available online: https://iris.who.int/bitstream/handle/10665/354574/WHO-EURO-2022-5290-45054-64214-eng.pdf (accessed on 18 May 2025).
- Fang, J.; Liu, Q.; Yang, J.; Kang, X.; Mei, Y.; Liu, J.; Wang, G.; Xiang, T. Functional Portrait and Genomic Feature of Carbapenem-Resistant Pseudomonas mendocina Harboring blaNDM-1 and blaIMP-1 in China. Foodborne Pathog. Dis. 2023, 20, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kumar, S.; Verma, S.K. Arsenic Resistance Mechanisms in Pseudomonas mendocina SMSKVR-3 Strain Isolated from Khetri Copper Mines, Rajasthan, India. Curr. Microbiol. 2022, 79, 69. [Google Scholar] [CrossRef]
- Miranda-Carrazco, A.; Vigueras-Cortés, J.M.; Villa-Tanaca, L.; Hernández-Rodríguez, C. Cyanotrophic and arsenic oxidizing activities of Pseudomonas mendocina P6115 isolated from mine tailings containing high cyanide concentration. Arch. Microbiol. 2018, 200, 1037–1048. [Google Scholar] [CrossRef]
- Gullberg, E.; Albrecht, L.M.; Karlsson, C.; Sandegren, L.; Andersson, D.I. Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. mBio 2014, 5, e01918-14. [Google Scholar] [CrossRef]
- Carballo, M.; Heydrich, M.; Rojas, N.; Salgado, I.; Romeu, B.; Manzano, A.; Larrea, J.; Dominguez, O.; Martínez, A.; Sánchez, M.; et al. Impact of microbial and chemical pollution in Cuban freshwater ecosystems: Strategies for environmental recovery. Biotecnol. Apl. 2011, 28, 276–279. [Google Scholar]
- Klümper, U.; Riber, L.; Dechesne, A.; Sannazzarro, A.; Hansen, L.H.; Sørensen, S.J.; Smets, B.F. Broad host range plasmids can invade an unexpectedly diverse fraction of a soil bacterial community. ISME J. 2015, 9, 934–945. [Google Scholar] [CrossRef]
- Rankin, D.J.; Rocha, E.P.C.; Brown, S.P. What traits are carried on mobile genetic elements, and why? Heredity 2011, 106, 1–10. [Google Scholar] [CrossRef]
- Medina-Pizzali, M.L.; Hartinger, S.M.; Salmon-Mulanovich, G.; Larson, A.; Riveros, M.; Mäusezahl, D. Antimicrobial Resistance in Rural Settings in Latin America: A Scoping Review with a One Health Lens. Int. J. Environ. Res. Public Health 2021, 18, 9837. [Google Scholar] [CrossRef]
- Brisola, M.C.; Crecencio, R.B.; Bitner, D.S.; Frigo, A.; Rampazzo, L.; Stefani, L.M.; Faria, G.A. Escherichia coli used as a biomarker of antimicrobial resistance in pig farms of Southern Brazil. Sci. Total Environ. 2019, 647, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Gambero, M.L.; Blarasin, M.; Bettera, S.; Albo, J.G. Tracing contamination sources through phenotypic characterization of Escherichia coli isolates from surface water and groundwater in an agro-ecosystem. Hydrol. Sci. J. 2018, 63, 1150–1161. [Google Scholar] [CrossRef]
- Vilchez, G.; Alonso, G. Alcances y limitaciones de los métodos de epidemiología molecular basados en el análisis de ácidos nucleicos. Rev. Soc. Ven. Microbiol. 2009, 29, 6–12. [Google Scholar]
- Samreen Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Strandén, A.; Frei, R.; Widmer, A.F. Molecular typing of methicillin-resistant Staphylococcus aureus: Can PCR replace pulsed-field gel electrophoresis? J. Clin. Microbiol. 2003, 41, 3181–3186. [Google Scholar] [CrossRef]
- Aman, M.; Fomda, B.A.; Roohi, S.; Qadri, U.; Wani, S.J.; Majid, U.; Dar, R.A. Pseudomonas mendocina bacteremia: A case study from Indian subcontinent. Indian J. Med. Microbiol. 2025, 55, 100827. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 34th Edition Informational Supplement M100-S27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- Lu, J.J.; Perng, C.L.; Lee, S.Y.; Wan, C.C. Use of PCR with universal primers and restriction endonuclease digestions for detection and identification of common bacterial pathogens in cerebrospinal fluid. J. Clin. Microbiol. 2000, 38, 2076–2080. [Google Scholar] [CrossRef]
- Versalovic, J.; Koeuth, T.; Lupski, J.R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991, 19, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C.; Arbeit, R.D.; Goering, R.V.; Mickelsen, P.A.; Murray, B.E.; Persing, D.H.; Swaminathan, B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995, 33, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Cho, Y.J.; Yong, D.; Chun, J. Genome sequence of Escherichia coli J53, a reference strain for genetic studies. J. Bacteriol. 2012, 194, 3742–3743. [Google Scholar] [CrossRef] [PubMed]
| Kirby-Bauer Test (μg/mL) by Antibiotic/Interpretive Categories | |||||||
|---|---|---|---|---|---|---|---|
| Strain ID | AK 1 | CN 1 | CAZ 2 | IMP 2 | CTX 2 | ETP 2 | CIP 3 |
| L3-1 | ≤2 (S) | ≤1 (S) | ≤1 (S) | 16 (I) | ≤1 (S) | ≤1 (S) | ≤0.25 (S) |
| L3-3 | ≤2 (S) | 14 (I) | ≤1 (S) | ≤0.25 (S) | ≤1 (S) | ≤1 (S) | ≤0.25 (S) |
| L3-6 | ≤2 (S) | 2 (S) | 2 (S) | 0.5 (S) | 2 (S) | 2 (S) | ≤0.25 (S) |
| L3-8 | ≤2 (S) | ≤1 (S) | 2 (S) | ≤0.25 (S) | 2 (S) | 2 (S) | ≤0.25 (S) |
| L3-9 | ≤2 (S) | ≤1 (S) | 2 (S) | ≤0.25 (S) | 2 (S) | 2 (S) | ≤0.25 (S) |
| L3-10 | ≤2 (S) | ≤1 (S) | 2 (S) | ≤0.5 (S) | 2 (S) | 2 (S) | ≤0.25 (S) |
| L3-12 | 16 (I) | ≤1 (S) | ≤1 (S) | ≤0.5 (S) | ≤1 (S) | ≤1 (S) | ≤0.25 (S) |
| L3-16 | ≤2 (S) | ≤1 (S) | ≤1 (S) | 0.5 (S) | ≤1 (S) | ≤1 (S) | ≤0.25 (S) |
| L3-17 | ≤2 (S) | ≤1 (S) | ≤1 (S) | 0.5 (S) | ≤1 (S) | ≤1 (S) | ≤0.25 (S) |
| L3-20 | ≤2 (S) | ≤1 (S) | ≤1 (S) | ≤0.25 (S) | ≤1 (S) | ≤1 (S) | ≤0.25 (S) |
| Titers of Donor and Recipient Strains and Frequency of Transfer | |
|---|---|
| Strain | Transfer Frequency |
| E. coli J-53 (recipient) | Does not apply |
| E. coli 1646 (donor, control) | 0.53 |
| P. mendocina L3-1 (donor) | 6.4 × 104 |
| P. mendocina L3-3 (donor) | There were no transconjugants |
| P. mendocina L3-12 (donor) | 0.03 |
| Minimum Inhibitory Concentrations for Metals (mM) | |||||||
|---|---|---|---|---|---|---|---|
| Strain | Pb(NO2)3 | CoCl2 | ZnSO4 | NiSO4 | CuSO4 | CdCl2 | HgCl2 |
| P. aeruginosa ATCC 27853 | >50 | 6.25 | >12.5 | >6.25 | 0.19 | >6.25 | 6.25 |
| L3-1 | >50 | 2.5 | 12.5 | 12.5 | 0.19 | 6.25 | N/G |
| L3-3 | 25 | 2.5 | 12.5 | 12.5 | >3.125 | 1.56 | N/G |
| L3-6 | >50 | 2.5 | >12.5 | 12.5 | 1.56 | >6.25 | N/G |
| L3-8 | >50 | 2.5 | >12.5 | 6.25 | 1.56 | >6.25 | N/G |
| L3-9 | >50 | 2.5 | >12.5 | 6.25 | 0.39 | 6.25 | N/G |
| L3-10 | >50 | 2.5 | 6.25 | 6.25 | >3.125 | 6.25 | N/G |
| L3-12 | >50 | 2.5 | 3.125 | 3.125 | >3.125 | 6.25 | N/G |
| L3-16 | >50 | 5 | 6.125 | 12.5 | >3.125 | >6.25 | N/G |
| L3-17 | >50 | 5 | >6.25 | 12.5 | >3.125 | >6.25 | N/G |
| L3-20 | >50 | 5 | >6.25 | 12.5 | >3.125 | 6.25 | N/G |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falco, A.; Mondragón-Quiguanas, A.; Burbano, L.; Villaquirán-Muriel, M.Á.; Correa, A.; Aranaga, C. Profiles of Sensitivity to Antibiotics and Heavy Metals in Strains of Pseudomonas mendocina Isolates from Leachate Pond. Antibiotics 2025, 14, 781. https://doi.org/10.3390/antibiotics14080781
Falco A, Mondragón-Quiguanas A, Burbano L, Villaquirán-Muriel MÁ, Correa A, Aranaga C. Profiles of Sensitivity to Antibiotics and Heavy Metals in Strains of Pseudomonas mendocina Isolates from Leachate Pond. Antibiotics. 2025; 14(8):781. https://doi.org/10.3390/antibiotics14080781
Chicago/Turabian StyleFalco, Aura, Alejandra Mondragón-Quiguanas, Laura Burbano, Miguel Ángel Villaquirán-Muriel, Adriana Correa, and Carlos Aranaga. 2025. "Profiles of Sensitivity to Antibiotics and Heavy Metals in Strains of Pseudomonas mendocina Isolates from Leachate Pond" Antibiotics 14, no. 8: 781. https://doi.org/10.3390/antibiotics14080781
APA StyleFalco, A., Mondragón-Quiguanas, A., Burbano, L., Villaquirán-Muriel, M. Á., Correa, A., & Aranaga, C. (2025). Profiles of Sensitivity to Antibiotics and Heavy Metals in Strains of Pseudomonas mendocina Isolates from Leachate Pond. Antibiotics, 14(8), 781. https://doi.org/10.3390/antibiotics14080781







