Illuminating the Invisible: Green Fluorescent Protein as a Beacon for Antibiotic-Induced Phage Activity in Escherichia coli
Abstract
1. Introduction
2. Results
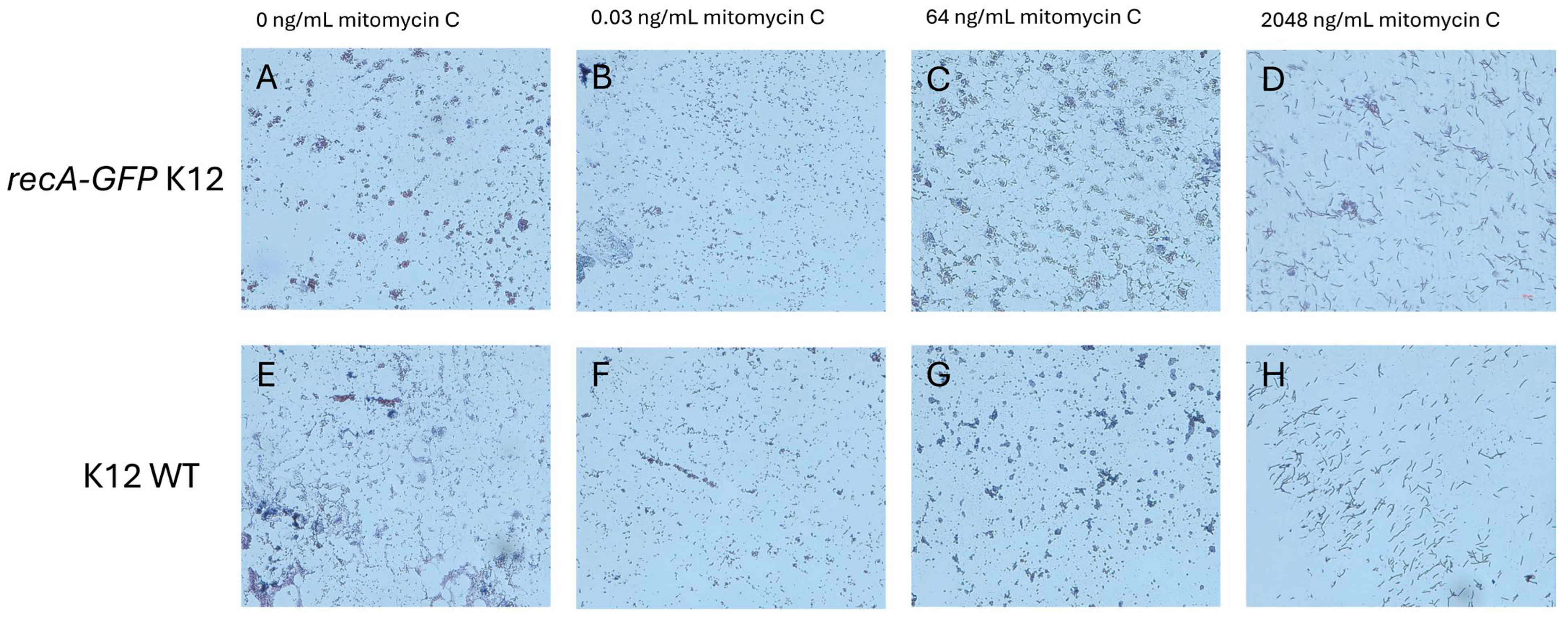
2.1. MIC Measurements Are Similar for recA-GFP and Wild-Type K12 Strains
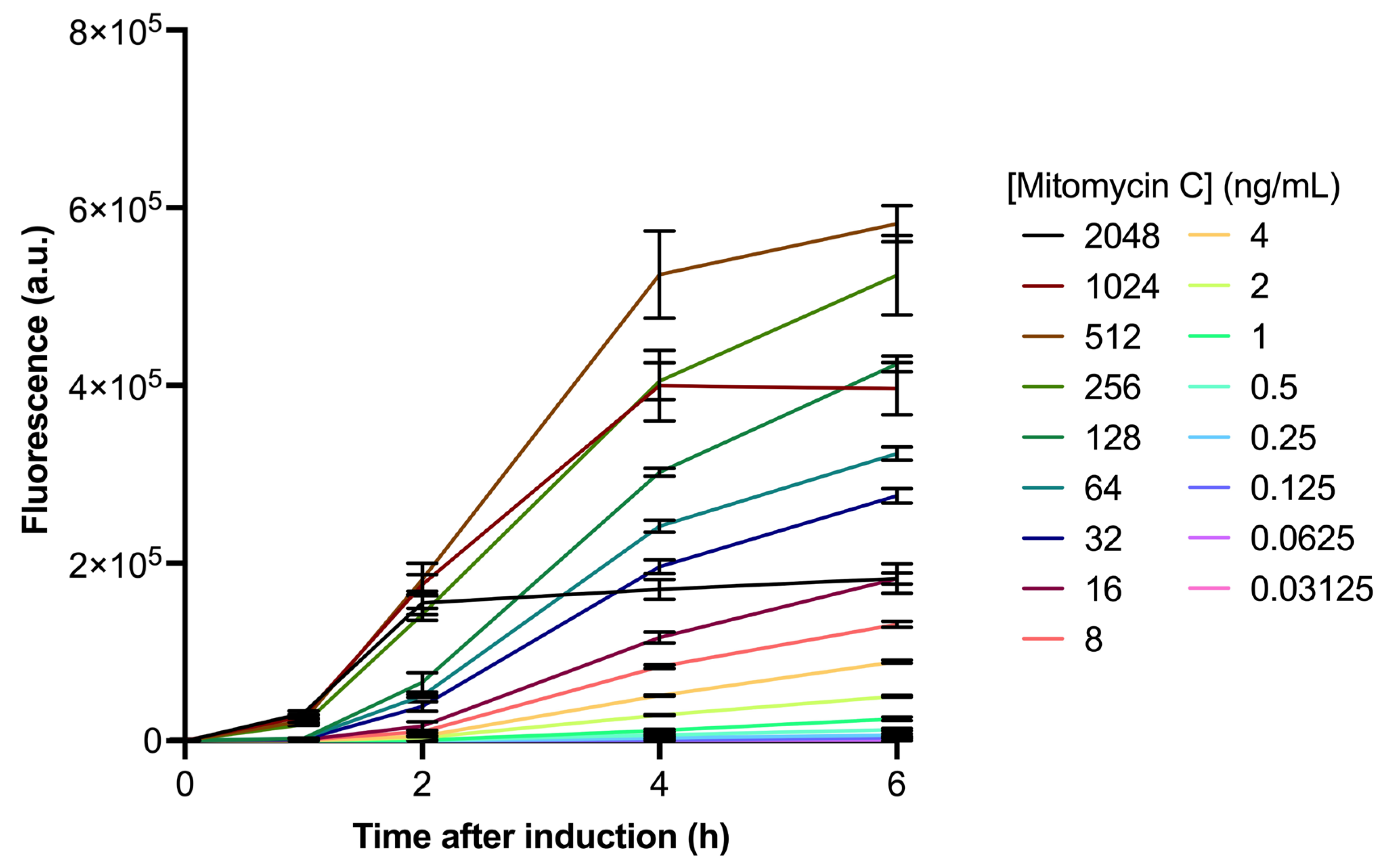
2.2. recA-Induction by Mitomycin C Is Dose- and Time-Dependent
2.3. Mitomycin C Induction of Bacteriophage Can Efficiently Be Quantified by ddPCR
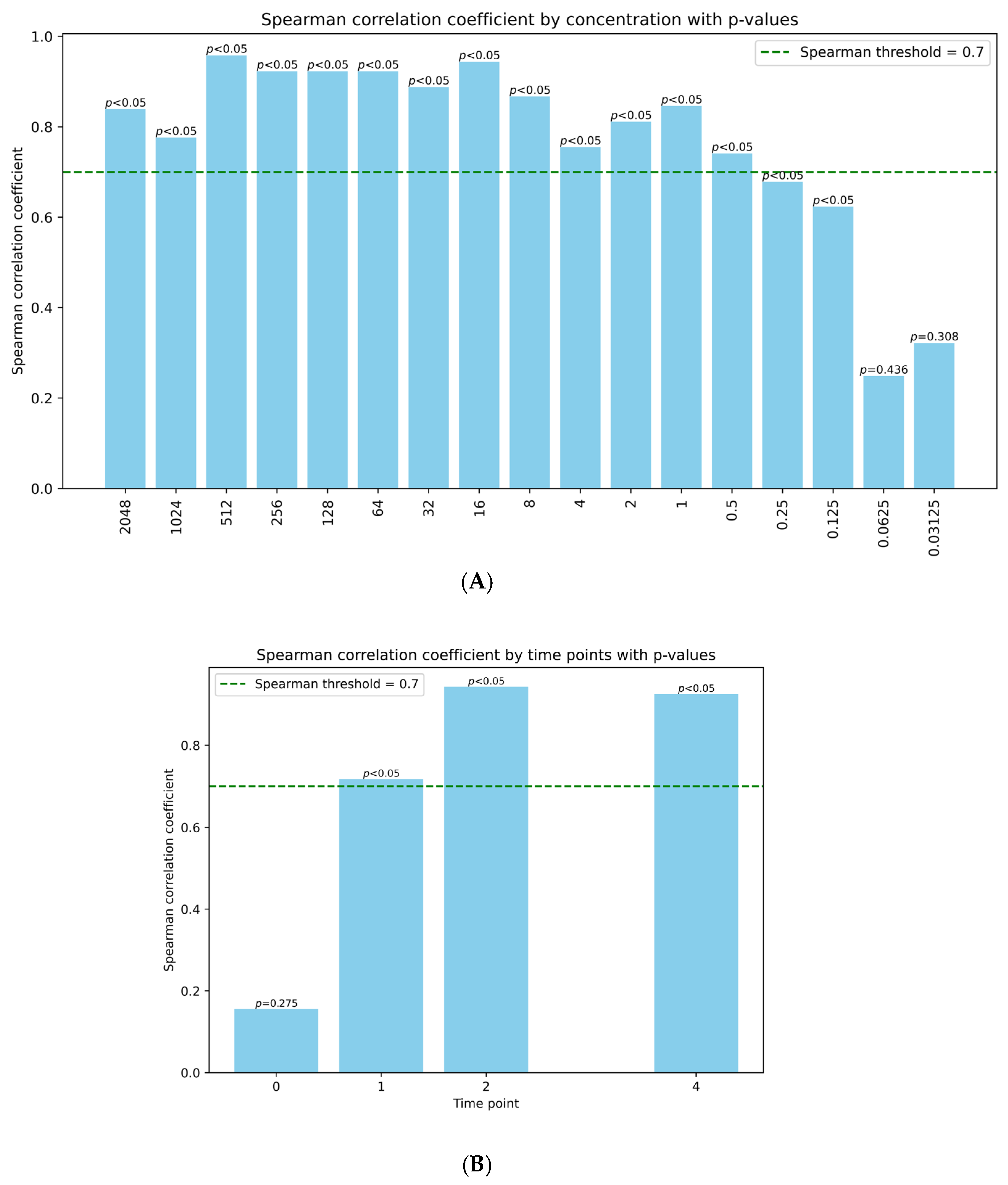
2.4. Phage Induction Correlates Well with recA Activation
3. Discussion
4. Materials and Methods
4.1. MIC Measurements
4.2. recA-GFP Induction in E. coli Cells
4.3. ddPCR Analysis of Bacteriophage Induction in E. coli
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| GFP | Green fluorescent protein |
| EC | Escherichia coli |
| ddPCR | Droplet digital PCR |
| WHO | World Health Organization |
| WT | Wild type |
| UV | Ultraviolet |
| ssDNA | Single-strand DNA |
| MIC | Minimum inhibitory concentration |
| λ | Lambda phage |
| PCR | Polymerase chain reaction |
| LB | Luria–Bertani broth |
| PBS | Phosphate-buffered saline |
| OD | Optical density |
Appendix A
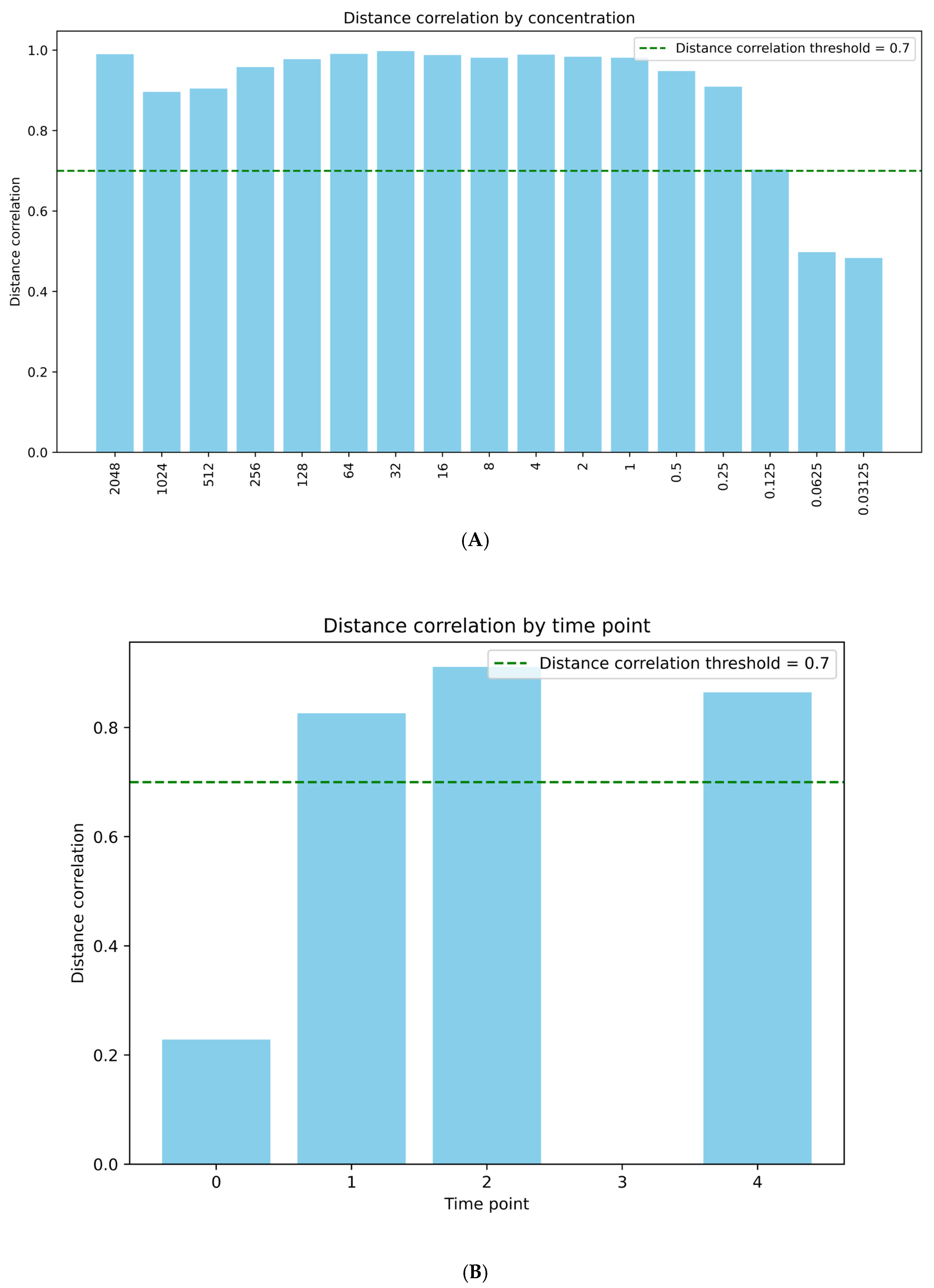

References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- WHO Antimicrobial Resistance. Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 24 June 2025).
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Roux, F.L.; Morand, S.; et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liu, C.; Zhou, S.; Liu, C.; Eldridge, D.J.; Ai, C.; Wilhelm, S.W.; Singh, B.K.; Liang, X.; Radosevich, M.; et al. Prophage-Encoded Antibiotic Resistance Genes Are Enriched in Human-Impacted Environments. Nat. Commun. 2024, 15, 8315. [Google Scholar] [CrossRef] [PubMed]
- Lood, R.; Ertürk, G.; Mattiasson, B. Revisiting Antibiotic Resistance Spreading in Wastewater Treatment Plants—Bacteriophages as a Much Neglected Potential Transmission Vehicle. Front. Microbiol. 2017, 8, 2298. [Google Scholar] [CrossRef] [PubMed]
- Ofir, G.; Sorek, R. Contemporary Phage Biology: From Classic Models to New Insights. Cell 2018, 172, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Quiles-Puchalt, N.; Chiang, Y.N.; Bacigalupe, R.; Fillol-Salom, A.; Chee, M.S.J.; Fitzgerald, J.R.; Penadés, J.R. Genome Hypermobility by Lateral Transduction. Science 2018, 362, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kawano, M.; Sugai, M. Distribution of Antimicrobial Resistance and Virulence Genes within the Prophage-Associated Regions in Nosocomial Pathogens. mSphere 2021, 6, e00452-21. [Google Scholar] [CrossRef] [PubMed]
- Balcazar, J.L. Bacteriophages as Vehicles for Antibiotic Resistance Genes in the Environment. PLoS Pathog. 2014, 10, e1004219. [Google Scholar] [CrossRef] [PubMed]
- Podlesek, Z.; Žgur Bertok, D. The DNA Damage Inducible SOS Response Is a Key Player in the Generation of Bacterial Persister Cells and Population Wide Tolerance. Front. Microbiol. 2020, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Payne-Dwyer, A.L.; Syeda, A.H.; Shepherd, J.W.; Frame, L.; Leake, M.C. RecA and RecB: Probing Complexes of DNA Repair Proteins with Mitomycin C in Live Escherichia Coli with Single-Molecule Sensitivity. J. R. Soc. Interface 2022, 19, 20220437. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Yekani, M.; Celenza, G.; Poortahmasebi, V.; Naghili, B.; Bellio, P.; Baghi, H.B. The Central Role of the SOS DNA Repair System in Antibiotics Resistance: A New Target for a New Infectious Treatment Strategy. Life Sci. 2020, 262, 118562. [Google Scholar] [CrossRef] [PubMed]
- Henrot, C.; Petit, M.A. Signals Triggering Prophage Induction in the Gut Microbiota. Mol. Microbiol. 2022, 118, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, J. Prophage Induction by Non-Antibiotic Compounds Promotes Transformation of Released Antibiotic Resistance Genes from Cell Lysis. Water Res. 2024, 263, 122200. [Google Scholar] [CrossRef] [PubMed]
- Stanczak-Mrozek, K.I.; Laing, K.G.; Lindsay, J.A. Resistance Gene Transfer: Induction of Transducing Phage by Sub-Inhibitory Concentrations of Antimicrobials Is Not Correlated to Induction of Lytic Phage. J. Antimicrob. Chemother. 2017, 72, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Tomasz, M. Mitomycin C: Small, Fast and Deadly (but Very Selective). Chem. Biol. 1995, 2, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Canchaya, C.; Desiere, F.; McShan, W.M.; Ferretti, J.J.; Parkhill, J.; Brüssow, H. Genome Analysis of an Inducible Prophage and Prophage Remnants Integrated in the Streptococcus Pyogenes Strain SF370. Virology 2002, 302, 245–258. [Google Scholar] [CrossRef] [PubMed]
- López-Leal, G.; Camelo-Valera, L.C.; Hurtado-Ramírez, J.M.; Verleyen, J.; Castillo-Ramírez, S.; Reyes-Muñoz, A. Mining of Thousands of Prokaryotic Genomes Reveals High Abundance of Prophages with a Strictly Narrow Host Range. mSystems 2022, 7, e00326-22. [Google Scholar] [CrossRef] [PubMed]
- Kadeřábková, N.; Mahmood, A.J.S.; Mavridou, D.A.I. Antibiotic Susceptibility Testing Using Minimum Inhibitory Concentration (MIC) Assays. NPJ Antimicrob. Resist. 2024, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Zaslaver, A.; Bren, A.; Ronen, M.; Itzkovitz, S.; Kikoin, I.; Shavit, S.; Leibermeister, W.; Surette, M.G.; Alon, U. A Comprehensive Library of Fluorescent Transcriptional Reporters for Escherichia Coli. Nat. Methods 2006, 3, 623–628. [Google Scholar] [CrossRef] [PubMed]

| Primer Target | Definition | Sequence |
|---|---|---|
| Lambda | Forward | TTGAATGCTGCCCTTCTTCA |
| Reverse | CTCTGGCGGTGTTGACATAA | |
| Probe (FAM) | GCGTCCTGCTGATGTGCTCAGTATCACCGC | |
| 16S (EC) | Forward | CATTGACGTTACCCGCAGAA |
| Reverse | CGCTTTACGCCCAGTAATTCC | |
| Probe (HEX) | CGTGCCAGCAGCCGCGGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.J.; Van Den Bossche, T.; Collin, M.; Lood, R. Illuminating the Invisible: Green Fluorescent Protein as a Beacon for Antibiotic-Induced Phage Activity in Escherichia coli. Antibiotics 2025, 14, 714. https://doi.org/10.3390/antibiotics14070714
Silva MJ, Van Den Bossche T, Collin M, Lood R. Illuminating the Invisible: Green Fluorescent Protein as a Beacon for Antibiotic-Induced Phage Activity in Escherichia coli. Antibiotics. 2025; 14(7):714. https://doi.org/10.3390/antibiotics14070714
Chicago/Turabian StyleSilva, Maria João, Tim Van Den Bossche, Mattias Collin, and Rolf Lood. 2025. "Illuminating the Invisible: Green Fluorescent Protein as a Beacon for Antibiotic-Induced Phage Activity in Escherichia coli" Antibiotics 14, no. 7: 714. https://doi.org/10.3390/antibiotics14070714
APA StyleSilva, M. J., Van Den Bossche, T., Collin, M., & Lood, R. (2025). Illuminating the Invisible: Green Fluorescent Protein as a Beacon for Antibiotic-Induced Phage Activity in Escherichia coli. Antibiotics, 14(7), 714. https://doi.org/10.3390/antibiotics14070714







