In Vitro Activity of Cefiderocol and Aztreonam/Avibactam Against Gram-Negative Non-Fermenting Bacteria: A New Strategy Against Highly Antibiotic-Resistant Infectious Agents
Abstract
1. Introduction
2. Results
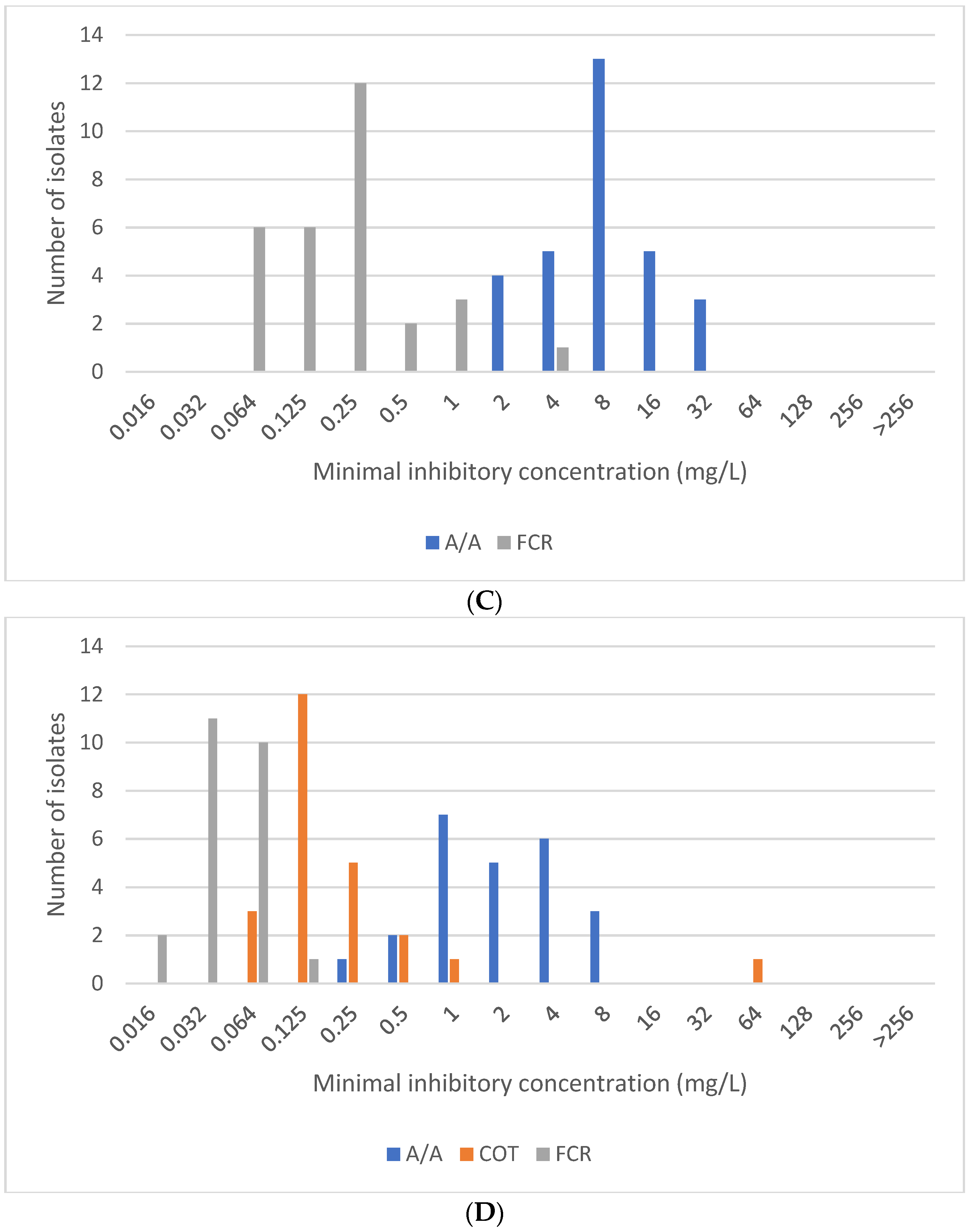
2.1. Stenotrophomonas maltophilia
2.2. Burkholderia cepacia Complex
2.3. Acinetobacter sp.
2.4. Pseudomonas aeruginosa
3. Discussion
4. Materials and Methods
4.1. Susceptibility Testing
4.1.1. Quality Control
4.1.2. Carbapenemase Production
4.1.3. Notes on the Result Interpretation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Pascale, R.; Corcione, S.; Bussini, L.; Pancaldi, L.; Giacobbe, D.R.; Ambretti, S.; Lupia, T.; Costa, C.; Marchese, A.; De Rosa, F.G.; et al. Non-fermentative gram-negative bloodstream infection in northern Italy: A multicenter cohort study. BMC. Infect. Dis. 2021, 21, 806. [Google Scholar] [CrossRef] [PubMed]
- Insuwanno, W.; Kiratisin, P.; Jitmuang, A. Stenotrophomonas maltophilia Infections: Clinical Characteristics and Factors Associated with Mortality of Hospitalized Patients. Infect. Drug. Resist. 2020, 13, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, L.; Kuo, S. Risk factors for mortality in Stenotrophomonas maltophilia bacteremia—A meta-analysis. Infect. Dis. 2024, 56, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.C.; Waters, V.J. Opportunistic Pathogens in Cystic Fibrosis: Epidemiology and Pathogenesis of Lung Infection. J. Pediatric. Infect. Dis. Soc. 2022, 11 (Suppl. S2), S3–S12. [Google Scholar] [CrossRef]
- Gottesdiener, L.S.; Satlin, M.J. Global impact of antibacterial resistance in patients with hematologic malignancies and hematopoietic cell transplant recipients. Transpl. Infect. Dis. 2023, 25 (Suppl. S1), e14169. [Google Scholar] [CrossRef]
- Lupia, T.; Carnevale-Schianca, F.; Vita, D.; Busca, A.; Caravelli, D.; Crisà, E.; Gregorc, V.; Curtoni, A.; Cerutti, A.; Shbaklo, N.; et al. Stenotrophomonas maltophilia Infections in Haematological Malignancies and Hematopoietic Stem Cell Transplantation: A Case Series including Cefiderocol-Based Regimens. Medicina 2024, 60, 88. [Google Scholar] [CrossRef]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, ciae403. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase-Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 15.0. 2025. Available online: http://www.eucast.org (accessed on 21 May 2025).
- Clinical and Laboratory Standards Institute. M100. Performance Standards for Antimicrobial Susceptibility Testing. 2025, 35th Edition. Available online: https://clsi.org (accessed on 21 May 2025).
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. 2018, 66, 1290–1297. [Google Scholar] [CrossRef]
- Barrasa, H.; Morán, M.A.; Fernández-Ciriza, L.; Isla, A.; Solinís, M.Á.; Canut-Blasco, A.; Rodríguez-Gascón, A. Optimizing Antibiotic Therapy for Stenotrophomonas maltophilia Infections in Critically Ill Patients: A Pharmacokinetic/Pharmacodynamic Approach. Antibiotics 2024, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Acosta, C.; Zarco-Márquez, S.; Jiménez-Andrade, M.C.; Volkow-Fernández, P.; Cornejo-Juárez, P. Stenotrophomonas maltophilia bacteremia and pneumonia at a tertiary-care oncology center: A review of 16 years. Support. Care. Cancer. 2018, 26, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ni, Y.; Guan, Q.; Li, R.; Cao, H.; Geng, Y.; You, Q. Stenotrophomonas maltophilia promotes lung adenocarcinoma progression by upregulating histone deacetylase 5. Front. Microbiol. 2023, 14, 1121863. [Google Scholar] [CrossRef] [PubMed]
- Schaumburg, F.; Idelevich, E.A.; Mellmann, A.; Kahl, B.C. Susceptibility of Burkholderia cepacia complex to Ceftazidime/Avibactam and Standard Drugs of Treatment for Cystic Fibrosis Patients. Microb. Drug. Resist. 2022, 28, 545–550. [Google Scholar] [CrossRef]
- Longshaw, C.; Manissero, D.; Tsuji, M.; Echols, R.; Yamano, Y. In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterized, carbapenem-non-susceptible Gram-negative bacteria from Europe. JAC. Antimicrob. Resist. 2020, 2, dlaa060. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chuang, Y.C.; Chen, P.Y.; Chou, P.C.; Wang, J.T. In vitro activity of cefiderocol and comparator antibiotics against multidrug-resistant non-fermenting Gram-negative bacilli. JAC. Antimicrob. Resist. 2024, 6, dlae006. [Google Scholar] [CrossRef]
- Sangiorgio, G.; Calvo, M.; Stefani, S. Aztreonam and avibactam combination therapy for metallo-β-lactamase-producing gram-negative bacteria: A Narrative Review. Clin. Microbiol. Infect. 2025, 31, 971–978. [Google Scholar] [CrossRef]
- Sader, H.S.; Mendes, R.E.; Ryan Arends, S.J.; Doyle, T.B.; Castanheira, M. Activity of Aztreonam-avibactam and other β-lactamase inhibitor combinations against Gram-negative bacteria isolated from patients hospitalized with pneumonia in United States medical centers (2020–2022). BMC. Pulm. Med. 2025, 25, 38. [Google Scholar] [CrossRef]
- Sader, H.S.; Mendes, R.E.; Doyle, T.B.; Winkler, M.L.; Castanheira, M. Antimicrobial susceptibility of clinical isolates of Stenotrophomonas maltophilia from Europe, Asia, and Latin America (2018–2023). Int. J Infect. Dis. 2025, 153, 107803. [Google Scholar] [CrossRef]
- Sethi, S.; Sharma, M.; Kumar, S.; Singhal, L.; Gautam, V.; Ray, P. Antimicrobial susceptibility pattern of Burkholderia cepacia complex & Stenotrophomonas maltophilia from North India: Trend over a decade (2007–2016). Indian. J. Med. Res. 2020, 152, 656–661. [Google Scholar] [CrossRef]
- Konca, C.; Tekin, M.; Geyik, M. Susceptibility Patterns of Multidrug-Resistant Acinetobacter baumannii. Indian. J. Pediatr. 2021, 88, 120–126. [Google Scholar] [CrossRef]
- Chahal, J.; Junko, G. Trimethoprim-Sulfamethoxazole-Induced Pancytopenia: A Case Report. Cureus 2024, 16, e75113. [Google Scholar] [CrossRef] [PubMed]
- Preyra, R.; Eddin, L.E.; Ahmadi, F.; Jafari, A.; Muanda, F.T. Safety of sulfamethoxazole-trimethoprim for the treatment of bacterial infection in outpatient settings: A systematic review and meta-analysis with active comparator disproportionality analysis. Br. J. Clin. Pharmacol. 2025, 91, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Lamm, D.; Meyer, K.; Vialichka, A.; Jurkovic, M.; Patel, S.; Mendes, R.E.; Bulman, Z.P.; Wenzler, E. Activity of Aztreonam in Combination with Avibactam, Clavulanate, Relebactam, and Vaborbactam against Multidrug-Resistant Stenotrophomonas maltophilia. Antimicrob. Agents. Chemother. 2020, 64, e00297-20. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zou, H.; Chen, X.; Wu, M.; Ma, D.; Yu, H.; Niu, S.; Huang, S. Avibactam potentiated the activity of both ceftazidime and aztreonam against S. maltophilia clinical isolates in vitro. BMC. Microbiol. 2021, 21, 60. [Google Scholar] [CrossRef]
- Calvopiña, K.; Hinchliffe, P.; Brem, J.; Heesom, K.J.; Johnson, S.; Cain, R.; Lohans, C.T.; Fishwick, C.W.; Schofield, C.J.; Spencer, J.; et al. Structural/mechanistic insights into the efficacy of nonclassical β-lactamase inhibitors against extensively drug resistant Stenotrophomonas maltophilia clinical isolates. Mol. Microbiol. 2017, 106, 492–504. [Google Scholar] [CrossRef]
- Delgado-Valverde, M.; Conejo, M.D.C.; Serrano, L.; Fernández-Cuenca, F.; Pascual, Á. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2020, 75, 1840–1849. [Google Scholar] [CrossRef]
- Hsueh, S.C.; Lee, Y.J.; Huang, Y.T.; Liao, C.H.; Tsuji, M.; Hsueh, P.R. In vitro activities of cefiderocol, ceftolozane/tazobactam, ceftazidime/avibactam and other comparative drugs against imipenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii, and Stenotrophomonas maltophilia, all associated with bloodstream infections in Taiwan. J. Antimicrob. Chemother. 2019, 74, 380–386. [Google Scholar] [CrossRef]
- Sakoh, T.; Miyajima, E.; Endo, Y.; Baba, M.; Haraguchi, M.; Morishima, M.; Ogura, S.; Kimura, M.; Araoka, H. Cefiderocol susceptibility of 146 Stenotrophomonas maltophilia strains clinically isolated from blood in two Japanese hospitals over a 10-year period. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 2485–2488. [Google Scholar] [CrossRef]
- Stracquadanio, S.; Torti, E.; Longshaw, C.; Henriksen, A.S.; Stefani, S. In vitro activity of cefiderocol and comparators against isolates of Gram-negative pathogens from a range of infection sources: SIDERO-WT-2014-2018 studies in Italy. J. Glob. Antimicrob. Resist. 2021, 25, 390–398. [Google Scholar] [CrossRef]
- DeJonge, B.L.; Nguyen, S.T.; Bryowsky, J.J.; Longshaw, C.M.; Maher, J.; Mendes, R.E.; Takemura, M.; Yamano, Y. 2761. Activity of Cefiderocol and Comparator Agents Against Achromobacter and Burkholderia Isolates, Collected During 2020–2022 as Part of SENTRY Antimicrobial Surveillance Program. Open. Forum. Infect. Dis. 2023, 10, ofad500.2372. [Google Scholar] [CrossRef]
- Takemura, M.; Nakamura, R.; Ota, M.; Nakai, R.; Sahm, D.F.; Hackel, M.A.; Yamano, Y. In vitro and in vivo activity of cefiderocol against Achromobacter spp. and Burkholderia cepacia complex, including carbapenem-non-susceptible isolates. Antimicrob. Agents. Chemother. 2023, 67, e0034623. [Google Scholar] [CrossRef] [PubMed]
- Radolfová-Křížová, L.; Maixnerová, M.; Jakubů, V.; Nemec, A. Extenzivně rezistentní kmeny Acinetobacter baumannii nesoucí geny pro karbapenemázu OXA-23 a metylázu ArmA v nemocnicích České republiky [Extensively drug-resistant Acinetobacter baumannii strains carrying genes encoding carbapenemase OXA-23 and methylase ArmA in hospitals of the Czech Republic]. Zpr. CEM. 2016, 25, 231–234. Available online: https://apps.szu.cz/anemec/Radolfova-Krizova_et_al_2016_ZCEM.pdf (accessed on 26 May 2025).
- Xiong, L.; Deng, C.; Yang, G.; Shen, M.; Chen, B.; Tian, R.; Zha, H.; Wu, K. Molecular epidemiology and antimicrobial resistance patterns of carbapenem-resistant Acinetobacter baumannii isolates from patients admitted at ICUs of a teaching hospital in Zunyi, China. Front. Cell. Infect. Microbiol. 2023, 13, 1280372. [Google Scholar] [CrossRef]
- Lagadinou, M.; Amerali, M.; Michailides, C.; Chondroleou, A.; Skintzi, K.; Spiliopoulou, A.; Kolonitsiou, F.; Leonidou, L.; Assimakopoulos, S.F.; Marangos, M. Antibiotic Resistance Trends in Carbapenem-Resistant Gram-Negative Pathogens and Eight-Year Surveillance of XDR Bloodstream Infections in a Western Greece Tertiary Hospital. Pathogens 2024, 13, 1136. [Google Scholar] [CrossRef]
- Cercenado, E.; Cardenoso, L.; Penin, R.; Longshaw, C.; Henriksen, A.S.; Pascual, A. In vitro activity of cefiderocol and comparators against isolates of Gram-negative bacterial pathogens from a range of infection sources: SIDERO-WT-2014-2018 studies in Spain. J. Glob. Antimicrob. Resist. 2021, 26, 292–300. [Google Scholar] [CrossRef]
- Henriksen, A.S.; Jeannot, K.; Oliver, A.; Perry, J.D.; Pletz, M.W.; Stefani, S.; Morrissey, I.; Longshaw, C.; ARTEMIS Study Investigators. In vitro activity of cefiderocol against European Pseudomonas aeruginosa and Acinetobacter spp., including isolates resistant to meropenem and recent β-lactam/β-lactamase inhibitor combinations. Microbiol. Spectr. 2024, 12, e0383623. [Google Scholar] [CrossRef]
- Moynié, L.; Luscher, A.; Rolo, D.; Pletzer, D.; Tortajada, A.; Weingart, H.; Braun, Y.; Page, M.G.; Naismith, J.H.; Köhler, T. Structure and Function of the PiuA and PirA Siderophore-Drug Receptors from Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob. Agents. Chemother. 2017, 61, e02531-16. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Kazmierczak, K.M.; de Jonge, B.L.M.; Hackel, M.A.; Sahm, D.F.; Bradford, P.A. In Vitro Activity of Aztreonam-Avibactam against Enterobacteriaceae and Pseudomonas aeruginosa Isolated by Clinical Laboratories in 40 Countries from 2012 to 2015. Antimicrob. Agents. Chemother. 2017, 61, e00472-17. [Google Scholar] [CrossRef]
- Mauri, C.; Maraolo, A.E.; Di Bella, S.; Luzzaro, F.; Principe, L. The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases. Antibiotics 2021, 10, 1012. [Google Scholar] [CrossRef]
- Oliver, A.; Arca-Suárez, J.; Gomis-Font, M.A.; González-Pinto, L.; López-Causapé, C. Emerging resistance mechanisms to newer β-lactams in Pseudomonas aeruginosa. Clin. Microbiol. Infect. 2025, in press. [Google Scholar] [CrossRef]
- Liofilchem. MIC Test Strip Application Guide; Liofilchem, s.r.l.: Roseto degli Abruzzi, Italy, 2022; Available online: https://www.liofilchem.com/images/brochure/mic_test_strip_patent/MIC_APPLICATION_GUIDE.pdf (accessed on 18 July 2025).
- The European Committee on Antimicrobial Susceptibility Testing Routine Extended Internal Quality Control for MIC determination Disk Diffusion as Recommended by EUCAST. Version 15.0. 2025. Available online: http://www.eucast.org (accessed on 26 May 2025).

| Acinetobacter sp. (n = 30) | Burkholderia cepacia Complex (n = 10) | Pseudomonas aeruginosa (n = 30) | Stenotrophomonas maltophilia (n = 24) | ||
|---|---|---|---|---|---|
| A/A | MIC50 (mg/L) | NT | 2 | 8 | 2 |
| MIC90 (mg/L) | NT | 2 | 16 | 8 | |
| range (mg/L) | NT | 1 to 16 | 2 to 32 | 0.25 to 8 | |
| % resistant (n) | NT | 10 (1) | 10 (3) | 0 (0) | |
| COT | MIC50 (mg/L) | 0.5 | 1 | NT | 0.125 |
| MIC90 (mg/L) | 32 | 4 | NT | 0.5 | |
| range (mg/L) | 0.064 to 32 | 0.125 to 4 | NT | 0.064 to 64 | |
| % resistant (n) | 50 (15) | 20 (2) | NT | 4.2 (1) | |
| FCR | MIC50 (mg/L) | 0.25 | 0.016 | 0.25 | 0.032 |
| MIC90 (mg/L) | 0.5 | 0.016 | 1 | 0.064 | |
| range (mg/L) | 0.032 to 0.5 | 0.016 to 0.016 | 0.064 to 4 | 0.016 to 0.125 | |
| % resistant (n) | 0 (0) | 0 (0) | 3.3 (1) | 0 (0) | |
| Antibiotic | |||
|---|---|---|---|
| Aztreonam/Avibactam | Trimethoprim/Sulfamethoxazole | Cefiderocol | |
| Pseudomonas aeruginosa (n = 30) | |||
| Carbapenem—susceptible (n = 15) | 93% (14/15) | NA | 100% (15/15) |
| Carbapenem—resistant Carbapenemase—negative (n = 10) | 80% (8/10) | NA | 90% (9/10) |
| GES (n = 3) | 100% (3/3) | NA | 100% (3/3) |
| VIM (n = 1) | 100% (1/1) | NA | 100% (1/1) |
| NDM (n = 1) | 100% (1/1) | NA | 100% (1/1) |
| Acinetobacter sp. (n = 30) | |||
| Carbapenem—susceptible (n = 15) | NA | 100% (15/15) | 100% (15/15) |
| Carbapenem—resistant Carbapenemase—negative (n = 15) | NA | 0% (0/15) | 100% (15/15) |
| Acinetobacter sp. | Pseudomonas aeruginosa | Stenotrophomonas maltophilia | Burkholderia cepacia Complex | ||
|---|---|---|---|---|---|
| MIC breakpoints (mg/L) | A/A | NA | 16 | 8 | 8 |
| COT | 4 | NA | 2 | 2 | |
| FCR | 0.5 | 2 | 0.125 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Závora, J.; Adámková, V.; Studená, A.; Kroneislová, G. In Vitro Activity of Cefiderocol and Aztreonam/Avibactam Against Gram-Negative Non-Fermenting Bacteria: A New Strategy Against Highly Antibiotic-Resistant Infectious Agents. Antibiotics 2025, 14, 762. https://doi.org/10.3390/antibiotics14080762
Závora J, Adámková V, Studená A, Kroneislová G. In Vitro Activity of Cefiderocol and Aztreonam/Avibactam Against Gram-Negative Non-Fermenting Bacteria: A New Strategy Against Highly Antibiotic-Resistant Infectious Agents. Antibiotics. 2025; 14(8):762. https://doi.org/10.3390/antibiotics14080762
Chicago/Turabian StyleZávora, Jan, Václava Adámková, Alžběta Studená, and Gabriela Kroneislová. 2025. "In Vitro Activity of Cefiderocol and Aztreonam/Avibactam Against Gram-Negative Non-Fermenting Bacteria: A New Strategy Against Highly Antibiotic-Resistant Infectious Agents" Antibiotics 14, no. 8: 762. https://doi.org/10.3390/antibiotics14080762
APA StyleZávora, J., Adámková, V., Studená, A., & Kroneislová, G. (2025). In Vitro Activity of Cefiderocol and Aztreonam/Avibactam Against Gram-Negative Non-Fermenting Bacteria: A New Strategy Against Highly Antibiotic-Resistant Infectious Agents. Antibiotics, 14(8), 762. https://doi.org/10.3390/antibiotics14080762







