Abstract
Staphylococcus aureus (S. aureus) is a major cause of opportunistic infections in humans and animals, leading to severe systemic diseases. The rise of MDR strains associated with animal carriage poses significant health challenges, underscoring the need to investigate animal-derived S. aureus. Objectives: This study examined the genotypic relatedness and phenotypic profiles of antimicrobial resistance in S. aureus, previously sampled from nostril swabs of healthy horses from two geographically distant Brazilian states (Northeast and South), separated by over 3700 km. The study also sought to confirm the presence of methicillin-resistant (MRSA) and borderline oxacillin-resistant (BORSA) strains and to characterize the isolates through molecular typing using PCR. Methods: Among 123 screened staphylococci, 21 isolates were confirmed as S. aureus via biochemical tests and PCR targeting species-specific genes (femA, nuc, coa). Results: REP-PCR analysis generated genotypic profiles, revealing four antimicrobial resistance patterns, with MDR observed in ten isolates. Six isolates exhibited cefoxitin resistance, suggesting methicillin resistance, despite the absence of the mecA gene. REP-PCR demonstrated high discriminatory power, grouping the isolates into five major clusters. Conclusions: The genotyping indicated no clustering by geographical origin, highlighting significant genetic diversity among S. aureus strains colonizing horses’ nostrils in Brazil. These findings highlight the widespread and varied nature of S. aureus among horses, contributing to a deeper understanding of its epidemiology and resistance profiles in animals across diverse regions. Ultimately, this genetic diversity can pose a public health risk that the epidemiological surveillance services must investigate.
1. Introduction
Staphylococcus spp. comprises a group of mesophilic bacteria commonly found in the environment and colonizing the skin and membranes of humans and animals [1,2]. Staphylococcus aureus (S. aureus) is typically a coagulase-positive staphylococci (CoPS) and is considered the main causative agent of opportunistic staphylococcal infections in both humans and animals, ranging from localized self-limiting cutaneous lesions to severe systemic infections [2,3].
A major concern regarding staphylococcal infections is the emergence of multidrug-resistant (MDR) strains, particularly methicillin-resistant Staphylococcus aureus (MRSA) [1]. This emergence poses a significant threat to public health in all sectors, reducing the availability of antibiotics used in hospitals and making the treatment of bacterial infections more challenging [4]. In another aspect, antimicrobial-resistant bacteria have a significant economic impact in low-income countries, increasing the health burden and mortality among vulnerable populations, which could reach ten million annual deaths and cost a hundred trillion dollars by 2050 [5].
Reports have shown that farm and pet animals can be asymptomatic carriers of MRSA [3,6], as well as borderline oxacillin-resistant Staphylococcus aureus (BORSA) [7,8]. In this scenario, resistant S. aureus can spread depending on the movement of the animals and even in human–animal interactions, creating a risk of infection to the farmers, workers, and tutors even when in contact with healthy animals [9,10]; moreover, MRSA has been described as having a high potential for transfer between equine species [11]. On the other hand, Staphylococcus spp. can also be carried and spread by asymptomatic humans, completing the epidemiologic infection chain of this pathogen and making it a challenge to control [1,9]. Additionally, genetic approaches have identified these bacteria as the causative agents of infections in humans who come into contact with livestock, and they may represent a significant risk to public health [6,10].
Methicillin-sensitive S. aureus (MSSA), MRSA, and BORSA are often detected in the nostrils of healthy horses in developed countries. For instance, Pusterla et al. [12] presented the detection of MRSA in 22%, while Hryniewicz and Garbacz [13] reported a prevalence of more than 25% of BORSA in equines attended to by routine veterinary services; conversely, Mama et al. [14] found 90% staphylococci prevalence in horses intended for human consumption. Besides β-lactams, resistance to aminoglycosides, fluoroquinolones, folate pathway inhibitors, fusidic acid, macrolides, and tetracyclines has been reported in S. aureus from equines in recent years, in different parts of the world [11,15,16]. It is important to emphasize reports of healthy horses carrying heavy-metal-resistant S. aureus [16] and a high nasal colonization rate by MDR strains, which can reach one in every four equines [11].
However, unlike the existing data for other livestock species, there is a lack of information regarding the presence of S. aureus in low-income countries. In a study conducted in Brazil, a clonal similarity of S. aureus isolated from the nostrils of hospitalized horses and veterinary hospital staff was observed, reinforcing the need for surveillance measures to monitor MDR staphylococci [17] that can cause infections in both humans and animals. In this context, this study aimed to investigate the antimicrobial resistance patterns and genetic relatedness of Staphylococcus aureus isolates from healthy horses in two distant regions of Brazil. Key investigations include the confirmation of MRSA and BORSA strains and the identification of clonal relationships through molecular typing, contributing to the understanding of the genetic diversity of resistant S. aureus in horse populations.
2. Results
Out of the 123 isolates studied, 90 (73.17%) were confirmed as CoPS isolates via the tube-coagulase test, but only 21 were shown to be positive for at least one of the three S. aureus species-specific genes. The other isolates neither produced free coagulase nor were they positive for the S. aureus markers; therefore, they were identified as coagulase-negative staphylococci (CoNS).
In the molecular identification, the genes nuc and femA were identified in 16 isolates, whereas only 9 isolates were positive for the coa gene. As shown in Table 1, the concordance amongst the presence of these genes used to confirm S. aureus was low to fair. However, one of these genes was found exclusively in all S. aureus isolates, confirming the specificity of these genes in Staphylococcus species.

Table 1.
Cohen’s kappa agreement among nuc, femA, and coa genes, as well as these genes and tube coagulase.
The data in Table 2 present the frequency patterns observed in the present study, where the most common patterns were nuc-femA and nuc-femA-coa, shared by 33% and 28.5% of S. aureus strains, respectively. The gene coa was the least frequent gene found in S. aureus.

Table 2.
Frequency patterns of isolated forms from nuc, femA, and coa genes.
According to the Kirby–Bauer antimicrobial susceptibility test, higher rates of resistance were observed in the studied strains for ampicillin (47.6%; 10/21), penicillin G (47.6%; 10/21), tetracycline (38.10%; 8/21), and vancomycin (38.10%; 8/21). On the other hand, the lowest resistance rates were observed for amoxicillin-clavulanate and enrofloxacin (4.76%; 1/21). The other AMR rates found were clindamycin (33.3%; 7/21), cefoxitin (28.57%; 6/21), oxacillin (23.8%; 5/21), chloramphenicol (14.28%; 3/21), azithromycin (9.52%; 2/21), and gentamicin (9.525%; 2/21). Pan-susceptibility was observed in six (28.57%) isolates.
A total of four different antimicrobial-resistant patterns were observed, and ten (47.6%) MDR isolates were identified. Other results included six (28.6%) pan-susceptible isolates, three (14.3%) resistant to two antimicrobials, and two (9.5%) resistant to only one antimicrobial.
Regarding the genotype pattern of antimicrobial resistance, a total of 9 out of 123 Staphylococcus isolates (7.3%) were positive for the mecA gene. However, this gene was not found among the 21 S. aureus isolates, including the 6 isolates that were resistant to cefoxitin, indicating that these isolates were likely MRSA. No mecC was found in any of the 123 Staphylococcus isolates investigated in this study.
Unlike mec genes, blaZ, a penicillinase determinant, was found in 27 of 123 Staphylococcus spp., including 5 S. aureus. Interestingly, all S. aureus strains harboring the blaZ gene were phenotypically resistant to ampicillin, cefoxitin, oxacillin, and penicillin; three of them also accumulated vancomycin resistance (Table 3).

Table 3.
Resistance and gene patterns of 21 S. aureus, as well as origins.
Concerning the genotyping of S. aureus from this study, large genetic diversity was observed (Figure 1). The Rep-PCR using the primer RW3A showed a D-value of 0.96, indicating that it is highly discriminatory. Similar results were described by Silva et al. [18] and Leon et al. [19]. The S. aureus isolates were clustered in five major groups (A, B, C, D, and E). Cluster A comprised two S. aureus isolates from State B and the MRSA USA400. Except for cluster A, which indicates the presence of MRSA among the S. aureus investigated in the present study, the isolates were not clustered according to their geographical origin, indicating a large genotypic diversity of S. aureus colonizing the nasal cavities of horses in Brazil. Isolates from different geographical regions were identified in all clusters in the present study.
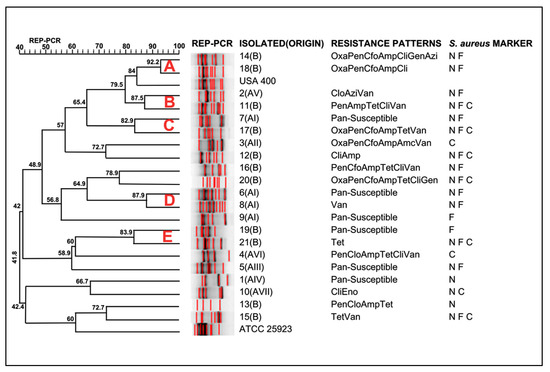
Figure 1.
Dendrogram of genotypic analysis made for RW3A from 21 S. aureus isolates. Amp = ampicillin; Cfo = cefoxitin; Pen = penicillin G; Clo = chloramphenicol; Gen = gentamicin; Oxa = oxacillin; Cli = clindamycin; Tet = tetracycline; Amc = amoxicillin/clavulanate; Azi = azithromycin; Van = vancomycin; Eno = enrofloxacin; N = Positive for nuc Marker; F = Positive for femA Marker; C = Positive for coa Marker.
3. Discussion
The low occurrence of S. aureus amongst staphylococci might be related to the fact that only healthy animals were sampled. Similar frequencies of S. aureus carriage in horses have been reported in Switzerland, Libya, and Brazil [20,21,22].
The unexpected frequency of the coa gene amongst isolates, even in coagulase-producing bacteria, could be related to the putative polymorphism of this gene. Variations in the coa gene have been previously observed amongst S. aureus [20,23]. Importantly, although PCR targeting the nuc gene has been typically used for S. aureus confirmation purposes due to its specificity for this bacterial species [24,25], our findings suggest that the use of more markers might contribute to improving S. aureus detection in strains exclusively sampling from horses.
Nosocomial infections in horses have been attributed to MDR S. aureus, and the high resistance rates against drugs commonly used are of great concern because of the therapeutic implications associated with multi-resistant strains in horses [14,20,22]. Furthermore, a worrying finding of our study was the coexistence of both β-lactam and vancomycin resistance. Concerning these two classes of antimicrobials, the β-lactam drugs have been indicated by the World Health Organization (WHO) as being critically important for human health [26], and vancomycin is the chosen drug for treating infections caused by methicillin-resistant staphylococci [27].
In this study, we found eight S. aureus strains resistant to ß-lactam and eight resistant to vancomycin (five of them were resistant to both). Although a limitation of the study may be the antimicrobial susceptibility test by Kirby–Bauer to identify vancomycin-resistant strains, previous reports using disk diffusion have established a high correlation between vancomycin-resistant S. aureus and both minimum inhibitory concentration (MIC ≥ 16 µg/mL) and the presence of genetic determinants (vanA and vanB) [28,29].
The correct identification of S. aureus strains is not a straightforward task, and it is believed to introduce a significant bias in studies aiming to assess antimicrobial resistance in S. aureus, especially in MRSA strains [8,25,30]. The low occurrence of the mec gene is not uncommon. In a previous study conducted in Brazil, only 1% of the Staphylococcus spp. isolated from horses presented the mecA gene, all of them identified in S. pseudintermedius [17]. Recent studies have demonstrated that the MRSA phenotype does not necessarily require the presence and expression of mecA, and other putative mechanisms for methicillin resistance, such as mecC, as well as hyperexpression of the bla-operon, have also been shown to occur in methicillin/cefoxitin-resistant S. aureus [13,31,32]. This is particularly important for animal-derived S. aureus, since the mecA gene was initially targeted as a marker for methicillin resistance in S. aureus from humans [33]. In this sense, pleomorphism in mec-operon might occur, making it challenging to confirm MRSA using PCR-mecA [34]; however, this hypothesis is based solely on previously published data, as no sequencing was performed in this study, which can be seen as a limitation of the study. Notably, the six cefoxitin-resistant isolates identified in the present study were also MDR isolates (Table 3), which have invariably been associated with MDR [33].
The potential of blaZ-positive S. aureus to show resistance to various β-lactam drugs leads to the misidentification of BORSA, MRSA, or MSSA. This condition implies the treatment failure of BORSA-associated infections when the bacterial identification is based on cefoxitin susceptibility [26]. Therefore, the correct identification of BORSA is crucial for the success of drug therapy in human medicine, as well as for the surveillance of bacteria in horse farms, focusing on its role in One Health [27].
Staphylococcosis in animals has been linked to pre-existing conditions associated with chronic diseases [35], and can cause serious diseases, especially when strains carry resistance determinants. MRSA infection caused by a strain carrying mec and blaZ genes can result in chronic obstructive pulmonary disease (COPD) and thrombophlebitis [31]. In that case, veterinarians and treaters are affected and need to be treated against S. aureus infection [31].
Although BORSA infections in humans are infrequently identified, a recent case report has been published, resulting in one death from a nosocomial infection, and observations of high genetic heterogeneity among S. aureus isolates, as well as the identification of blaZ gene expression. [36]. A more severe outbreak was reported by Huang et al. [37], with a high human mortality rate, reaching 24.6%, caused by BORSA strains in affected patients of bacteremia. Conversely, recent reports have found a high prevalence of MDR BORSA strains in healthy horses [38], which can serve as a source of infection for farmers and handlers.
BORSA strains have been reported in other animal species, such as asymptomatic swine [8] and in the nasal cavities of patients hospitalized in a neonatal intensive care unit [26]. Curiously, from both swine and human cases, sequence type 398, related to the LA-MRSA lineage, was reported, despite the absence of mecA, mecB, or mecC genes [8,26]. These previous reports and our results on the occurrence of S. aureus strains in humans and healthy animals with methicillin-resistant phenotypes highlight its potential impact on public health.
Although the occurrence of S. aureus in equines presenting no clinical signs is not uncommon, lineages originally from animals have been found more often in humans. For instance, the prevalence of ST398 could be 10-fold higher in the nasal cavities of humans than in horses [17], corroborating our findings and confirming that equines and humans are potential reservoirs of this pathogen.
Unlike our study, Santos et al. [8] identified a lower diversity of clusters in S. aureus isolates from swine, which is consistent with their study in a similar geographical region, suggesting that the reservoir for this bacterium may be more closely linked to the animal species than its origin. However, the genetic similarity of MRSA USA 400 with BORSA from swine [8], as found in horses, poses a potential health risk to handlers, animals, and individuals involved in management [39].
Although the study regions are approximately 3700 km apart, genetic compatibility was observed between clusters B and C of some S. aureus isolates (Figure 2). The identification of these genetically similar isolates from animals in different regions may indicate the dissemination of S. aureus-resistant strains in other states of the country, hosted by both humans and equines, due to their strong interaction [3]. However, our results are somewhat divergent from those obtained from other animal species. Recent studies focusing on S. aureus frequency from Brazil highlighted a large diversity of S. aureus strains from swine, carrying resistance determinants [8,40]. Therefore, we hypothesize that two factors could be involved in the dissemination of S. aureus in a continental Country: the selective pressure imposed by off-label antimicrobials used in farm animals [4], and the transport of animals and humans between different geographical regions, carrying MDR S. aureus strains [41]. Ultimately, although the lack of clustering by region may be unexpected, these results can help veterinarians and personnel handling horses in the prudent use of antimicrobials in veterinary medicine [3].
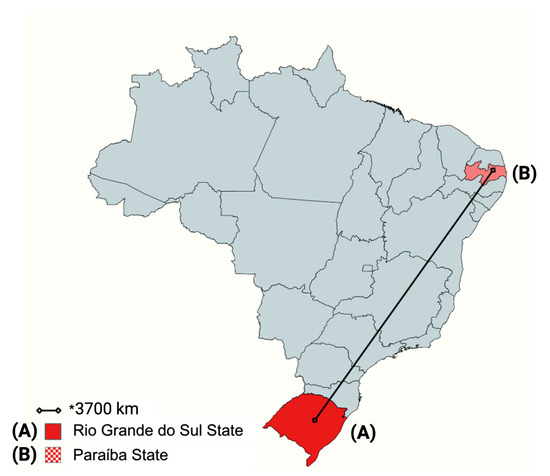
Figure 2.
Distance between the State of Rio Grande do Sul and the State of Paraíba in a map of Brazil. The bacteria were isolated from the nostrils of healthy horses originating from the regions highlighted on the map. * = The black line indicates approximately 3700 km of distance. Created in BioRender. Saraiva, M. (2025) https://BioRender.com/plog7ww.
4. Materials and Methods
4.1. Bacteria and Staphylococcus aureus Confirmation
Samples of nasal swabs from clinically healthy horses were previously and randomly collected from animals originating from different Brazilian states: seven cities in Rio Grande do Sul State (identified as AI to AVII) and one municipality in Paraiba State (identified as B). The approximate distance between them is 3700 km (Figure 2).
A total of 123 Staphylococcus spp. strains were received in the Laboratory for Animal-derived Foods (LAPOA) of the College for Agricultural Sciences, Federal University of Paraíba (CCA/UFPB): 87 isolates from State A, and 36 isolates from State B. S. aureus identification was performed using conventional morphological (stained Gram) and phenotypic (biochemical tests, including coagulase production, Voges–Proskauer, polymyxin B resistance, and mannitol fermentation) analysis. Isolates showing morphological and phenotypical S. aureus characteristics were confirmed by polymerase chain reaction (PCR) targeting three specific genes (nuc, femA, and coa), according to the work of Saraiva et al. [24] (Appendix A Table A1).
The extraction of genomic DNA from Staphylococcus spp. was conducted using the boiling-centrifugation method [42]. Briefly, five colonies of each isolate were suspended in 100 μL of ultrapure water, frozen for 10 min, and then boiled at 100 °C for an additional 10 min. The samples were centrifuged for 3 min at 12,000 rpm at 4 °C to remove any cellular debris. The supernatant of each sample was carefully removed, and, to avoid contaminants, 70 μL was transferred to another tube and then stored at 4 °C until use. After DNA extraction, the master mix was prepared in a 25 μL volume using 1 μL of Taq DNA polymerase (LGC Biotechnologies, Middlesex, England), two μL of MgCl2, 20 μM of each dNTP (Thermo Fisher Scientific, Agawam, MA, USA), and two μL of DNA template [24]. PCRs were performed in TC5000 thermal cycler (Techne, Essex, England); amplification products were electrophorized in 2% agarose gel, stained with Gelred (Biotium, Fremont, CA, USA), visualized under UV, and documented by Gel Logic 212 PRO (Carestream Molecular Imaging Software—Version 5.0, Carestream Health Inc., Rochester, NY, USA).
4.2. Antimicrobial Susceptibility Test
An antimicrobial susceptibility test was performed using the Kirby–Bauer disk-diffusion method, as described by the Clinical and Laboratory Standards Institute [43]. The antimicrobial drugs used and their respective concentrations were as follows: amoxicillin/clavulanate (AMC, 10 µg), ampicillin (AMP, 30 µg), azithromycin (AZI, 15 µg), cefoxitin (CFO, 30 µg), chloramphenicol (CLO, 30 µg), clindamycin (CLI, 2 μg), enrofloxacin (ENO, 10 µg), gentamicin (GEN, 10 µg), oxacillin (OXA, one µg), penicillin G (PEN, 10 UI), tetracycline (TET, 30 µg) and vancomycin (VAN, 30 µg) (CECON®, São Paulo, Brazil). The interpretation of antimicrobial susceptibility test results was performed according to the CLSI [43]. Isolates were considered MDR when showing resistance against three or more drugs derived from different antimicrobial classes.
4.3. MRSA and BORSA Confirmation, and Genotyping
Confirmed S. aureus isolates were extracted via the technique of phenol: chloroform: isoamyl alcohol, according to the Laboratory Manual of Sambrook et al. [44]; afterwards, investigation by PCR targeting mecA, mecC [8], and blaZ [45] genes was performed, using the same master mix above (Appendix A Table A1). Two strains (USA 400 and ATCC 25923) were used as positive and negative controls. Amplification products were electrophorized in 2% agarose gel, stained with Gelred (Biotium, Fremont, CA, USA), visualized under UV, and documented by Gel Logic 212 PRO (Carestream Molecular Imaging Software—Version 5.0, Carestream Health Inc., Rochester, NY, USA). Additionally, these were genotyped by Repetitive Extragenic Palindromic-PCR (Rep-PCR), targeting the RW3A, according to the work of Zee et al. [46]. All PCRs were performed into TC5000 thermal cycler (Techne, Essex, England), and amplification products were electrophorized in 2% agarose gel, stained with Gelred (Biotium, Fremont, CA, USA) visualized under ultraviolet light, and documented by Gel Logic 212 PRO (Carestream Molecular Imaging Software—Version 5.0, Carestream Health Inc., Rochester, NY, USA).
4.4. Data Analyses
The concordance amongst the presence of the genes nuc, femA, and coa was determined by the Cohen’s kappa index of concordance according to the criteria previously established by Landis and Koch [47] (Appendix A Table A2).
The genetic relatedness among S. aureus was determined by analyzing the similarity among Rep-PCR fingerprints using the Dice coefficient with a 2% tolerance for genetic distance measurement calculations. Cluster analysis was performed using the unweighted pair-group method with average linkages (UPGMA) via commercial software (BioNumerics 7.1, Applied Maths, Sint-Martens-Latem, Belgium), and the results were presented in a dendrogram. An 80% similarity coefficient was used as a threshold for clustering. The discriminatory power (D value) was calculated as described by Hunter [48].
5. Conclusions
In conclusion, our findings indicate a high genetic diversity of S. aureus colonizing the nasal cavities of horses from Brazil, as well as the presence of BORSA among the samples. A special concern is the high frequency of MDR pathogens harbored by horses, which could affect not only animals but also human health. Our results demonstrate that the MRSA phenotype does not require the presence or expression of the mecA gene. Based on the literature, this indicates pleomorphism in the presence of the mec-operon and bla-operon, making it difficult to confirm whether a strain is carrying the gene.
Author Contributions
M.M.S.S.: Writing—original draft, Methodology, Data curation; H.L.d.S.R.: Writing—original draft, Data curation; V.P.B.: Writing—original draft, Data curation; C.M.C.G.d.L.—Methodology; S.C.L.S.: Methodology; D.T.S.: Writing—review and editing, Methodology; P.E.N.G.: Supervision, Writing—review and editing; R.F.C.V.: Supervision, Writing—review and editing; C.J.B.O.: Supervision, Project administration, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior— Brasil (CAPES)—Finance Code 001, Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), and Financiadora de Estudos e Projetos (FINEP).
Institutional Review Board Statement
All procedures were approved by the Ethical Committee of Animal Use in Research of the Federal University of Paraiba (CEUA-UFPB).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used in this study are available upon request from the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CoPS | Coagulase-positive Staphylococcus |
| CoNS | Coagulase-negative Staphylococcus |
| MDR | Multidrug-resistant |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| BORSA | Borderline oxacillin-resistant Staphylococcus aureus |
| S. aureus | Staphylococcus aureus |
Appendix A
In this section, we provide tables and appendix data from this study.

Table A1.
Primers and cycling conditions used in the identification of Staphylococcus aureus.
Table A1.
Primers and cycling conditions used in the identification of Staphylococcus aureus.
| Gene | Amplicon Size (bp) | Primer Sequence (5′–3′) | Cycling | Reference |
|---|---|---|---|---|
| coa | (variable) | F: ATA GAG ATG CTG GTA CAG G R: GCT TCC GAT TGT TCG ATG C | 1 | [24] |
| blaZ | (517 bp) | F: AAG AGA TTT GCC TAT GCT TC R: GCT TGA CCA CTT TTA TCA GC | 2 | [45] |
| femA | (132 bp) | F: AAA AAA GCA CAT AAC AAG CG R: GAT AAA GAA GAA ACC AGC AG | 3 | [24] |
| mecA | (162 bp) | F: TCC AGA TTA CAA CTT CAC CAG G R: CCA CTT CAT ATC TTG TAA CG | 4 | [8] |
| mecC | (138 bp) | F: GAA AAA AAG GCT TAG AAC GCC TC R: GAA GAT CTT TTC CGT TTT CAG C | 5 | [8] |
| nuc | (270 bp) | F: GCG ATT GAT GGT GAT ACG GTT R: AGC CAA GCC TTG ACG AAC TAA AGC | 3 | [24] |
Legend: (1) 94 °C 4 min (94 °C 1 min, 60 °C 1 min, 72 °C 1 min) × 30 and 72 °C 5 min; (2) 95 °C 5 min (95 °C 30 s, 55 °C 30 s, 72 °C 30 s) × 30 and 72 °C 5 min; (3) 94 °C 5 min (94 °C 40 s, 58 °C 40 s, 72 °C 1 min) × 10 (94 °C 1 min, 50 °C 1 min, 72 °C 2 min) × 25 and 72 °C 10 min; (4) 94 °C 4 min (94 °C 30 s, 53 °C 30 s, 72 °C 1 min) × 30 and 72 °C 4 min; (5) 94 °C 5 min (94 °C 30 s, 59 °C 1 min, 72 °C 1 min) × 30 and 72 °C 10 min. F: forward primer; R: reverse primer.

Table A2.
Interpretation criteria for the Cohen’s kappa index of correlation according to Landis and Koch [47].
Table A2.
Interpretation criteria for the Cohen’s kappa index of correlation according to Landis and Koch [47].
| Kappa Values | Concordance |
|---|---|
| <0 | No Agreement |
| 0–0.20 | Poor Agreement |
| 0.21–0.40 | Fair Agreement |
| 0.41–0.60 | Moderate Agreement |
| 0.61–0.80 | Substantial Agreement |
References
- Lee, A.S.; Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. 2018, 4, 18033. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Wu, J.; Chen, S.; Jin, Y.; Long, J.; Duan, G.; Yang, H. Transmission of livestock-associated methicillin-resistant Staphylococcus aureus between animals, environment, and humans in the farm. Environ. Sci. Pollut. Res. 2023, 30, 86521–86539. [Google Scholar] [CrossRef]
- Marsella, R. Antibiotic resistance in equine dermatology: What should we do? J. Am. Veter Med. Assoc. 2025, 1, 1–5. [Google Scholar] [CrossRef]
- Saraiva, M.M.S.; Lim, K.; Monte, D.F.M.; Givisiez, P.E.N.; Rodrigues Alves, L.B.; Freitas Neto, O.C.; Kariuki, S.; Berchieri Junior, A.; Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 5 February 2025).
- Kizerwetter-Świda, M.; Chrobak-Chmiel, D.; Rzewuska, M. High-level mupirocin resistance in methicillin-resistant staphylococci isolated from dogs and cats, United Kingdom. BMC Vet. Res. 2019, 15, 238. [Google Scholar] [CrossRef]
- Maksimovic, Z.; Dizdarevic, J.; Babic, S.; Rifatbegovic, M. Antimicrobial resistance in coagulase-positive Staphylococci isolated from various animals in Bosnia and Herzegovina. Microbiol. Drug Resist. 2022, 28, 136–142. [Google Scholar] [CrossRef]
- Santos, S.C.L.; Saraiva, M.M.S.; Moreira Filho, A.L.B.; Silva, N.M.V.; De Leon, C.M.C.G.; Pascoal, L.A.F.; Givisiez, P.E.N.; Gebreyes, W.A.; Oliveira, C.J.B. Swine as reservoirs of zoonotic borderline oxacillin-resistant Staphylococcus aureus ST398. Comp. Immunol. Microbiol. Infect. Dis. 2021, 79, 101697. [Google Scholar] [CrossRef]
- Bastard, J.; Andraud, M.; Chauin, C.; Glaser, P.; Opatowski, L.; Temime, L. Dynamics of livestock-associated methicillin resistant Staphylococcus aureus in pig movement networks: Insight from mathematical modeling and french data, France. Epidemics 2020, 31, 100389–100401. [Google Scholar] [CrossRef]
- Jiang, J.H.; Cameron, D.R.; Nethercott, C.; Aires-de-Souza, M.; Peleg, A.Y. Virulence atributes of successful methicillin-resistant Staphylococcus aureus lineages. Clin. Microbiol. Rev. 2023, 36, e00148-22. [Google Scholar] [CrossRef]
- Anwaar, F.; Ijaz, M.; Rasheed, H.; Shah, S.F.A.; Haider, S.A.R.; Sabir, M.J. Evidence and molecular characterization of multidrug resistant Staphylococcus aureus isolated from equines in Pakistan. J. Equine Vet. Sci. 2023, 126, 104498. [Google Scholar] [CrossRef]
- Pusterla, N.; Rice, M.; Henry, T.; Barnum, S.; James, K. Investigation of the Shedding of Selected Respiratory Pathogens in Healthy Horses Presented for Routine Dental Care, United States. J. Vet. Dent. 2020, 37, 88–93. [Google Scholar] [CrossRef]
- Hryniewicz, M.M.; Garbacz, K. Borderline oxacillin-resistant Staphylococcus aureus (BORSA)–a more common problem than expected? J. Med. Microbiol. 2017, 66, 1367–1373. [Google Scholar] [CrossRef]
- Mama, O.M.; Gómez, P.; Ruiz-Ripa, L.; Gómez-Sanz, E.; Zarazaga, M.; Torres, C. Antimicrobial Resistance, Virulence, and Genetic Lineages of Staphylococci from Horses Destined for Human Consumption: High Detection of S. aureus Isolates of Lineage ST1640 and Those Carrying the lukPQ Gene, Switzerland. Animals 2019, 9, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Isgren, C.M.; Williams, N.J.; Fletcher, O.D.; Timofte, D.; Newton, R.J.; Maddox, T.W.; Clegg, P.D.; Pinchbeck, G.L. Antimicrobial resistance in clinical bacterial isolates from horses in the UK. Equine Vet. J. 2022, 54, 390–414. [Google Scholar] [CrossRef] [PubMed]
- Nwobi, O.C.; Anyanwu, M.U.; Jaja, I.F.; Nwankwo, I.O.; Okolo, C.C.; Nwobi, C.A.; Ezenduka, E.V.; Oguttu, J.W. Staphylococcus aureus in horses in Nigeria: Occurrence, antimicrobial, methicillin and heavy metal resistance and virulence potentials. Antibiotics 2023, 12, 242. [Google Scholar] [CrossRef]
- Olivo, G.; Zakia, L.S.; Ribeiro, M.G.; Cunha, M.L.R.S.; Riboli, D.F.M.; Mello, P.L.; Teixeira, N.B.; Araújo, C.E.T.; Oliveira-Filho, J.P.; Borges, A.S. Methicillin-resistant Staphylococcus spp. investigation in hospitalized horses and contacting personnel in a teaching veterinary hospital. J. Equine Vet. Sci. 2024, 134, 105031. [Google Scholar] [CrossRef]
- Silva, A.T.F.; Silva, J.G.; Aragão, B.B.; Silva, N.M.V.; Vasconcelos, P.C.; Oliveira, C.J.B.; Mota, R.A. Genetic traceability of Staphylococcus aureus strains isolated from primiparous dairy cows mastitis, humans and environment in the Northeast region of Brazil. Microbiology: Cienc. Rural. 2021, 51, e20200679. [Google Scholar] [CrossRef]
- De Leon, C.M.C.G.; Sousa, F.G.C.; Saraiva, M.M.S.; Givisiez, P.E.N.; Silva, N.M.V.; Vieira, R.F.C.; Oliveira, C.J.B. Equipment contact surfaces as sources of Staphylococcus carrying enterotoxin-encoding genes in goat milk dairy plants. Int. Dairy J. 2020, 111, 104827. [Google Scholar] [CrossRef]
- Mota, S.L.; Santos, L.O.; Vidaletti, M.R.; Rodrigues, R.O.; Coppola, M.M.; Mayer, F.Q. Antimicrobial resistance of Coagulase-positive Staphylococcus isolated from healthy crioulo horses and associated risk factors. J. Equine Vet. Sci. 2021, 107, 103779. [Google Scholar] [CrossRef]
- Hurni, J.I.; Kaiser-Thom, S.; Gerber, V.; Keller, J.; Colaude, U.; Fernández, J.; Schwendener, S.; Perreten, V. Prevalence and whole genome-based phylogenetic, virulence, and antibiotic-resistance characteristics of nasal Staphylococcus aureus in healthy Swiss horses. Schweiz Arch Tierheilkd 2022, 164, 499–512. [Google Scholar] [CrossRef]
- Othman, A.A.; Hiblu, M.A.; Abbassi, M.S.; Abouzeed, Y.M.; Ahmed, M.O. Nasal colonization and antibiotic resistance patterns of Staphylococcus species isolated from healthy horses in Tripoli, Libya. J. Equine Vet. Sci. 2021, 32, 61–65. [Google Scholar] [CrossRef]
- Locatelli, C.; Gattolin, S.; Monistero, V.; Castiglioni, B.; Moroni, P.; Addis, M.F.; Cremonesi, P. Staphylococcus aureus coa gene sequence analysis can prevent misidentification of coagulase-negative strains and contribute to their control in dairy cow herds. Front. Microbiol. 2023, 14, 1120305. [Google Scholar] [CrossRef]
- Saraiva, M.M.S.; De Leon, C.M.C.G.; Santos, S.C.L.; Stipp, D.T.; Souza, M.M.; Santos Filho, L.; Gebreyes, W.A.; Oliveira, C.J.B. Accuracy of PCR targeting different markers for Staphylococcus aureus identification: A comparative study using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry as the gold standard. J. Vet. Diagn. Investig. 2018, 30, 252–255. [Google Scholar] [CrossRef]
- Srisrattakarn, A.; Panpru, P.; Tippayawat, P.; Chanawong, A.; Tavichakorntrakool, R.; Daduang, J.; Wonglakorn, L.; Lulitanond, A. Rapid detection of methicillin-resistant Staphylococcus aureus in positive blood-cultures by recombinase polymerase amplification combined with lateral flow strip. PLoS ONE 2022, 17, e0270686. [Google Scholar] [CrossRef] [PubMed]
- Sawhney, S.S.; Ransom, E.M.; Wallace, M.A.; Reich, P.J.; Dantas, G.; Burnham, C.A.D. Comparative genomics of Borderline Oxacillin-Resistant Staphylococcus aureus detected during a pseudo-outbreak of Methicillin-Resistant S. aureus in a Neonatal Intensive Care Unit. mBio 2022, 13, e03196-21. [Google Scholar] [CrossRef]
- Salemi, R.; Zega, A.; Aguglia, E.; Lo Verde, F.; Pigola, G.; Stefani, S.; Cafiso, V. Balancing the virulence and antimicrobial resistance in VISA DAP-R CA-MRSA Superbug. Antibiotics 2022, 11, 1159. [Google Scholar] [CrossRef]
- Saber, T.; Samir, M.; Mekkawy, R.M.; Ariny, E.; El-Sayed, S.R.; Enan, G.; Abdelatif, S.H.; Askora, A.; Merwad, A.M.A.; Tartor, Y.H. Methicillin and Vancomycin-Resistant Staphylococcus aureus from humans and ready-to-eat meat: Characterization of antimicrobial resistance and biofilm formation ability. Front. Microbiol. 2022, 12, 735494. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Ando, R.; Matsumoto, T.; Ishii, Y.; Tateda, K. Association between cell growth and vancomycin resistance in clinical community-associated Methicillin-Resistant Staphylococcus aureus. Infect. Drug Resist. 2019, 12, 2379–2390. [Google Scholar] [CrossRef]
- Walter, C.; Weissert, C.; Gizewski, E.; Burckhardt, I.; Mannsperger, H.; Hanselmann, S.; Busch, W.; Zimmermann, S.; Noite, O. Performance evaluation of machine-assisted interpretation of Gram strains from positive blood cultures. J. Clin. Microbiol. 2024, 62, e0087623. [Google Scholar] [CrossRef]
- Albert, E.; Sahin-Tóth, J.; Horváth, A.; Papp, M.; Biksi, I.; Dobay, O. Genomic evidence for direct transmission of mecC-MRSA between a horse and its veterinarian. Antibiotics 2023, 12, 408. [Google Scholar] [CrossRef]
- Ciesielczuk, H.; Xenophontos, M.; Lambourne, J. Methicillin-Resistant Staphylococcus aureus Harboring mecC Still Eludes Us in East London, United Kingdom. J. Clin. Microbiol. 2019, 57, e00020-19. [Google Scholar] [CrossRef]
- Algammal, A.M.; Hetta, H.F.; Elkelish, A.; Alkhalifah, D.H.H.; Hozzein, W.N.; Batiha, G.E.; Nahhas, N.E.; Mabrok, M.A. Methicillin-resistant Staphylococcus aureus (MRSA): One Health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Dovepress 2020, 13, 3255–3265. [Google Scholar] [CrossRef]
- Tabuchi, F.; Lulitanond, A.; Lulitanond, V.; Thunyaharn, S.; Kaito, C. Epidemiological study on the relationship between toxin production and psm-mec mutations in MRSA isolates in Thailand. Microbiol. Immunol. 2020, 64, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.C.; Lefebvre, S.L.; Rankin, S.C.; Aceto, H.; Morley, P.S.; Caron, J.P.; Galês, R.D.; Holbrook, T.C.; Moore, B.; Taylor, D.R.; et al. Retrospective multicentre study of methicillin-resistant Staphylococcus aureus infections in 115 horses. Equine Vet. J. 2009, 41, 401–405. [Google Scholar] [CrossRef]
- Konstantinovski, M.M.; Veldkamp, K.E.; Lavrijsen, A.P.M.; Bosch, T.; Kraakman, M.E.M.; Nooij, S.; Claas, E.C.J.; Gooskens, J. Hospital transmission of borderline oxacillin-resistant Staphylococcus aureus evaluated by whole-genome sequencing. J. Med. Microbiol. 2021, 70, 001384. [Google Scholar] [CrossRef]
- Huang, Y.T.; Liao, C.H.; Chen, S.Y.; Hsu, H.S.; Teng, L.J.; Hsueh, P.R. Emergence of multidrug-resistant sequence type 45 strains among mecA-positive borderline oxacillin-resistant Staphylococcus aureus causing bacteraemia in a medical centre in Taiwan. Int. J. Antim. A 2018, 52, 70–75. [Google Scholar] [CrossRef]
- Scholtzek, A.D.; Hanke, D.; Walther, B.; Eichhorn, I.; Stockle, S.D.; Klein, K.S.; Gehlen, H.; Lubke-Becker, A.; Scwartz, S.; Febler, A.T. Molecular characterization of equine Staphylococcus aureus isolates exhibiting reduced oxacillin susceptibility. Toxins 2019, 11, 535. [Google Scholar] [CrossRef]
- Little, S.V.; Hillhouse, A.E.; Lawhon, S.D.; Bryan, L.K. Analysis of virulence and antimicrobial resistance gene carriage in Staphylococcus aureus infections in equids using whole-genome sequencing. Msphere 2021, 6, e00196-20. [Google Scholar] [CrossRef]
- Nobre, M.L.M.; Santos, L.S.; Silva, D.R.P.; Oliveira, F.A.A.; Araújo, A.R.; Campos, M.A.S.; Sousa, B.C.; Figuerêdo, A.V.; Muratori, M.C.S.; Soares, M.J.S. Multiresistance and virulence factors of Staphylococcus aureus isolated from pigs. Arq. Bras. Med. Vet. E Zootec. 2021, 73, 343–351. [Google Scholar] [CrossRef]
- Arntzen, V.H.; Feenstra, S.G.; Beninca, E.; Le, T.T.N.; Mascini, E.M.; Nabuurs-Franssen, M.H.; Voss, A.; Marik, A.M.; Jong, E.; Silvis, W.; et al. Spatial analysis of methicillin-resistant Staphylococcus aureus carriage (MRSA) at hospital admission in a livestock dense region. MedRxiv 2023. [Google Scholar] [CrossRef]
- Ajibade, O.A.; Akinduro, A.O.; Omojufehinsi, G.; Odetoyin, B.; Olaniyi, O.O. Molecular characterization of antibiotic-resistant bacteria associated with maggots obtained from chicken droppings. Environm. Sci. Eur. 2024, 36, 26. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI document VET08Ed4E; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024; ISBN 978-1-68440-011-9. [Google Scholar]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: New York, NY, USA, 2001. [Google Scholar]
- Gao, J.; Ferreri, M.; Liu, X.Q.; Chen, L.B.; Su, J.L.; Han, B. Development of multiplex polymerase chain reaction assay for rapid detection of Staphylococcus aureus and selected antibiotic resistance genes in bovine mastitic milk samples. J. Vet. Diagn. Invest. 2011, 23, 894–901. [Google Scholar] [CrossRef]
- Zee, A.V.D.; Verbakel, H.; Zon, J.C.V.; Frenay, I.; Belkum, A.V.; Peeters, M.; Buiting, A.; Bergmans, A. Molecular Genotyping of Staphylococcus aureus Strains: Comparison of Repetitive Element Sequence-Based PCR with Various Typing Methods and Isolation of a Novel Epidemicity Marker, Netherlands. J. Clin. Microbiol. 1999, 37, 342–349. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. An Application of Hierarchical Kappa-type Statistics in the Assessment of Majority Agreement among Multiple Observers, United Kigndom. Biometrics 1977, 33, 363–374. [Google Scholar]
- Hunter, P.R. Reproducibility, and indices of discriminatory power of microbial typing methods, United Kingdom. J. Clin. Microbiol. 1990, 28, 1903–1905. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).