Clinicians’ Reasons for Non-Visit-Based, No-Infectious-Diagnosis-Documented Antibiotic Prescribing: A Sequential Mixed-Methods Study
Abstract
1. Introduction
2. Results
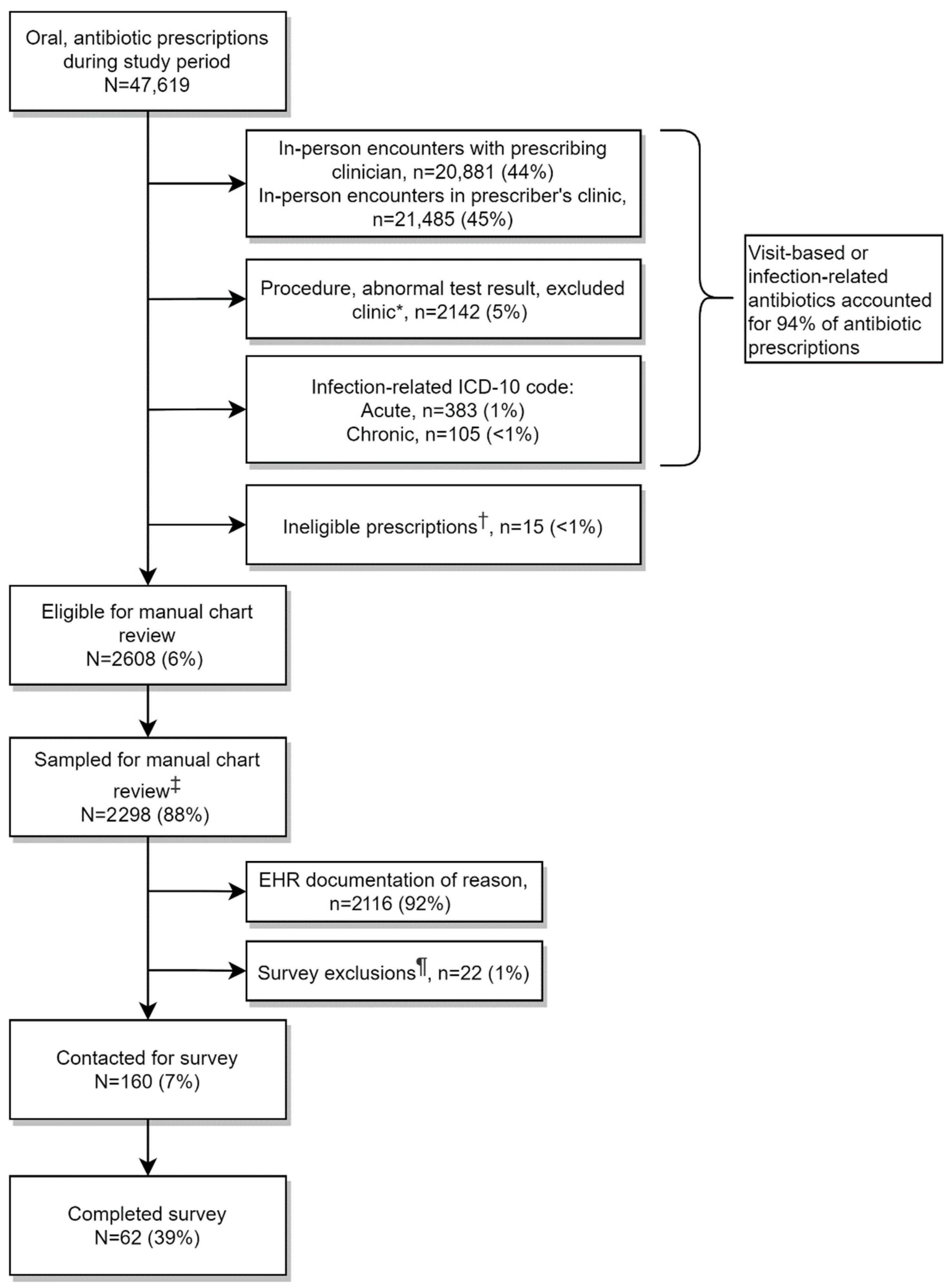
2.1. Prescription Flow
2.2. Prescription, Patient, and Clinician Characteristics
2.3. Manual Chart Review
2.4. Clinician Survey Data
2.5. Appropriateness of Survey-Described Antibiotic Prescribing
3. Discussion
4. Materials and Methods
4.1. Setting and Overview
4.2. Data Extraction
4.3. Identifiable Reasons for Antibiotic Prescribing
4.4. Manual Chart Review Form
4.5. Selection for Chart Review
4.6. Clinician Survey
4.7. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Chart Abstraction Form



References
- Duffy, E.; Ritchie, S.; Metcalfe, S.; Van Bakel, B.; Thomas, M.G. Antibacterials Dispensed in the Community Comprise 85%-95% of Total Human Antibacterial Consumption. J. Clin. Pharm. Ther. 2018, 43, 59–64. [Google Scholar] [CrossRef]
- Suda, K.J.; Hicks, L.A.; Roberts, R.M.; Hunkler, R.J.; Matusiak, L.M.; Schumock, G.T. Antibiotic Expenditures by Medication, Class, and Health Care Setting in the United States, 2010–2015. Clin. Infect. Dis. 2018, 66, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.M.; Brown, D.S.; Durkin, M.J.; Sahrmann, J.M.; Nickel, K.B.; O’Neil, C.A.; Olsen, M.A.; Hyun, D.Y.; Zetts, R.M.; Newland, J.G. Association of Inappropriate Outpatient Pediatric Antibiotic Prescriptions With Adverse Drug Events and Health Care Expenditures. JAMA Netw. Open 2022, 5, e2214153. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.M.; Brown, D.S.; Newland, J.G.; Nickel, K.B.; Sahrmann, J.M.; O’Neil, C.A.; Olsen, M.A.; Zetts, R.M.; Hyun, D.Y.; Durkin, M.J. Comparative Safety and Attributable Health Care Expenditures Following Inappropriate versus Appropriate Outpatient Antibiotic Prescriptions among Adults with Upper Respiratory Infections. Clin. Infect. Dis. 2023, 76, 986–995. [Google Scholar] [CrossRef]
- Geller, A.I.; Lovegrove, M.C.; Shehab, N.; Hicks, L.A.; Sapiano, M.R.P.; Budnitz, D.S. National Estimates of Emergency Department Visits for Antibiotic Adverse Events Among Adults-United States, 2011–2015. J. Gen. Intern. Med. 2018, 33, 1060–1068. [Google Scholar] [CrossRef]
- Estany-Gestal, A.; Salgado-Barreira, A.; Vazquez-Lago, J.M. Antibiotic Use and Antimicrobial Resistance: A Global Public Health Crisis. Antibiotics 2024, 13, 900. [Google Scholar] [CrossRef]
- Kastrin, T.; Mioč, V.; Mahnič, A.; Čižman, M. Slovenian Meningitidis Study Group Impact of the COVID-19 Pandemic on Community Consumption of Antibiotics for Systemic Use and Resistance of Invasive Streptococcus pneumoniae in Slovenia. Antibiotics 2023, 12, 945. [Google Scholar] [CrossRef]
- Batenburg, D.; Verheij, T.; Van’t Veen, A.; van der Velden, A. Practice-Level Association between Antibiotic Prescribing and Resistance: An Observational Study in Primary Care. Antibiotics 2020, 9, 470. [Google Scholar] [CrossRef]
- Fischer, M.A.; Mahesri, M.; Lii, J.; Linder, J.A. Non-Infection-Related and Non-Visit-Based Antibiotic Prescribing Is Common among Medicaid Patients. Health Aff. 2020, 39, 280–288. [Google Scholar] [CrossRef]
- Fischer, M.A.; Mahesri, M.; Lii, J.; Linder, J.A. Non-Visit-Based and Non-Infection-Related Antibiotic Use in the US: A Cohort Study of Privately Insured Patients During 2016–2018. Open Forum Infect. Dis. 2021, 8, ofab412. [Google Scholar] [CrossRef]
- Brown, T.; Lee, J.Y.; Guzman, A.; Fischer, M.A.; Friedberg, M.W.; Chua, K.-P.; Linder, J.A. Prevalence and Appropriateness of In-Person versus Not-in-Person Ambulatory Antibiotic Prescribing in an Integrated Academic Health System: A Cohort Study. PLoS ONE 2023, 18, e0289303. [Google Scholar] [CrossRef]
- Riedle, B.N.; Polgreen, L.A.; Cavanaugh, J.E.; Schroeder, M.C.; Polgreen, P.M. Phantom Prescribing: Examining the Frequency of Antimicrobial Prescriptions Without a Patient Visit. Infect. Control Hosp. Epidemiol. 2017, 38, 273–280. [Google Scholar] [CrossRef]
- Chua, K.P.; Fischer, M.A.; Linder, J.A. Appropriateness of Outpatient Antibiotic Prescribing among Privately Insured US Patients: ICD-10-CM Based Cross Sectional Study. BMJ 2019, 364, k5092. [Google Scholar] [CrossRef]
- Chua, K.-P.; Linder, J.A. Prevalence of Inappropriate Antibiotic Prescribing by Antibiotic Among Privately and Publicly Insured Non-Elderly US Patients, 2018. J. Gen. Intern. Med. 2021, 36, 2861–2864. [Google Scholar] [CrossRef]
- Linder, J.A. Breaking the Ambulatory Antibiotic Prescribing Cycle with All-Antibiotic Stewardship, Patient Stewardship, and Visit Stewardship. Clin. Infect. Dis. 2021, 73, e1680–e1683. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.; Huang, J.; Somers, M.; Hsueh, L.; Graetz, I.; Millman, A.; Muelly, E.; Gopalan, A. Telemedicine Versus In-Person Primary Care: Treatment and Follow-up Visits. Ann. Intern. Med. 2023, 176, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Mehrotra, A.; Gidengil, C.A.; Poon, S.J.; Uscher-Pines, L.; Ray, K.N. Quality of Care for Acute Respiratory Infections during Direct-to-Consumer Telemedicine Visits for Adults. Health Aff. 2018, 37, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.C.; Cosgrove, S.E.; Miller, M.A.; Tamma, P. A Framework for Implementing Antibiotic Stewardship in Ambulatory Care: Lessons Learned from the Agency for Healthcare Research and Quality Safety Program for Improving Antibiotic Use. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e109. [Google Scholar] [CrossRef]
- Sanchez, G.V.; Kabbani, S.; Tsay, S.V.; Bizune, D.; Hersh, A.L.; Luciano, A.; Hicks, L.A. Antibiotic Stewardship in Outpatient Telemedicine: Adapting Centers for Disease Control and Prevention Core Elements to Optimize Antibiotic Use. Telemed. J. E. Health. 2024, 30, 951–962. [Google Scholar] [CrossRef]
- WHO AWaRe (Access, Watch, Reserve) Classification of Antibiotics for Evaluation and Monitoring of Use. 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 22 April 2025).
- Bent, S.; Nallamothu, B.K.; Simel, D.L.; Fihn, S.D.; Saint, S. Does This Woman Have an Acute Uncomplicated Urinary Tract Infection? JAMA 2002, 287, 2701–2710. [Google Scholar] [CrossRef]
- Travelers’ Diarrhea. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/preparing/travelers-diarrhea#treatment (accessed on 28 October 2024).
- American Medical Association Treating Self or Family. Available online: https://code-medical-ethics.ama-assn.org/ethics-opinions/treating-self-or-family (accessed on 20 July 2025).
- Poetker, D.M.; Smith, T.L. What Rhinologists and Allergists Should Know about the Medico-Legal Implications of Antibiotic Use: A Review of the Literature: The Medico-Legal Implications of Antibiotics. Int. Forum Allergy Rhinol. 2015, 5, 104–110. [Google Scholar] [CrossRef]
- Buehrle, D.J.; Wagener, M.M.; Nguyen, M.H.; Clancy, C.J. Trends in Outpatient Antibiotic Prescriptions in the United States During the COVID-19 Pandemic in 2020. JAMA Netw. Open 2021, 4, e2126114. [Google Scholar] [CrossRef]
- Norman, C.; Svensson, M.; Schmidt, I.; Bergfeldt, V.S.; Obeid, R.; Ternhag, A.; Struwe, J.L. Reduced Dispensing of Prescribed Antibiotics during the Covid-19 Pandemic Has Not Increased Severe Complications from Common Infections. BMC Public Health 2022, 22, 252. [Google Scholar] [CrossRef]
- King, L.M.; Tsay, S.V.; Hicks, L.A.; Bizune, D.; Hersh, A.L.; Fleming-Dutra, K. Changes in Outpatient Antibiotic Prescribing for Acute Respiratory Illnesses, 2011 to 2018. Antimicrob. Steward. Healthc. Epidemiol. 2021, 1, e66. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.-P.; Fischer, M.A.; Rahman, M.; Linder, J.A. Changes in the Appropriateness of US Outpatient Antibiotic Prescribing After the Coronavirus Disease 2019 Outbreak: An Interrupted Time Series Analysis of 2016–2021 Data. Clin. Infect. Dis. 2024, 79, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Goss, F.R.; Bookman, K.; Barron, M.; Bickley, D.; Landgren, B.; Kroehl, M.; Williamson, K.; Zane, R.; Wiler, J. Improved Antibiotic Prescribing Using Indication-Based Clinical Decision Support in the Emergency Department. J. Am. Coll. Emerg. Physicians Open 2020, 1, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Schiff, G.D.; Seoane-Vazquez, E.; Wright, A. Incorporating Indications into Medication Ordering--Time to Enter the Age of Reason. N. Engl. J. Med. 2016, 375, 306–309. [Google Scholar] [CrossRef]
- Linder, J.A.; Meeker, D.; Fox, C.R.; Friedberg, M.W.; Persell, S.D.; Goldstein, N.J.; Doctor, J.N. Effects of Behavioral Interventions on Inappropriate Antibiotic Prescribing in Primary Care 12 Months After Stopping Interventions. JAMA 2017, 318, 1391–1392. [Google Scholar] [CrossRef]
- Kronman, M.P.; Gerber, J.S.; Grundmeier, R.W.; Zhou, C.; Robinson, J.D.; Heritage, J.; Stout, J.; Burges, D.; Hedrick, B.; Warren, L.; et al. Reducing Antibiotic Prescribing in Primary Care for Respiratory Illness. Pediatrics 2020, 146, e20200038. [Google Scholar] [CrossRef]
- Cals, J.W.; de Bock, L.; Beckers, P.J.; Francis, N.A.; Hopstaken, R.M.; Hood, K.; de Bont, E.G.; Butler, C.C.; Dinant, G.J. Enhanced Communication Skills and C-Reactive Protein Point-of-Care Testing for Respiratory Tract Infection: 3.5-Year Follow-up of a Cluster Randomized Trial. Ann. Fam. Med. 2013, 11, 157–164. [Google Scholar] [CrossRef]
- Meeker, D.; Knight, T.K.; Friedberg, M.W.; Linder, J.A.; Goldstein, N.J.; Fox, C.R.; Rothfeld, A.; Diaz, G.; Doctor, J.N. Nudging Guideline-Concordant Antibiotic Prescribing: A Randomized Clinical Trial. JAMA Intern. Med. 2014, 174, 425–431. [Google Scholar] [CrossRef]
- Richards, A.R.; Linder, J.A. Behavioral Economics and Ambulatory Antibiotic Stewardship: A Narrative Review. Clin. Ther. 2021, 43, 1654–1667. [Google Scholar] [CrossRef]
- Creswell, J.W.; David Creswell, J. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches; SAGE Publications: Thousand Oaks, CA, USA, 2017; ISBN 9781506386713. [Google Scholar]
- Starren, J.B.; Winter, A.Q.; Lloyd-Jones, D.M. Enabling a Learning Health System through a Unified Enterprise Data Warehouse: The Experience of the Northwestern University Clinical and Translational Sciences (NUCATS) Institute. Clin. Transl. Sci. 2015, 8, 269–271. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Hsieh, H.F.; Shannon, S.E. Three Approaches to Qualitative Content Analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
| Patient Characteristics | All Patients (n = 41,935) 1 | Patients with Manual Chart Review | |
|---|---|---|---|
| All Antibiotics (n = 2201) 2 | “Watch” Antibiotics (n = 1208) 3 | ||
| Age in years, mean (SD) | 42 (23) | 52 (20) | 54 (19) |
| Female, n (%) | 26,519 (63) | 1490 (68) | 749 (62) |
| Ethnicity—Hispanic or Latino, n (%) | 3208 (8) | 98 (4) | 40 (3) |
| Race, n (%) | |||
| Asian | 1623 (4) | 53 (2) | 33 (3) |
| Black | 2728 (7) | 124 (6) | 53 (4) |
| White | 32,681 (78) | 1805 (82) | 1000 (83) |
| Other/Unknown | 4903 (12) | 219 (10) | 122 (10) |
| Insurance, n (%) | |||
| Private Medicaid Medicare Self-pay/other | 18,288 (44) 15,482 (37) 7422 (18) 743 (2) | 838 (38) 701 (32) 607 (28) 55 (3) | 469 (39) 354 (29) 353 (29) 32 (3) |
| Comorbidities, median (IQR) | 0 (0, 1) | 0 (0, 2) | 0 (0, 2) |
| # of other prescriptions, median (IQR) | 2 (0, 5) | 4 (2, 7) | 4 (2, 8) |
| # of Physician visits in period, median (IQR) | 4 (1, 8) | 5 (2, 9) | 4 (2, 9) |
| # of ED visits in period, n (%) | |||
| 0 1 2+ | 41,420 (99) 457 (1) 58 (<1) | 2165 (98) 35 (2) 1 (<1) | 1187 (98) 20 (2) 1 (<1) |
| # of hospitalizations in period, n (%) | |||
| 0 1 2+ | 38,071 (91) 2585 (6) 1279 (3) | 1967 (89) 165 (8) 69 (3) | 1068 (88) 99 (8) 41 (3) |
| Primary care clinician listed, n (%) | 36,108 (86) | 2043 (93) | 1136 (94) |
| Clinician Characteristics | All Prescribers (n = n = 1177) 4 | Clinicians with Manual Chart Review | |
| All Antibiotics (n = 500) 5 | “Watch” Antibiotics (n = 326) 6 | ||
| Female, n (%) | 721 (61) | 310 (62) | 187 (57) |
| Clinician type, n (%) | |||
| Physician APN/NP/midwife 7 Physician assistant | 928 (79) 161 (14) 88 (8) | 412 (82) 54 (11) 34 (7) | 284 (87) 23 (7) 19 (6) |
| Limited to Physicians | N = 928 | N = 412 | N = 284 |
| Specialty, n (%) | |||
| Primary care Medical Surgical Other | 392 (42) 485 (52) 40 (4) 11 (1) | 242 (59) 150 (36) 16 (4) 4 (1) | 191 (67) 82 (29) 8 (3) 3 (1) |
| Clinical full-time equivalent, n (%) | |||
| ≤25% 26–50% 51–75% 76–100% | 351 (38) 147 (16) 208 (22) 222 (24) | 73 (18) 66 (16) 116 (28) 157 (38) | 49 (17) 33 (12) 72 (25) 130 (46) |
| Years since medical school graduation, mean (SD) | 22 (11) 8 | 23 (11) 9 | 23 (11) 10 |
| Reason | All Antibiotics (n = 2116) 1 | % | “Watch” Antibiotics (n = 1146) 2 | % |
|---|---|---|---|---|
| Patient-reported symptoms | 1500 | 71 | 790 | 69 |
| Respiratory | 984 | 658 | ||
| Urinary | 306 | 83 | ||
| Skin/soft tissue | 140 | 14 | ||
| Fever | 56 | 40 | ||
| GI | 53 | 29 | ||
| Dental | 7 | 1 | ||
| Other symptoms | 4 | 0 | ||
| Persistence of symptoms after initial management | 376 | 18 | 191 | 17 |
| Travel | 260 | 12 | 225 | 20 |
| Lab/imaging results | 242 | 11 | 75 | 7 |
| Evaluated in clinic | 195 | 9 | 111 | 10 |
| Refill request | 42 | 2 | 8 | 1 |
| Peri-procedural prophylaxis | 15 | 1 | 5 | <1 |
| Other reasons 3 | 166 | 8 | 81 | 7 |
| Category | Description | Illustrative Extracted Explanation from EHR |
|---|---|---|
| Patient-reported symptoms | Patient initiates contact with clinician and reports symptoms/seeks treatment. | “Patient says she is having annual sinus congestion symptoms, going on vacation and does not want to go in for office visit” [ID 84] “Has had LLE extremity pain and swelling × 3 days. Has pain in her R leg. No fevers.” [ID 223] |
| Lab/imaging results | Clinician receives lab or imaging results and places order in response. | “Urine culture shows urinary infection with a bacteria called Klebsiella… It will respond more fully to cefixime once daily instead of the nitrofurantoin she already started.” [ID 2034] “Pap did show BV and will need treatment to prevent preterm contractions.” [ID 1793] |
| Evaluated in clinic | Patient seen by another clinician or by the prescribing clinician but not documented as an in-person encounter | “Pt had office visit in hematology where she presented with ongoing URI symptoms including sore throat, cough, adenopathy, and today low grade fever. Advised she follow up with her PCP again to discuss if she needs additional course of antibiotics.” [PCP then prescribes antibiotics via orders only.] [ID 1895] |
| Peri-procedural prophylaxis | Patient has recently completed or upcoming scheduled procedure | “Scheduled for Mohs surgery…abx pre op due to mtx and pred. Immunocompromised.” [ID 2457] “Patient is calling requesting amoxicillin before her dentist appointment.” [ID 1297] |
| Travel | Patient reports current or planned domestic or international travel. Includes both requests due to symptoms and “just in case” requests. | “[Patient] called from out of town complaining of three weeks of sinus pain worsening…” [ID 350] “Patient called in, states is leaving this evening [OUT OF COUNTRY]. Just had a pedicure completed and foot was cut and does not want to seek treatment out there if an infection develops. Patient asking if a script can be called in for antibiotic just in case.” [ID 863] “I am going to Mexico on Friday, to a resort where I have never gotten sick before, but just in case can you give me a prescription for the antibiotic I should take…” [ID 243] |
| Refill request | Patient requests refill or clinician uses “refill” order mechanism to prescribe. | “Spoke to pt and he said you usually send him refill with antibiotic.” [ID 1700] [From patient email]: “I’m having diverticulitis symptoms again and am wondering if you can call in a refill or prescription for Ciprofloxin which is what you’ve prescribed for past flare ups.” [ID 1896] |
| Persistence of symptoms after initial management | Follow-up about a condition/symptom that patient/clinician dyad has previously discussed. | “Pt has finished 10 days of abx for sinus infection. Was feeling better while on abx, but now sx are coming back. Pt says he usually has to do a double dose of abx for sinus this time of year.” [ID 937] [From RN]: “Pt finished her antibiotics ordered [15 days ago]. Bactrim DS for 5 days. She still feels like ‘something isn’t right’ and discomfort.” [From MD]: “UCx resistant to bactrim. Treat with cipro.” [ID 1282] |
| Theme | Description | Illustrative Clinician Quote |
|---|---|---|
| Clinic Staff | Antibiotic prescribed for staff in prescriber’s clinic. | “[Patient] is one of our staff at the office. I gave her a zpak for an upper respiratory infection on the side without an office visit.” [ID 628] |
| Continuation of previous condition | Follow-up about a condition/symptom that patient/clinician dyad has previously discussed. | “[Patient] was seen 10 days earlier with bronchitis—he was starting to improve 7 days after starting Zpak when symptoms returned on day 8 a change in antibiotics was warranted as he was having the same symptoms as he presented with a week prior” [ID 1420] |
| Diagnosis made, but not documented in EHR | Clinician referenced patient diagnosis but did not document it in EHR. | “Sinusitis for two weeks.” [ID 620] “He had recurrent diverticulitis over the holidays…” [ID 1263] |
| Evaluated in clinic | Patient evaluated in clinic without EHR documented encounter. | “The patient brought his kid in. Had strep throat. [Patient] said when he was leaving ‘I have the same symptoms’. Looked in his throat, he had a red exudative throat. Patient was put on an antibiotic. I was slammed that day, did not have time to see him, nor did I have time to put anything on his chart.” [ID 1036] |
| Evaluated outside of clinic | Patient evaluated by clinician outside of clinic (e.g., patient’s home). | “Did not put my notes in yet but he was a home visit.” [ID 46] “I did see her on my weekly dialysis rounds and sent a urinalysis and urine culture prior to prescribing antibiotics.” [ID 2295] |
| Family | Patient related to prescribing clinician. | “He is my son and I examined him at home.” [ID 2138] “My wife has had repeated UTIs that have responded to cipro. We were [OUT OF STATE] when she began to have the same sx and I prescribed the Cipro for her.” [ID 1874] |
| Infection Exposure | Patient came in contact with contagious infection. | “I saw the sib that day with strep. Rapid test positive in my office. This sib was at home with same symptoms—fever, ST no uri symptoms so I told mom I would treat her too. This is a single mom, 3 kids, works full time—my discretion—my decision to treat sib.” [ID 825] |
| Lab/imaging results | Clinician receives lab or imaging results and places order in response. | “This UA was ordered by cardiology and patient was scheduled for an angiogram and we being the PCP they wanted us to treat her UTI prior to the angiogram. The UA was done by them that’s why there was no documentation in her file by her cardiologist.” [ID 1820] |
| Patient-reported symptoms | Patient initiates contact with clinician and reports symptoms/seeks treatment. | “I forgot to document telephone encounter… I since have added details to her chart. Pt had been sick for 5–6 days with cough following flu like symptoms… I offered to send in medication without being seen due to extreme weather.” [ID 1790] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, T.; Guzman, A.; Lee, J.Y.; Fischer, M.A.; Friedberg, M.W.; Linder, J.A. Clinicians’ Reasons for Non-Visit-Based, No-Infectious-Diagnosis-Documented Antibiotic Prescribing: A Sequential Mixed-Methods Study. Antibiotics 2025, 14, 740. https://doi.org/10.3390/antibiotics14080740
Brown T, Guzman A, Lee JY, Fischer MA, Friedberg MW, Linder JA. Clinicians’ Reasons for Non-Visit-Based, No-Infectious-Diagnosis-Documented Antibiotic Prescribing: A Sequential Mixed-Methods Study. Antibiotics. 2025; 14(8):740. https://doi.org/10.3390/antibiotics14080740
Chicago/Turabian StyleBrown, Tiffany, Adriana Guzman, Ji Young Lee, Michael A. Fischer, Mark W. Friedberg, and Jeffrey A. Linder. 2025. "Clinicians’ Reasons for Non-Visit-Based, No-Infectious-Diagnosis-Documented Antibiotic Prescribing: A Sequential Mixed-Methods Study" Antibiotics 14, no. 8: 740. https://doi.org/10.3390/antibiotics14080740
APA StyleBrown, T., Guzman, A., Lee, J. Y., Fischer, M. A., Friedberg, M. W., & Linder, J. A. (2025). Clinicians’ Reasons for Non-Visit-Based, No-Infectious-Diagnosis-Documented Antibiotic Prescribing: A Sequential Mixed-Methods Study. Antibiotics, 14(8), 740. https://doi.org/10.3390/antibiotics14080740







