Environmental Dispersion of Multiresistant Enterobacteriaceae in Aquatic Ecosystems in an Area of Spain with a High Density of Pig Farming
Abstract
1. Introduction
2. Results
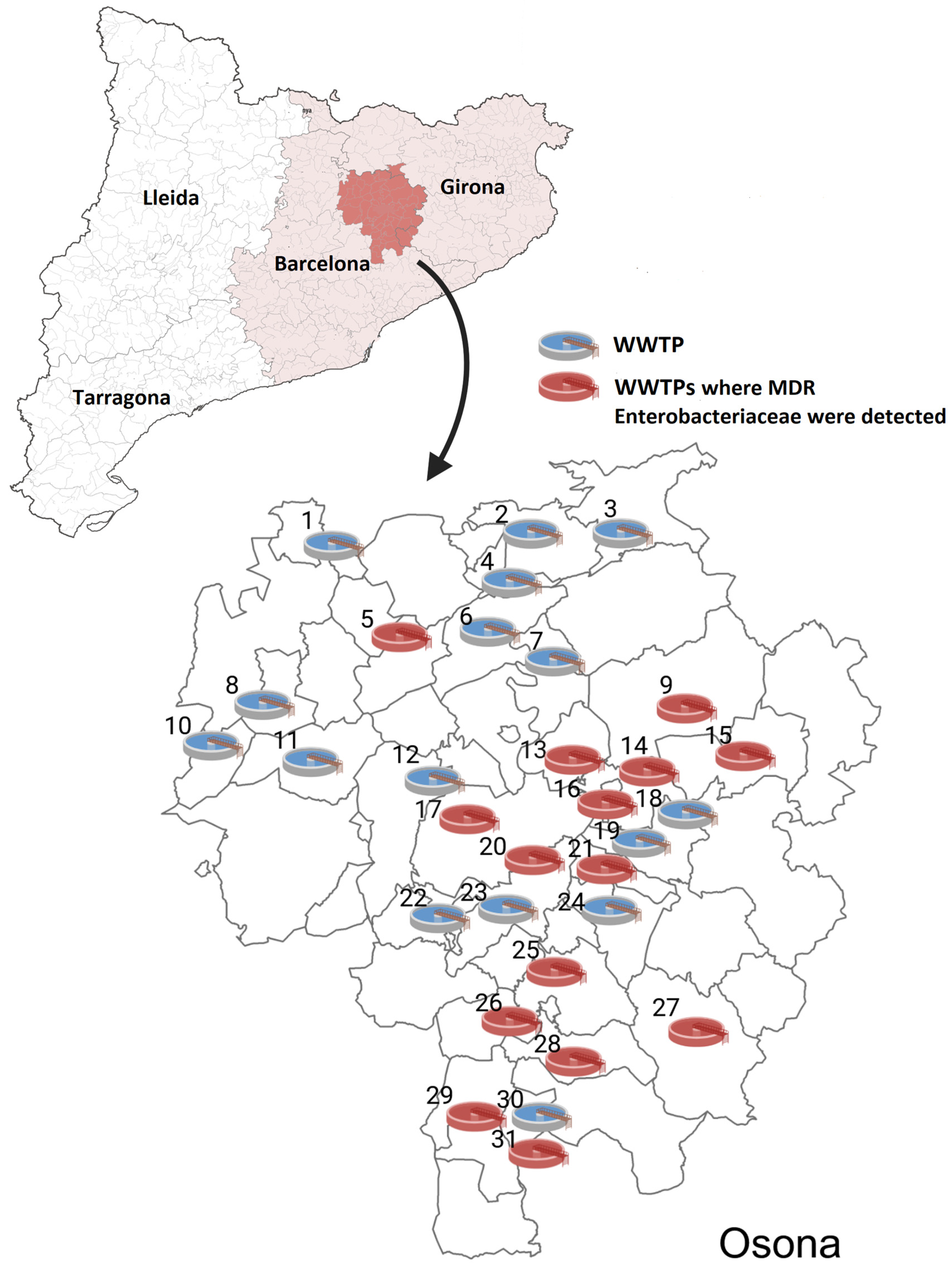
2.1. Prevalence Rates
2.2. Species/Strain Distribution
2.2.1. From WWTP Influent
2.2.2. From WWTP Effluent
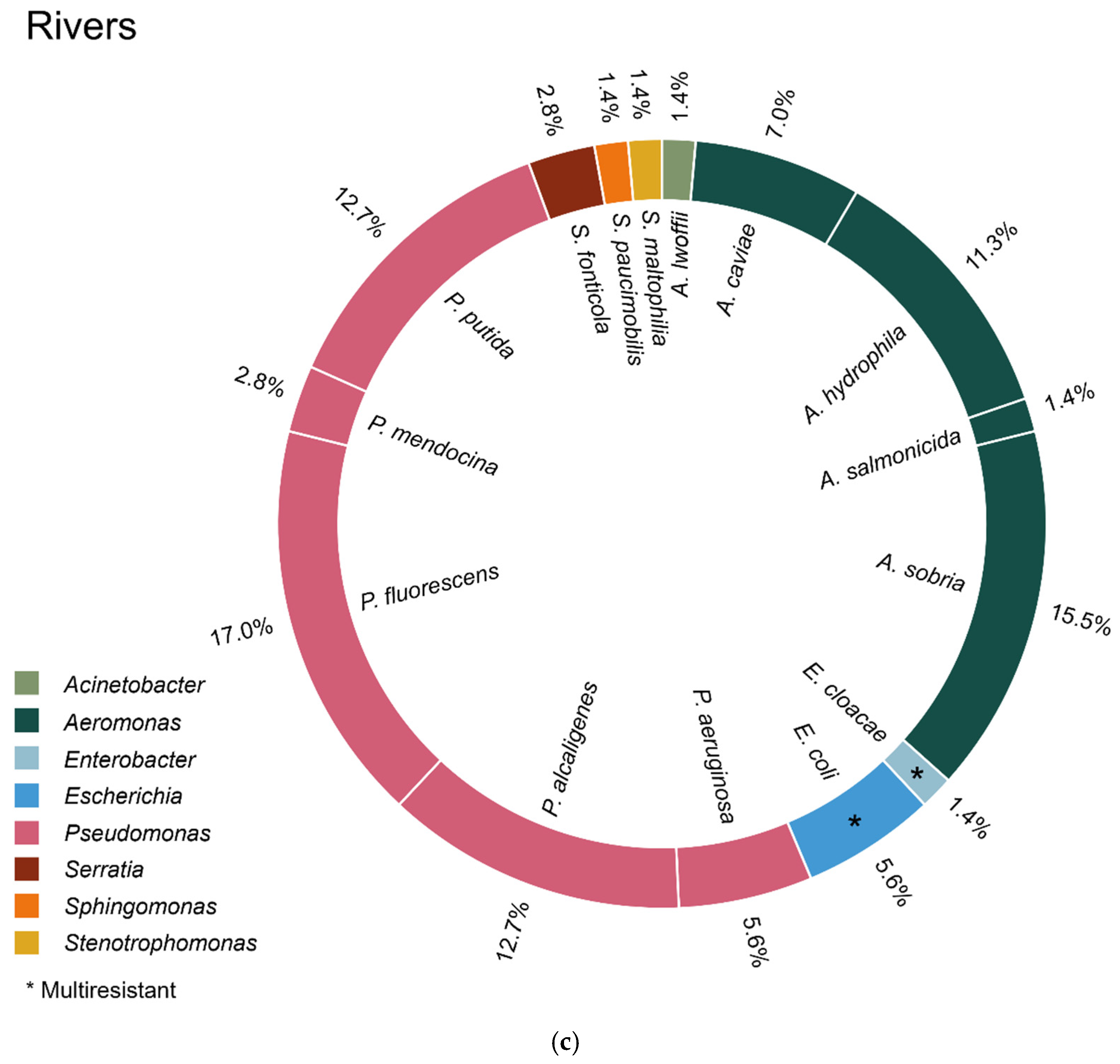
2.2.3. From River Water
2.2.4. From the General Pupulation
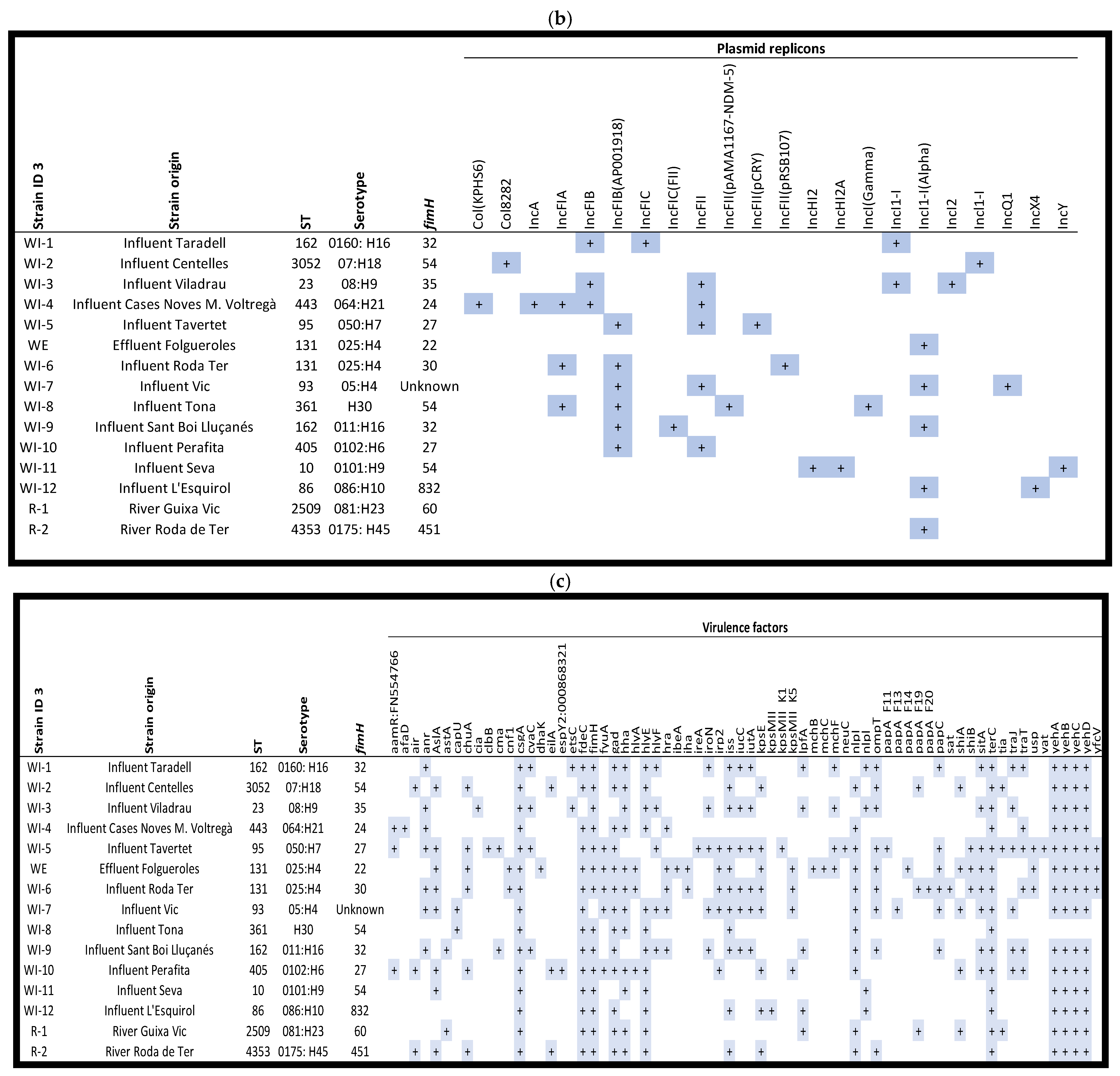
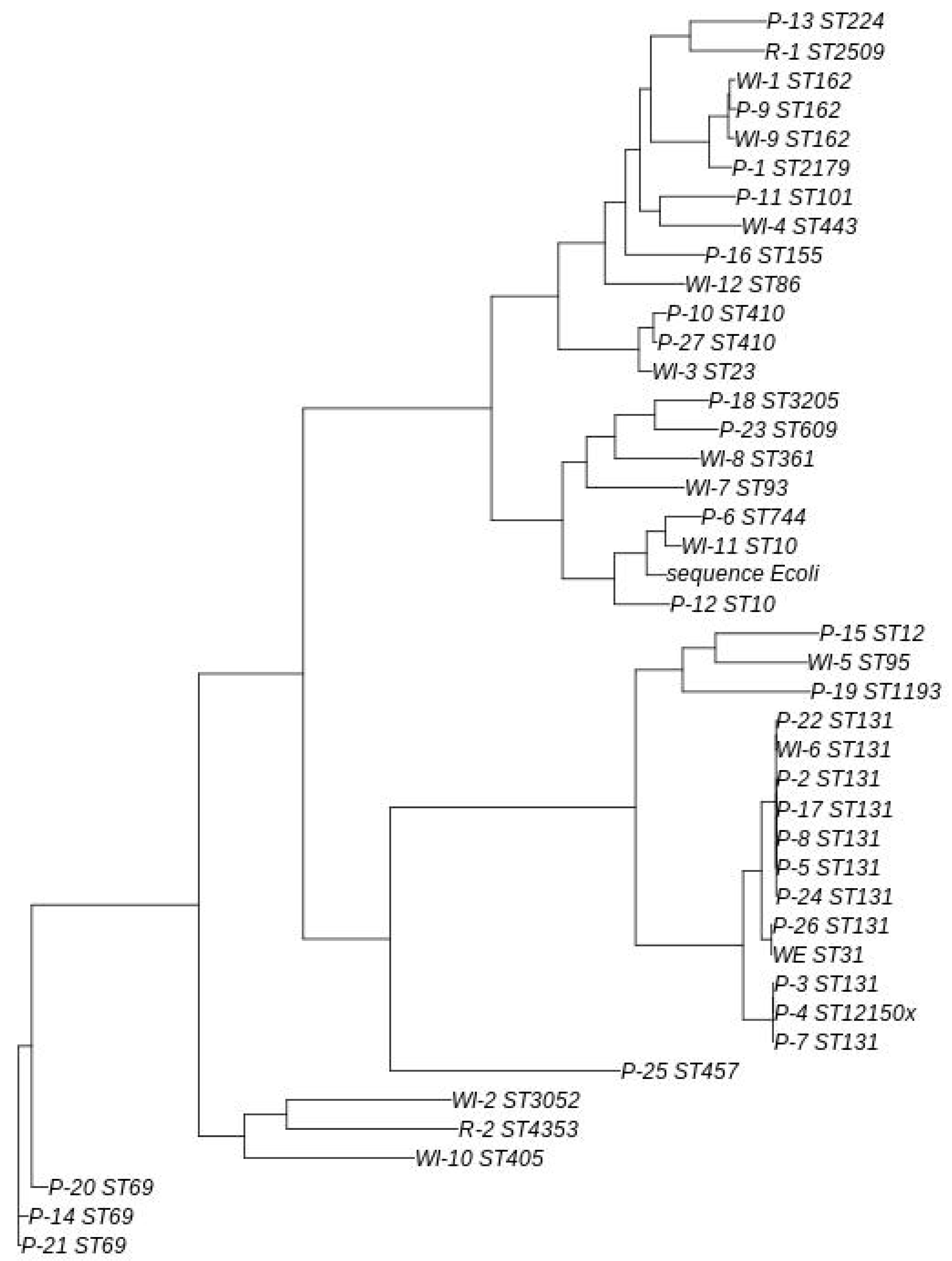
2.3. Genomic/Phylogenetic Analysis of Multiresistant Enterobacteriaceae
2.3.1. From WWTPs and Rivers
2.3.2. From the General Population
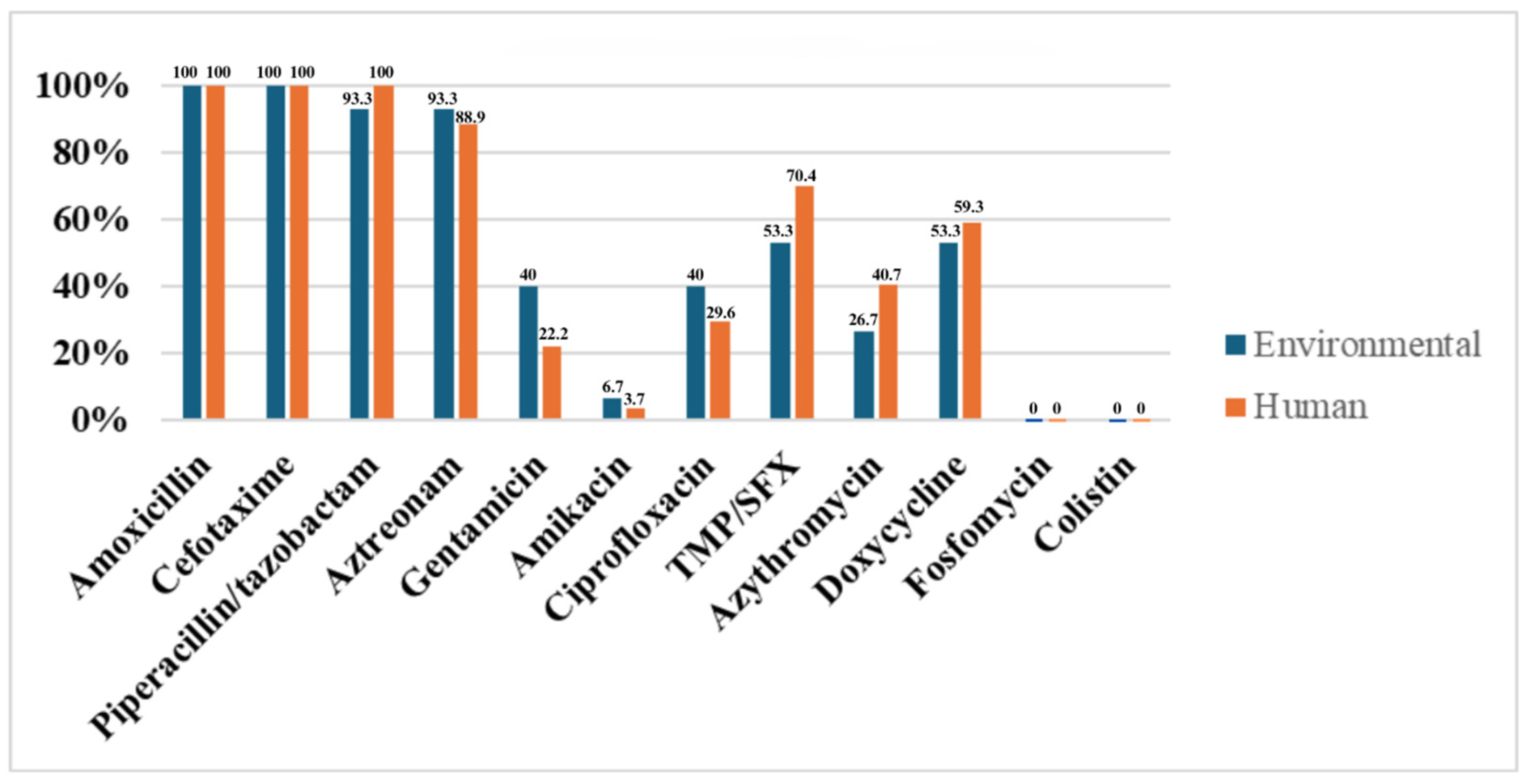
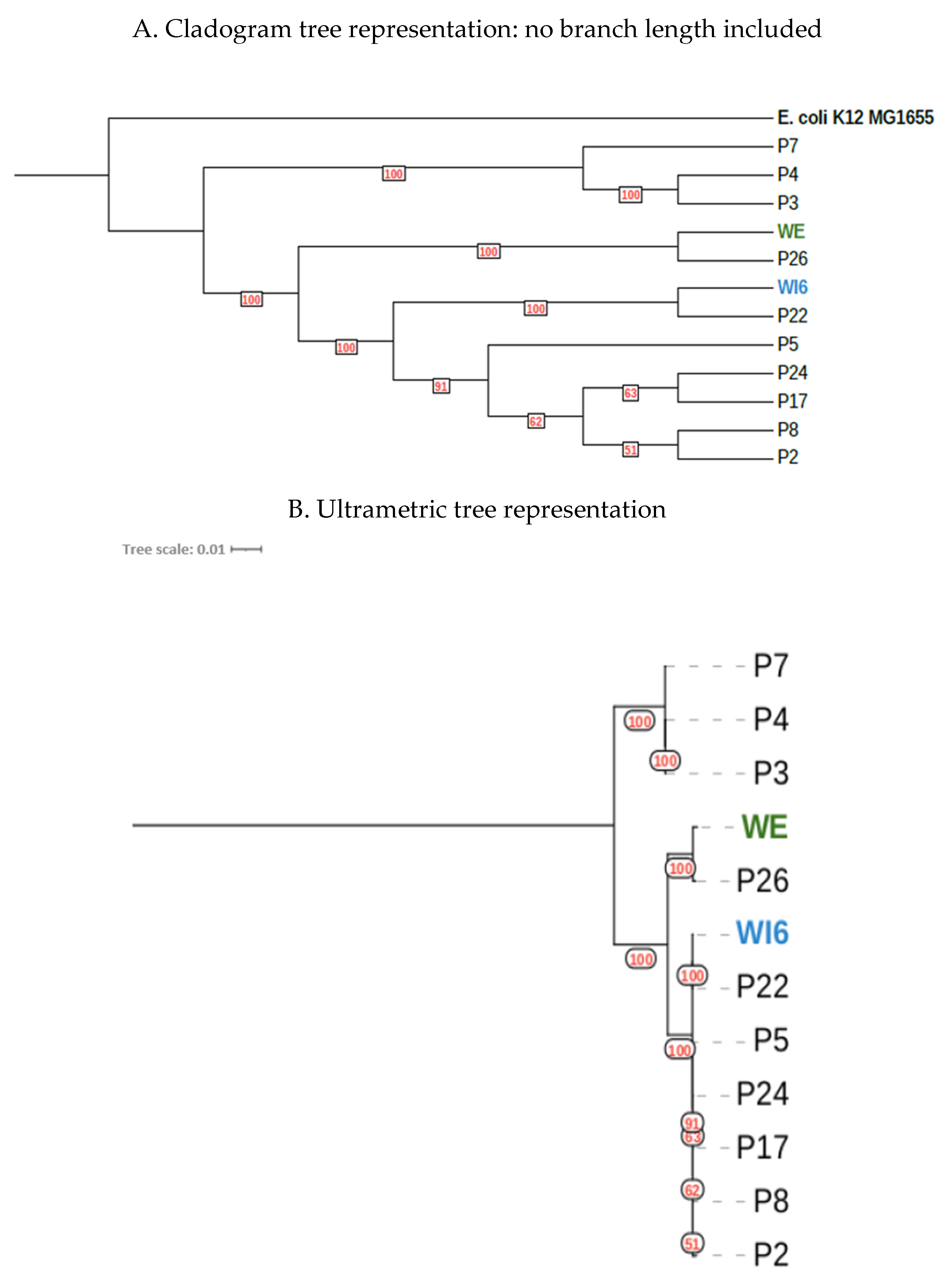
2.4. Comparison of Environmental and Human ESBL-Producing E. coli Strains
3. Discussion
4. Materials and Methods
4.1. Collection of Aquatic Samples
4.2. Microbiological Analysis of Environmental Samples
4.3. Rectal Carriage of Multiresistant-Enterobacteriaceae Among the General Population
4.4. Microbiological Analysis of Rectal Swabs
4.5. DNA Extraction and Quantification
4.6. Whole Genome Sequencing (WGS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARG | Antibiotic resistant gene |
| E. coli | Escherichia coli |
| ESBL | Extended-spectrum β-lactamase |
| K. pneumoniae | Klebsiella pneumoniae |
| MLST | Multi Locus Sequence Typing |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| SEIMC | Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica |
| ST | SpaType |
| VF | Virulence factor |
| VIM | Verona integron-encoded metallo-beta-lactamase |
| WWTP | Wastewater treatment plant |
References
- Mairi, A.; Pantel, A.; Sotto, A.; Lavigne, J.P.; Touati, A. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, L.; Jouini, A.; Alonso, C.A.; Klibi, N.; Dziri, R.; Boudabous, A.; Ben Slama, K.; Torres, C. Characteristics of extended-spectrum β-lactamase (ESBL)- and pAmpC beta-lactamase-producing Enterobacteriaceae of water samples in Tunisia. Sci. Total Environ. 2016, 550, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Zurfluh, K.; Hächler, H.; Nüesch-Inderbinen, M.; Stephan, R. Characteristics of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae isolates from rivers and lakes in Switzerland. Appl. Environ. Microbiol. 2013, 79, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Ojer-Usoz, E.; González, D.; García-Jalón, I.; Vitas, A.I. High dissemination of extended-spectrum β-lactamase-producing Enterobacteriaceae in effluents from wastewater treatment plants. Water Res. 2014, 56, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspot for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Manaia, C.M.; Rocha, J.; Scaccia, N.; Marano, R.; Radu, E.; Biancullo, F.; Cerqueira, F.; Fortunato, G.; Iakovides, I.C.; Zammit, I.; et al. Antibiotic resistance in wastewater treatment plants: Tackling the black box. Environ. Int. 2018, 115, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Masarikova, M.; Sukkar, I.; Jamborova, I.; Medvecky, M.; Papousek, I.; Literak, I.; Cizek, A.; Dolejska, M. Antibiotic-resistant Escherichia coli from treated municipal wastewaters and Black-headed Gull nestlings on the recipient river. One Health 2024, 19, 100901. [Google Scholar] [CrossRef] [PubMed]
- Sabri, N.A.; Schmitt, H.; Van Der Zaan, B.; Gerritsen, H.W.; Zuidema, T.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Prevalence of antibiotics and antibiotic resistance genes in a wastewater effluent-receiving river in the Netherlands. J. Environ. Chem. Eng. 2020, 8, 102245. [Google Scholar] [CrossRef]
- Nasser-Ali, M.; Aja-Macaya, P.; Conde-Pérez, K.; Trigo-Tasende, N.; Rumbo-Feal, S.; Fernández-González, A.; Bou, G.; Poza, M.; Vallejo, J.A. Emergence of Carbapenemase Genes in Gram-Negative Bacteria Isolated from the Wastewater Treatment Plant in A Coruña, Spain. Antibiotics 2024, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Pino-Hurtado, M.S.; Fernández-Fernández, R.; Campaña-Burguet, A.; González-Azcona, C.; Lozano, C.; Zarazaga, M.; Torres, C. A Surveillance Study of Culturable and Antimicrobial-Resistant Bacteria in Two Urban WWTPs in Northern Spain. Antibiotics 2024, 13, 955. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, C.M.; Delgado-Blas, J.F.; Calero-Caceres, W.; Muniesa, M.; Gonzalez-Zorn, B. Spread of mcr-1-carrying Enterobacteriaceae in sewage water from Spain. J. Antimicrob. Chemother. 2017, 72, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Lekunberri, I.; Villagrasa, M.; Balcázar, J.L.; Borrego, C.M. Contribution of bacteriophage and plasmid DNA to the mobilization of antibiotic resistance genes in a river receiving treated wastewater discharges. Sci. Total Environ. 2017, 601–602, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Lluch, M.; Mora, A.; López, C.; Mamani, R.; Dahbi, G.; Marzoa, J.; Herrera, A.; Viso, S.; Blanco, J.E.; Blanco, M.; et al. Detection of quinolone-resistant Escherichia coli isolates belonging to clonal groups O25b:H4-B2-ST131 and O25b:H4-D-ST69 in raw sewage and river water in Barcelona, Spain. J. Antimicrob. Chemother. 2013, 68, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Reynaga, E.; Navarro, M.; Vilamala, A.; Roure, P.; Quintana, M.; Garcia-Nuñez, M.; Figueras, R.; Torres, C.; Lucchetti, G.; Sabrià, M. Prevalence of colonization by methicillin-resistant Staphylococcus aureus ST398 in pigs and pig farm workers in an area of Catalonia, Spain. BMC Infect. Dis. 2016, 16, 716. [Google Scholar] [CrossRef] [PubMed]
- Biggel, M.; Hoehn, S.; Frei, A.; Dassler, K.; Jans, C.; Stephan, R. Dissemination of ESBL-producing E. coli ST131 through wastewater and environmental water in Switzerland. Environ. Pollut. 2023, 337, 122476. [Google Scholar] [CrossRef] [PubMed]
- Díez de los Ríos, J.; Hernández-Meneses, M.; Navarro, M.; Montserrat, S.; Perissinotti, A.; Miró, J.M. Staphylococcus caprae: An emerging pathogen related to infective endocarditis. Clin. Microbiol. Infect. 2023, 29, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Shibuki, R.; Nishiyama, M.; Mori, M.; Baba, H.; Kanamori, H.; Watanabe, T. Characterization of extended-spectrum β-lactamase-producing Escherichia coli isolated from municipal and hospital wastewater in Japan. J. Glob. Antimicrob. Resist. 2023, 32, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.H.; Bertrand, X.; Madec, J.Y. Escherichia coli st131, an intriguing clonal group. Clin. Microbiol. Rev. 2014, 27, 543–574. [Google Scholar] [CrossRef] [PubMed]
- Dolejska, M.; Frolkova, P.; Florek, M.; Jamborova, I.; Purgertova, M.; Kutilova, I.; Cizek, A.; Guenther, S.; Literak, I. CTX-M-15-producing Escherichia coli clone B2-O25b-ST131 and Klebsiella spp. isolates in municipal wastewater treatment plant effluents. J. Antimicrob. Chemother. 2011, 66, 2784–2790. [Google Scholar] [CrossRef] [PubMed]
- Davidova-Gerzova, L.; Lausova, J.; Sukkar, I.; Nesporova, K.; Nechutna, L.; Vlkova, K.; Chudejova, K.; Krutova, M.; Palkovicova, J.; Kaspar, J.; et al. Hospital and community wastewater as a source of multidrug-resistant ESBL-producing Escherichia coli. Front. Cell. Infect. Microbiol. 2023, 13, 1184081. [Google Scholar] [CrossRef] [PubMed]
- Heljanko, V.; Tyni, O.; Johansson, V.; Virtanen, J.P.; Räisänen, K.; Lehto, K.M.; Lipponen, A.; Oikarinen, S.; Pitkänen, T.; WastPan Study Group; et al. Clinically relevant sequence types of carbapenemase-producing Escherichia coli and Klebsiella pneumoniae detected in Finnish wastewater in 2021–2022. Antimicrob. Resist. Infect. Control 2024, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.I.; Gheorghe, I.; Barbu, I.C.; Surleac, M.; Paraschiv, S.; Măruţescu, L.; Popa, M.; Pîrcălăbioru, G.G.; Talapan, D.; Niţă, M.; et al. Multidrug Resistant Klebsiella pneumoniae ST101 Clone Survival Chain From Inpatients to Hospital Effluent After Chlorine Treatment. Front. Microbiol. 2021, 11, 610296. [Google Scholar] [CrossRef] [PubMed]
- Galler, H.; Feierl, G.; Petternel, C.; Reinthaler, F.F.; Haas, D.; Grisold, A.J.; Luxner, J.; Zarfel, G. KPC-2 and OXA-48 carbapenemase-harbouring Enterobacteriaceae detected in an Austrian wastewater treatment plant. Clin. Microbiol. Infect. 2014, 20, O132–O134. [Google Scholar] [CrossRef] [PubMed]
- Piedra-Carrasco, N.; Fàbrega, A.; Calero-Cáceres, W.; Cornejo-Sánchez, T.; Brown-Jaque, M.; Mir-Cros, A.; Muniesa, M.; González-López, J.J.; Chang, Y.-F. Carbapenemase-producing enterobacteriaceae recovered from a Spanish river ecosystem. PLoS ONE 2017, 12, e0175246. [Google Scholar] [CrossRef] [PubMed]
- Monge-Olivares, L.; Peñalva, G.; Pulido, M.R.; Garrudo, L.; Ángel Doval, M.; Ballesta, S.; Merchante, N.; Rasero, P.; Cuberos, L.; Carpes, G.; et al. Quantitative study of ESBL and carbapenemase producers in wastewater treatment plants in Seville, Spain: A culture-based detection analysis of raw and treated water. Water Res. 2025, 281, 123706. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; González-Alba, J.M.; Galán, J.C. CTX-M enzymes: Origin and diffusion. Front. Microbiol. 2012, 3, 110. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Raza, S.; Schaufler, K.; Roschanski, N.; Sarwar, F.; Semmler, T.; Schierack, P.; Guenther, S. High prevalence of CTX-M-15-Type ESBL-Producing E. coli from migratory avian species in Pakistan. Front. Microbiol. 2017, 8, 2476. [Google Scholar] [CrossRef] [PubMed]
- Rozwandowicz, M.; Brouwer, M.S.M.; Fischer, J.; Wagenaar, J.A.; Gonzalez-Zorn, B.; Guerra, B.; Mevius, D.J.; Hordijk, J. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Bezabih, Y.M.; Sabiiti, W.; Alamneh, E.; Bezabih, A.; Peterson, G.M.; Bezabhe, W.M.; Roujeinikova, A. The global prevalence and trend of human intestinal carriage of ESBL-producing Escherichia coli in the community. J. Antimicrob. Chemother. 2021, 76, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Proia, L.; Anzil, A.; Subirats, J.; Borrego, C.; Farrè, M.; Llorca, M.; Balcázar, J.L.; Servais, P. Antibiotic resistance along an urban river impacted by treated wastewaters. Sci. Total. Environ. 2018, 628–629, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Limayem, A.; Wasson, S.; Mehta, M.; Pokhrel, A.R.; Patil, S.; Nguyen, M.; Chen, J.; Nayak, B. High-Throughput Detection of Bacterial Community and Its Drug-Resistance Profiling From Local Reclaimed Wastewater Plants. Front. Cell. Infect. Microbiol. 2019, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2016, Version 6.0 EUCAST. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 30 June 2025).
- Red Nacional de Vigilancia Epidemiológica (RENAVE). Protocolo de Vigilancia y Control de Microorganismos Multirresistentes o de Especial Relevancia Clínico-Epidemiológica (Protocolo MMR); Red Nacional de Vigilancia Epidemiológica: Madrid, Spain, 2016. [Google Scholar]
- Arkin, A.P.; Cottingham, R.W.; Henry, C.S.; Harris, N.L.; Stevens, R.L.; Maslov, S.; Dehal, P.; Ware, D.; Perez, F.; Canon, S.; et al. KBase: The United States department of energy systems biology knowledgebase. Nat. Biotechnol. 2018, 36, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.V.; Cosentino, S.; Rasmussen, S.; Friis, C.; Hasman, H.; Marvig, R.L.; Jelsbak, L.; Sicheritz-Pontéen, T.; Ussery, D.W.; Aarestrup, F.M.; et al. Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 2012, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Bertels, F.; Silander, O.K.; Pachkov, M.; Rainey, P.B.; Van Nimwegen, E. Automated reconstruction of whole-genome phylogenies from short-sequence reads. Mol. Biol. Evol. 2014, 31, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Tseemann/Snippy: Scissors: Rapid Haploid Variant Calling and Core Genome Alignment. Available online: https://github.com/tseemann/snippy (accessed on 6 May 2024).
- Garrison, E.; Marth, G. Haplotype-Based Variant Detection from Short-Read Sequencing. Available online: https://arxiv.org/abs/1207.3907v2 (accessed on 22 May 2024).
- López Causapé, C.; González Candelas, F.; Carmona, M.; Oliver Palomo, A. Aplicaciones de las técnicas de secuenciación masiva en la Microbiología Clínica. In Procedimientos en Microbiología Clínica; Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica: Madrid, Spain, 2021; Chapter 71; ISBN 978-84-09-31350-1. [Google Scholar]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]


| Sample Site | Species | SpaType | ARG(s) Present | Plasmids Replicons |
|---|---|---|---|---|
| WWTP influent | OXA-48-producing K. pneumoniae | 101 | blaOXA-48, blaOXA-1, blaTEM-1B, blaSHV-106, blaCTX-M-15, aac(6′)-Ib-cr, aac(3)-IId, sul2, OqxB, OqxA, dfrA14, tet(D) and fosA | Col440I, ColpVC, IncFIB(K), IncFII(pKP91), IncL and IncR |
| River | VIM-producing E. cloacae complex | 45 | blaVIM-1, blaACT-15, blaTEM-1B, blaOXA-1, blaCTX-M-9, ant (2″)-Ia, aac(6′)-Ib3, aac(6′)-Ib-cr, qnrB5, qnrA1, qnrB81, qnrB19, sul1, sul2, dfrA14, dfrB1, tet(A), catB3 and mcr-9 | Col(pHAD28), IncFIB(pECLA), IncFII(pECLA), IncHI2, and IncHI2A |
| P17 | P22 | P24 | P26 | P2 | P3 | P4 | P5 | P7 | P8 | Ref. | WE | WI6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P17 | 0 | - | - | - | - | - | - | - | - | - | - | - | - |
| P22 | 179 | 0 | - | - | - | - | - | - | - | - | - | - | - |
| P24 | 86 | 165 | 0 | - | - | - | - | - | - | - | - | - | - |
| P26 | 4651 | 4680 | 4661 | 0 | - | - | - | - | - | - | - | - | - |
| P2 | 440 | 513 | 452 | 4708 | 0 | - | - | - | - | - | - | - | 1 |
| P3 | 10,520 | 10,530 | 10,520 | 10,630 | 10,700 | 0 | - | - | - | - | - | - | - |
| P4 | 10,510 | 10,520 | 10,520 | 10,620 | 10,700 | 84 | 0 | - | - | - | - | - | - |
| P5 | 89 | 148 | 89 | 4638 | 423 | 10,480 | 10,490 | 0 | - | - | - | - | - |
| P7 | 10,500 | 10,510 | 10,490 | 10,610 | 10,690 | 113 | 101 | 10,470 | 0 | - | - | - | - |
| P8 | 96 | 159 | 108 | 4661 | 430 | 10,510 | 10,500 | 79 | 10,490 | 0 | - | - | - |
| Ref. | 95,810 | 95,850 | 95,800 | 96,980 | 95,620 | 95,620 | 95,630 | 95,810 | 95,600 | 95,820 | 0 | - | - |
| WE | 4794 | 4779 | 4782 | 798 | 4820 | 10,770 | 10,770 | 4761 | 10,760 | 4766 | 97,120 | 0 | - |
| WI6 | 141 | 146 | 149 | 4660 | 503 | 10,510 | 10,510 | 134 | 10,500 | 147 | 95,810 | 4779 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez de los Ríos, J.; Párraga-Niño, N.; Navarro, M.; Serra-Pladevall, J.; Vilamala, A.; Arqué, E.; Baldà, M.; Blanco, T.N.; Pedro-Botet, L.; Mascaró, Ó.; et al. Environmental Dispersion of Multiresistant Enterobacteriaceae in Aquatic Ecosystems in an Area of Spain with a High Density of Pig Farming. Antibiotics 2025, 14, 753. https://doi.org/10.3390/antibiotics14080753
Díez de los Ríos J, Párraga-Niño N, Navarro M, Serra-Pladevall J, Vilamala A, Arqué E, Baldà M, Blanco TN, Pedro-Botet L, Mascaró Ó, et al. Environmental Dispersion of Multiresistant Enterobacteriaceae in Aquatic Ecosystems in an Area of Spain with a High Density of Pig Farming. Antibiotics. 2025; 14(8):753. https://doi.org/10.3390/antibiotics14080753
Chicago/Turabian StyleDíez de los Ríos, Javier, Noemí Párraga-Niño, María Navarro, Judit Serra-Pladevall, Anna Vilamala, Elisenda Arqué, María Baldà, Tamar Nerea Blanco, Luisa Pedro-Botet, Óscar Mascaró, and et al. 2025. "Environmental Dispersion of Multiresistant Enterobacteriaceae in Aquatic Ecosystems in an Area of Spain with a High Density of Pig Farming" Antibiotics 14, no. 8: 753. https://doi.org/10.3390/antibiotics14080753
APA StyleDíez de los Ríos, J., Párraga-Niño, N., Navarro, M., Serra-Pladevall, J., Vilamala, A., Arqué, E., Baldà, M., Blanco, T. N., Pedro-Botet, L., Mascaró, Ó., & Reynaga, E. (2025). Environmental Dispersion of Multiresistant Enterobacteriaceae in Aquatic Ecosystems in an Area of Spain with a High Density of Pig Farming. Antibiotics, 14(8), 753. https://doi.org/10.3390/antibiotics14080753






