In Vitro and Ex Vivo Evaluation of Rifampicin Cytotoxicity in Human Skin Models
Abstract
1. Introduction
2. Results
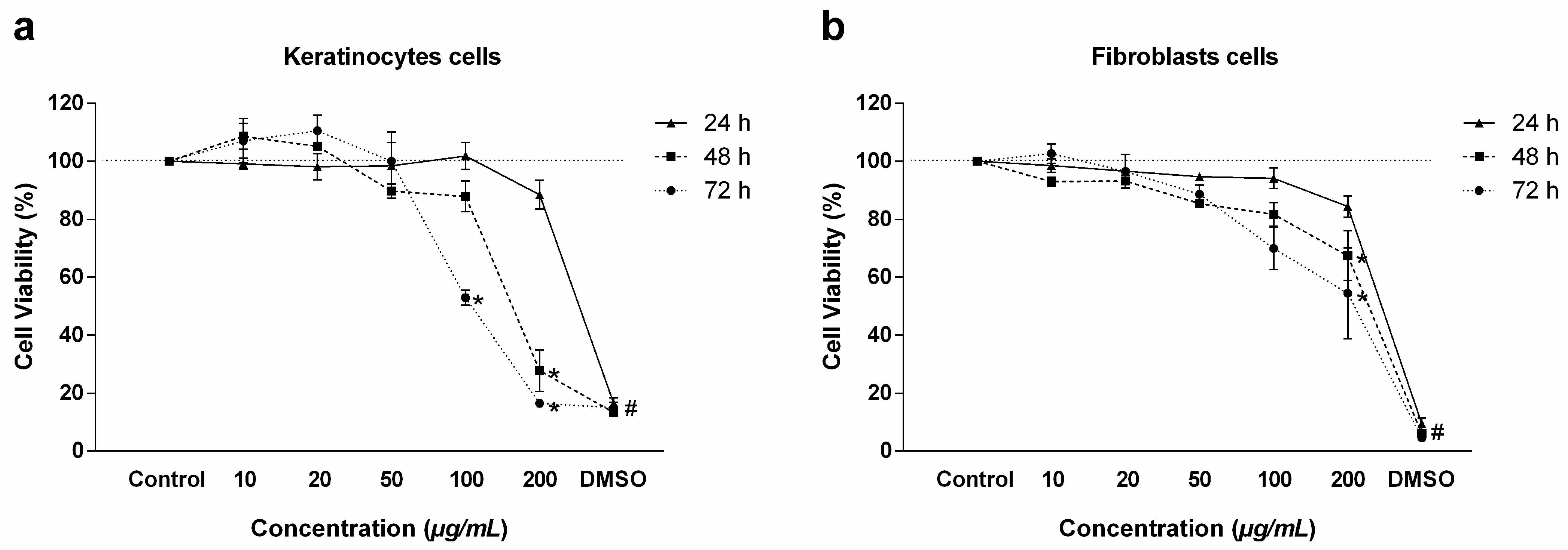
2.1. Cell Viability
2.2. Tissue Viability
2.3. Histological Analyses and Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Lines
4.2.1. Primary Cell Line
4.2.2. Immortalized Cells
4.3. Tissue Culture—hOSEC
4.4. Drug and Dilution
4.5. Cell Viability Assay
4.6. Tissue Viability Assay
4.7. Histological and Apoptosis Studies
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [PubMed]
- Hendriksen, C.F. Replacement, reduction and refinement alternatives to animal use in vaccine potency measurement. Expert Rev. Vaccines 2009, 8, 313–322. [Google Scholar] [PubMed]
- Giacomotto, J.; Ségalat, L. High-throughput screening and small animal models, where are we? Br. J. Pharmacol. 2010, 160, 204–216. [Google Scholar]
- Quantin, P.; Thélu, A.; Catoire, S.; Ficheux, H. Perspectives and strategies of alternative methods used in the risk assessment of personal care products. Ann. Pharm. Fr. 2015, 73, 422–435. [Google Scholar] [PubMed]
- Ferreira, L.M.; Hochman, B.; Barbosa, M.V. Modelos experimentais em pesquisa Experimental models in research. Acta Cir. Bras. 2005, 20, 28–34. [Google Scholar]
- Lebonvallet, N.; Jeanmaire, C.; Danoux, L.; Sibille, P.; Pauly, G.; Misery, L. The evolution and use of skin explants: Potential and limitations for dermatological research. Eur. J. Dermatol. 2010, 20, 671–684. [Google Scholar]
- Ranganatha, N.; Kuppast, I.J. A review on alternatives to animal testing methods in drug development. Int. J. Pharm. Pharm. Sci. 2012, 4, 28–32. [Google Scholar]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar]
- Fell, H.B. Tissue culture and its contribution to biology and medicine. J. Exp. Biol. 1972, 57, 1–13. [Google Scholar]
- Stenn, K.S.; Dvoretzky, I. Human serum and epithelial spread in tissue culture. Arch. Dermatol. Res. 1979, 264, 3–15. [Google Scholar]
- Mathes, S.H.; Ruffner, H.; Graf-Hausner, U. The use of skin models in drug development. Adv. Drug Deliv. Rev. 2014, 69–70, 81–102. [Google Scholar]
- Frade, M.A.C.; Andrade, T.A.M.; Aguiar, A.F.C.L.; Guedes, F.A.; Leite, M.N.; Passos, W.R.; Coelho, E.B.; Das, P.K. Prolonged viability of human organotypic skin explant in culture method (hOSEC). An. Bras. Dermatol. 2015, 90, 347–350. [Google Scholar] [PubMed]
- Leite, M.N.; Viegas, J.S.R.; Praça, F.S.G.; de Paula, N.A.; Ramalho, L.N.Z.; Bentley, M.V.L.B.; Frade, M.A.C. Ex vivo model of human skin (hOSEC) for assessing the dermatokinetics of the anti-melanoma drug Dacarbazine. Eur. J. Pharm. Sci. 2021, 160, 105769. [Google Scholar] [PubMed]
- Andrade, T.A.; Aguiar, A.F.; Guedes, F.A.; Leite, M.N.; Caetano, G.F.; Coelho, E.B.; Das, P.K.; Frade, M.A.C. Ex vivo model of human skin (hOSEC) as alternative to animal use for cosmetic tests. Procedia Eng. 2015, 110, 67–73. [Google Scholar]
- Maggi, N.; Pasqualucci, C.R.; Ballotta, R.; Sensi, P. Rifampicin: A new orally active rifamycin. Chemotherapy 1966, 11, 285–292. [Google Scholar]
- Bacchi, A.; Pelizzi, G.; Nebuloni, M.; Ferrari, P. Comprehensive study on structure-activity relationships of rifamycins: Discussion of molecular and crystal structure and spectroscopic and thermochemical properties of rifamycin O. J. Med. Chem. 1998, 41, 2319–2332. [Google Scholar]
- Zhang, Y. The magic bullets and tuberculosis drug targets. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 529–564. [Google Scholar]
- WHO, World Health Organization. Leprosy (Hansen’s Disease). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/leprosy (accessed on 20 May 2025).
- Andrade, V. Implementação da PQT/OMS no Brazil. Hansen. Int. 2006, 31, 23–31. Available online: https://periodicos.saude.sp.gov.br/index.php/hansenologia/article/view/35212 (accessed on 20 May 2025).
- Cambau, E.; Saunderson, P.; Matsuoka, M.; Cole, S.T.; Kai, M.; Suffys, P.; Rosa, P.S.; Williams, D.; Gupta, U.D.; Lavania, M.; et al. Antimicrobial resistance in leprosy: Results of the first prospective open survey conducted by a WHO surveillance network for the period 2009–15. Clin. Microbiol. Infect. 2018, 24, 1305–1310. [Google Scholar]
- Blass, B.E. (Ed.) In vitro Screening Systems. In Basic Principles of Drug Discovery and Development; Academic Press: Cambridge, MA, USA, 2015; pp. 143–202. [Google Scholar]
- Segeritz, C.P.; Vallier, L. Cell Culture: Growing Cells as Model Systems In Vitro. In Basic Science Methods for Clinical Researchers; Jalali, M., Saldanha, F.Y.L., Jalali, M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 151–172. [Google Scholar]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar]
- Goh, J.-Y.; Weaver, R.J.; Dixon, L.; Platta, N.J.; Roberts, R.A. Development and use of in vitro alternatives to animal testing by the pharmaceutical industry 1980–2013. Toxicol. Res. 2015, 4, 1297–1307. [Google Scholar]
- WHO, World Health Organization. Weekly Epidemiological Record. Nº 35. 2017, Volume 92, pp. 501–520. Available online: http://www.who.int/wer/en/ (accessed on 25 May 2025).
- Grobbelaar, M.; Louw, G.E.; Sampson, S.L.; van Helden, P.D.; Donald, P.R.; Warren, R.M. Evolution of rifampicin treatment for tuberculosis. Infect. Genet. Evol. 2019, 74, 103937. [Google Scholar]
- Niemi, M.; Backman, J.T.; Fromm, M.F.; Neuvonen, P.J.; Kivistö, K.T. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin. Pharmacokinet. 2003, 42, 819–850. [Google Scholar] [PubMed]
- Pastor, D.M.; Poritz, L.S.; Olson, T.L.; Kline, C.L.; Harris, L.R.; Koltun, W.A.; Chinchilli, V.M.; Irby, R.B. Primary cell lines: False representation or model system? a comparison of four human colorectal tumors and their coordinately established cell lines. Int. J. Clin. Exp. Med. 2010, 3, 69–83. [Google Scholar]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar]
- Deyrieux, A.F.; Wilson, V.G. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology 2007, 54, 77–83. [Google Scholar] [PubMed]
- Radko, L.; Olejnik, M.; Posyniak, A. Primary Human Hepatocytes, but Not HepG2 or Balb/c 3T3 Cells, Efficiently Metabolize Salinomycin and Are Resistant to Its Cytotoxicity. Molecules 2020, 25, 1174. [Google Scholar]
- Singh, M.; Sasi, P.; Rai, G.; Gupta, V.H.; Amarapurkar, D.; Wangikar, P.P. Studies on toxicity of antitubercular drugs namely isoniazid, rifampicin, and pyrazinamide in an in vitro model of HepG2 cell line. Med. Chem. Res. 2011, 20, 1611–1615. [Google Scholar]
- Parmar, R.; Misra, R.; Mohanty, S. In vitro controlled release of Rifampicin through liquid-crystalline folate nanoparticles. Colloids Surf. B Biointerfaces 2015, 129, 198–205. [Google Scholar]
- Vostálová, J.; Cukr, M.; Zálešák, B.; Lichnovská, R.; Ulrichová, J.; Svobodová, A.R. Comparison of various methods to analyse toxic effects in human skin explants: Rediscovery of TTC assay. J. Photochem. Photobiol. B 2018, 178, 530–536. [Google Scholar]
- Wang, H.; Maeda, Y.; Fukutomi, Y.; Makino, M. An in vitro model of Mycobacterium leprae induced granuloma formation. BMC Infect. Dis. 2013, 13, 279. [Google Scholar]
- Williams, D.L.; Gillis, T.P. Molecular detection of drug resistance in Mycobacterium leprae. Lepr. Rev. 2004, 75, 118–130. [Google Scholar] [PubMed]
- Davis, G.L.; Ray, N.A.; Lahiri, R.; Gillis, T.P.; Krahenbuhl, J.L.; Williams, D.L.; Adams, L.B. Molecular assays for determining Mycobacterium leprae viability in tissues of experimentally infected mice. PLoS Negl. Trop. Dis. 2013, 7, e2404. [Google Scholar]
- Souto, L.R.M.; Rehder, J.; Vassallo, J.; Cintra, M.L.; Kraemer, M.H.S.; Puzzi, M.B. Model for human skin reconstructed in vitro composed of associated dermis and epidermis. São Paulo Med. J. 2006, 124, 71–76. [Google Scholar]
- Krishnan, K.; Mani, A.; Jasmine, S. Cytotoxic Activity of Bioactive Compound 1,2-Benzene Dicarboxylic Acid, Mono 2-Ethylhexyl Ester Extracted from a Marine Derived Streptomyces sp. VITSJK8. Int. J. Mol. Cell. Med. 2014, 3, 246–254. [Google Scholar] [PubMed]
- Qiao, J.; Wang, S.; Wen, Y.; Jia, H. Photodynamic effects on human periodontal-related cells in vitro. Photodiagnosis Photodyn. Ther. 2014, 11, 290–299. [Google Scholar]
- Mohammadi, E.; Vatanpour, H.; Shirazi, F.H. Immunomodulatory effects of bee venom in human synovial fibroblast cell line. Iran. J. Pharm. Res. 2015, 14, 313–320. [Google Scholar]
- de Lavor, M.S.; Binda, N.S.; Fukushima, F.B.; Caldeira, F.M.; da Silva, J.F.; Silva, C.M.; de Oliveira, K.M.; Martins, B.C.; Torres, B.B.J.; Rosado, I.R.; et al. Ischemia-reperfusion model in rat spinal cord: Cell viability and apoptosis signaling study. Int. J. Clin. Exp. Pathol. 2015, 8, 9941–9949. [Google Scholar]
- Andrade, T.A.M.; Masson-Meyers, D.S.; Caetano, G.F.; Terra, V.A.; Ovidio, P.P.; Jordão-Júnior, A.A.; Frade, M.A.C. Skin changes in streptozotocin-induced diabetic rats. Biochem. Biophys. Res. Commun. 2017, 490, 1154–1161. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leite, M.N.; de Paula, N.A.; Ramalho, L.N.Z.; Frade, M.A.C. In Vitro and Ex Vivo Evaluation of Rifampicin Cytotoxicity in Human Skin Models. Antibiotics 2025, 14, 691. https://doi.org/10.3390/antibiotics14070691
Leite MN, de Paula NA, Ramalho LNZ, Frade MAC. In Vitro and Ex Vivo Evaluation of Rifampicin Cytotoxicity in Human Skin Models. Antibiotics. 2025; 14(7):691. https://doi.org/10.3390/antibiotics14070691
Chicago/Turabian StyleLeite, Marcel Nani, Natália Aparecida de Paula, Leandra Náira Zambelli Ramalho, and Marco Andrey Cipriani Frade. 2025. "In Vitro and Ex Vivo Evaluation of Rifampicin Cytotoxicity in Human Skin Models" Antibiotics 14, no. 7: 691. https://doi.org/10.3390/antibiotics14070691
APA StyleLeite, M. N., de Paula, N. A., Ramalho, L. N. Z., & Frade, M. A. C. (2025). In Vitro and Ex Vivo Evaluation of Rifampicin Cytotoxicity in Human Skin Models. Antibiotics, 14(7), 691. https://doi.org/10.3390/antibiotics14070691





