Outcomes of Implementing a Multidimensional Antimicrobial Stewardship Program in a Medical Ward in a Third-Level University Hospital in Northern Italy
Abstract
1. Introduction
2. Results
2.1. Patient Profiles and Infection Rates
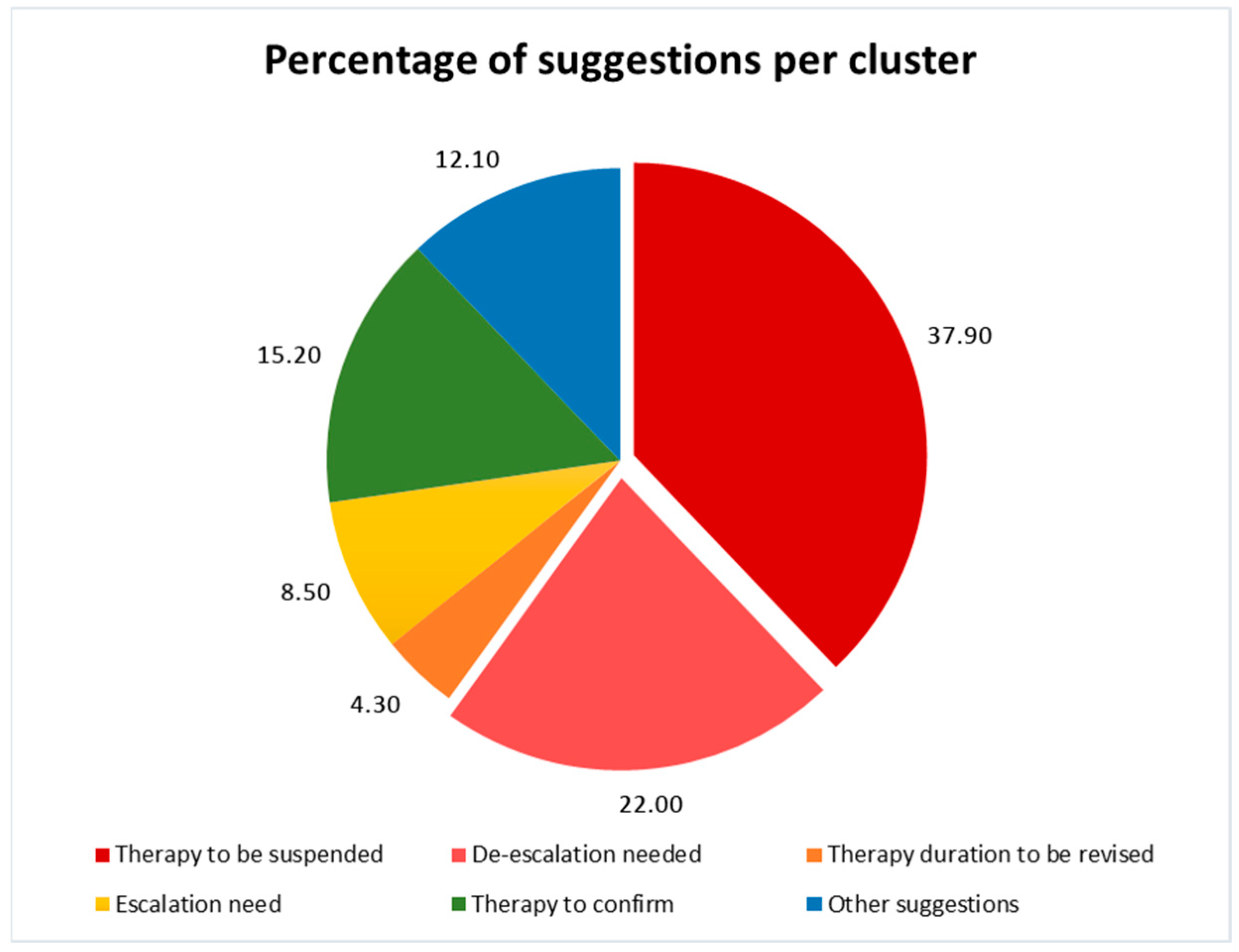
2.2. The Stewardship Program’s Actions and Effects
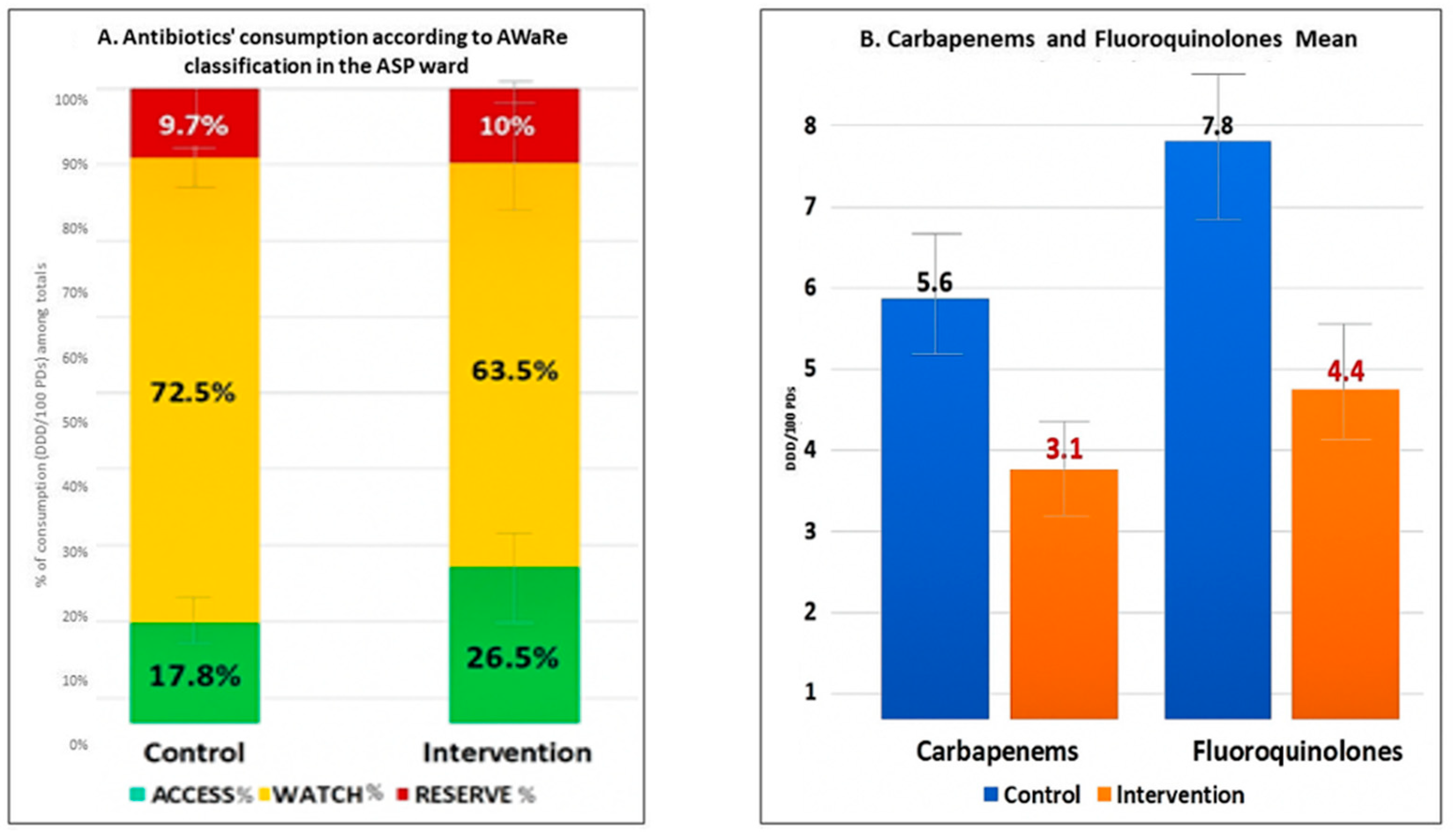
2.3. Improvements in Prescription Quality and Costs
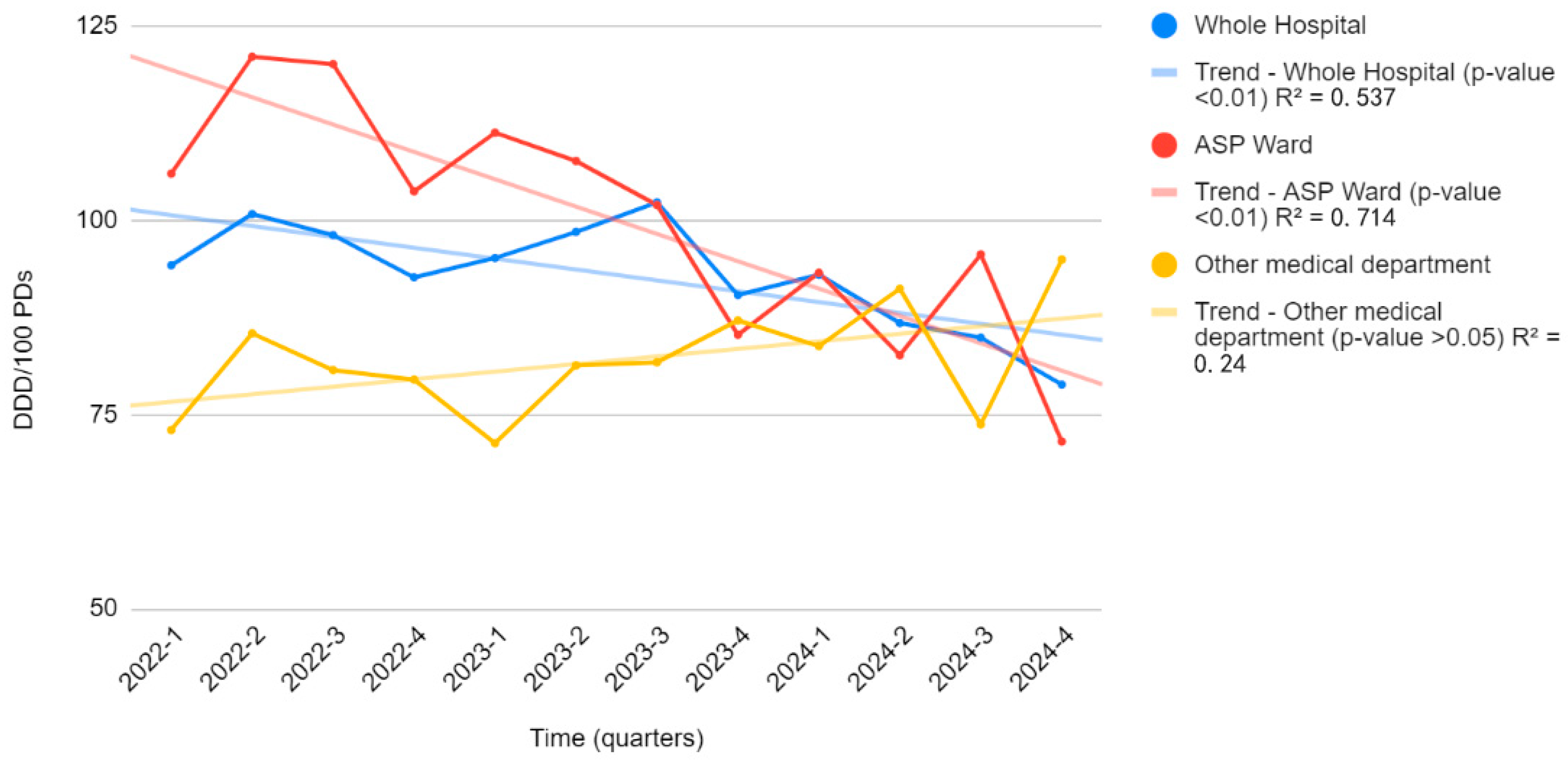
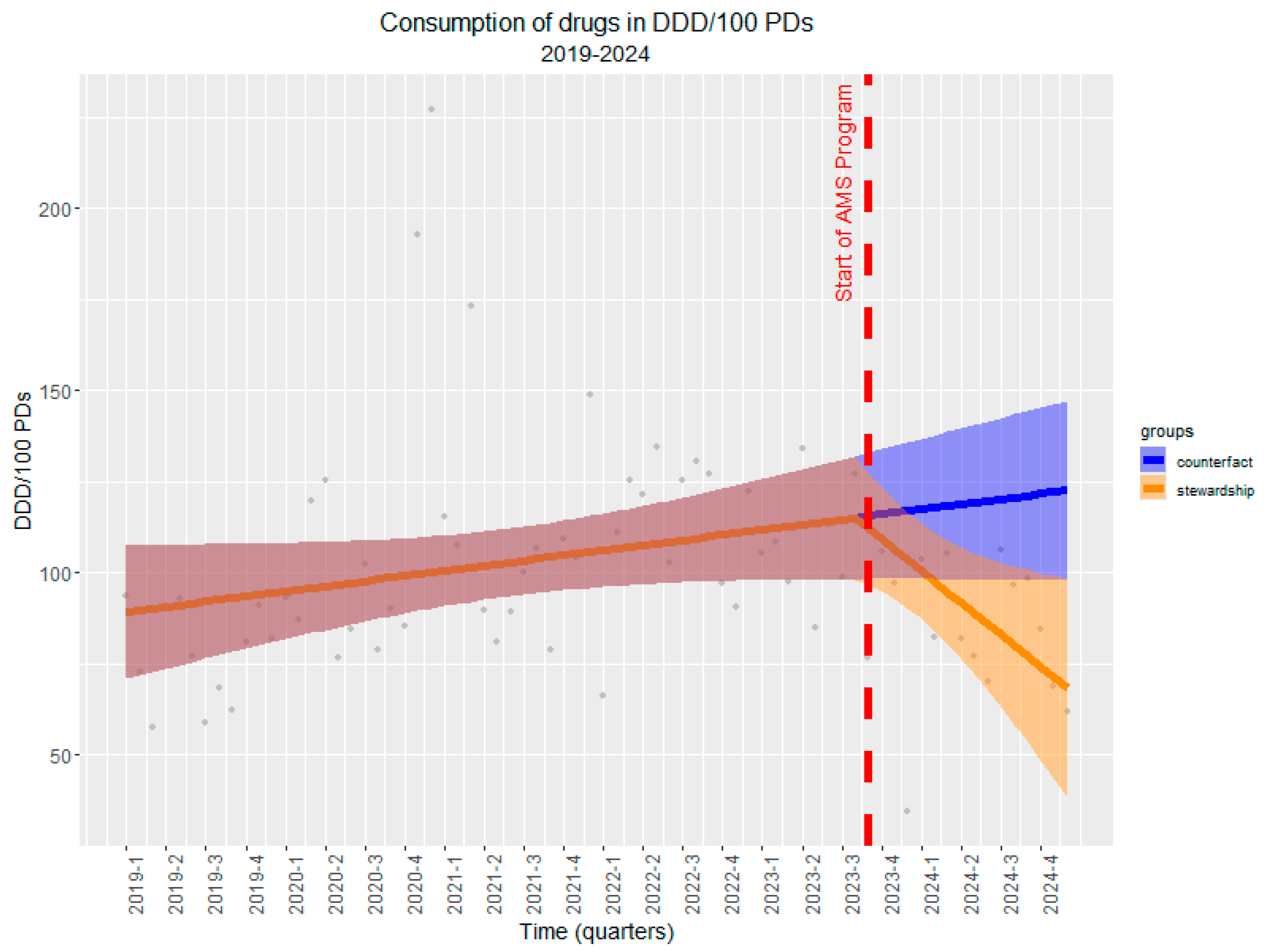
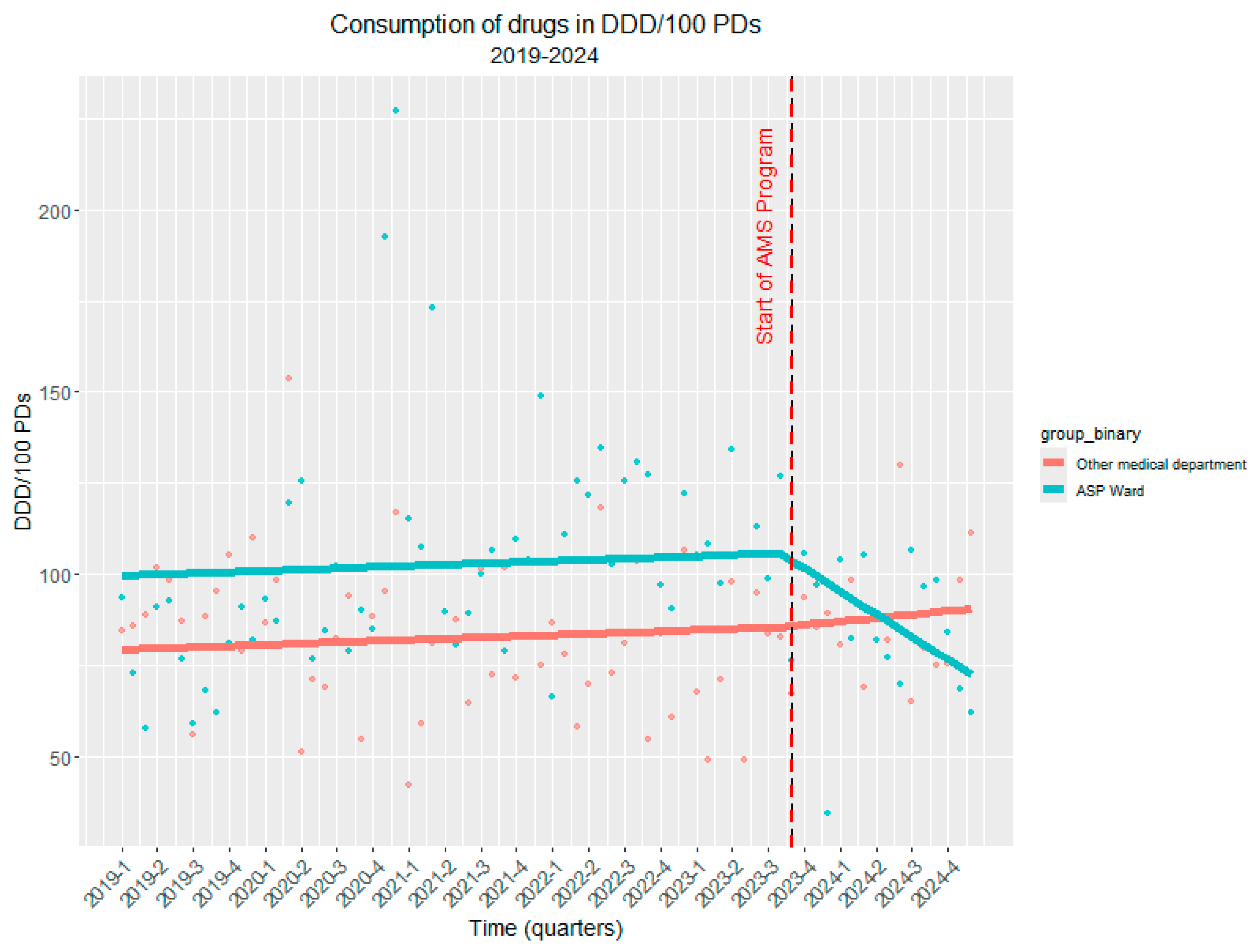
2.4. Long-Term Impact (Interrupted Time Series Analysis)
3. Discussion
Study Limitations and Future Perspectives
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Castagna, T.; Pantolini, B.; Campanardi, M.C.; Roperti, M.; Grotto, A.; Fattori, M.; Dal Maso, L.; Carrara, F.; Zambarbieri, G.; et al. The challenge of antimicrobial resistance (AMR): Current status and future prospects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 9603–9615. [Google Scholar] [CrossRef]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control 2018, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Miyakis, S.; Brentnall, S.; Masso, M.; Reynolds, G.; Byrne, M.K.; Newton, P.; Crawford, S.; Fish, J.; Nicholas, B.; Hill, T.; et al. Key predictors and burden of meticillin-resistant Staphylococcus aureus infection in comparison with meticillin-susceptible S. aureus infection in an Australian hospital setting. J. Hosp. Infect. 2022, 129, 41–48. [Google Scholar] [CrossRef]
- Tsuzuki, S.; Yu, J.; Matsunaga, N.; Ohmagari, N. Length of stay, hospitalisation costs and in-hospital mortality of methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia in Japan. Public Health 2021, 198, 292–296. [Google Scholar] [CrossRef]
- Inagaki, K.; Lucar, J.; Blackshear, C.; Hobbs, C.V. Methicillin-susceptible and Methicillin-resistant Staphylococcus aureus Bacteremia: Nationwide Estimates of 30-Day Readmission, In-hospital Mortality, Length of Stay, and Cost in the United States. Clin. Infect. Dis. 2019, 69, 2112–2118. [Google Scholar] [CrossRef]
- Adebisi, Y.A.; Ogunkola, I.O. The global antimicrobial resistance response effort must not exclude marginalised populations. Trop. Med. Health 2023, 51, 33. [Google Scholar] [CrossRef]
- Zhou, C.; Jin, L.; Wang, Q.; Wang, X.; Chen, F.; Gao, Y.; Zhao, C.; Chen, H.; Cao, B.; Wang, H. Bloodstream Infections Caused by Carbapenem-Resistant Enterobacterales: Risk Factors for Mortality, Antimicrobial Therapy and Treatment Outcomes from a Prospective Multicenter Study. Infect. Drug Resist. 2021, 14, 731–742. [Google Scholar] [CrossRef]
- So-Ngern, A.; Osaithai, N.; Meesing, A.; Chumpangern, W. Mortality rate and factors associated with mortality of carbapenem-resistant Enterobacteriaceae infection. Drug Target Insights 2023, 17, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Endale, H.; Mathewos, M.; Abdeta, D. Potential Causes of Spread of Antimicrobial Resistance and Preventive Measures in One Health Perspective—A Review. Infect. Drug Resist. 2023, 16, 7515–7545. [Google Scholar] [CrossRef]
- Samreen Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Baur, D.; Gladstone, B.P.; Burkert, F.; Carrara, E.; Foschi, F.; Döbele, S.; Tacconelli, E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 990–1001. [Google Scholar] [CrossRef]
- Sartelli, M.; Marini, C.P.; McNelis, J.; Coccolini, F.; Rizzo, C.; Labricciosa, F.M.; Petrone, P. Preventing and Controlling Healthcare-Associated Infections: The First Principle of Every Antimicrobial Stewardship Program in Hospital Settings. Antibiotics 2024, 13, 896. [Google Scholar] [CrossRef] [PubMed]
- Carrara, E.; Sibani, M.; Barbato, L.; Mazzaferri, F.; Salerno, N.D.; Conti, M.; Azzini, A.M.; Dalbeni, A.; Pellizzari, L.; Fontana, G.; et al. How to ‘SAVE’ antibiotics: Effectiveness and sustainability of a new model of antibiotic stewardship intervention in the internal medicine area. Int. J. Antimicrob. Agents 2022, 60, 106672. [Google Scholar] [CrossRef]
- Fortini, A.; Faraone, A.; Di Pietro, M.; Cappugi, C.; Magnante, G.; Boccadori, C.; Bartolini, S.; Rabatti, L. Antimicrobial stewardship in an Internal Medicine ward: Effects on antibiotic consumption and on the use of carbapenems. Intern. Emerg. Med. 2018, 13, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Zay Ya, K.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2253806. [Google Scholar] [CrossRef]
- Naylor, N.R.; Zhu, N.; Hulscher, M.; Holmes, A.; Ahmad, R.; Robotham, J.V. Is antimicrobial stewardship cost-effective? A narrative review of the evidence. Clin. Microbiol. Infect. 2017, 23, 806–811. [Google Scholar] [CrossRef]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef]
- Ku, T.S.N.; Al Mohajer, M.; Newton, J.A.; Wilson, M.H.; Monsees, E.; Hayden, M.K.; Messacar, K.; Kisgen, J.; Diekema, D.J.; Morgan, D.J.; et al. Improving antimicrobial use through better diagnosis: The relationship between diagnostic stewardship and antimicrobial stewardship. Infect. Control Hosp. Epidemiol. 2023, 44, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use; WHO: Geneva, Switzerland, 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 3 January 2025).
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef]
- Mandelli, G.; Dore, F.; Langer, M.; Garbero, E.; Alagna, L.; Bianchin, A.; Ciceri, R.; Paolo, A.D.; Giani, T.; Giugni, A.; et al. Effectiveness of a Multifaced Antibiotic Stewardship Program: A Pre-Post Study in Seven Italian ICUs. J. Clin. Med. 2022, 11, 4409. [Google Scholar] [CrossRef]
- Cox, J.A.; Vlieghe, E.; Mendelson, M.; Wertheim, H.; Ndegwa, L.; Villegas, M.V.; Gould, I.; Levy Hara, G. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017, 23, 812–818. [Google Scholar] [CrossRef]
- Lawes, T.; Lopez-Lozano, J.M.; Nebot, C.A.; Macartney, G.; Subbarao-Sharma, R.; Wares, K.D.; Sinclair, C.; Gould, I.M. Effect of a national 4C antibiotic stewardship intervention on the clinical and molecular epidemiology of Clostridium difficile infections in a region of Scotland: A non-linear time-series analysis. Lancet Infect. Dis. 2017, 17, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Maraolo, A.E.; Mazzitelli, M.; Zappulo, E.; Scotto, R.; Granata, G.; Andini, R.; Durante-Mangoni, E.; Petrosillo, N.; Gentile, I. Oral Vancomycin Prophylaxis for Primary and Secondary Prevention of Clostridioides difficile Infection in Patients Treated with Systemic Antibiotic Therapy: A Systematic Review, Meta-Analysis and Trial Sequential Analysis. Antibiotics 2022, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Huttner, B.; Harbarth, S.; Nathwani, D.; ESCMID Study Group for Antibiotic Policies (ESGAP). Success stories of implementation of antimicrobial stewardship: A narrative review. Clin. Microbiol. Infect. 2014, 20, 954–962. [Google Scholar] [CrossRef]
- Li, H.; Gong, Y.; Han, J.; Zhang, S.; Chen, S.; Xu, X.; Lu, Z.; Yin, X. Interrupted Time-Series Analysis to Evaluate the Impact of a National Antimicrobial Stewardship Campaign on Antibiotic Prescribing: A Typical Practice in China’s Primary Care. Clin. Infect. Dis. 2021, 73, e4463–e4471. [Google Scholar] [CrossRef]
- Morgan, B.L.; Bettencourt, H.; May, L. Interrupted time-series analysis to evaluate the impact of a behavioral change outpatient antibiotic stewardship intervention. Antimicrob. Steward. Healthc. Epidemiol. 2021, 1, e37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ten Oever, J.; Harmsen, M.; Schouten, J.; Ouwens, M.; van der Linden, P.D.; Verduin, C.M.; Kullberg, B.J.; Prins, J.M.; Hulscher, M.E.J.L.; The SWAB Working Group on Antimicrobial Stewardship. Human resources required for antimicrobial stewardship teams: A Dutch consensus report. Clin. Microbiol. Infect. 2018, 24, 1273–1279. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 March 2025).
- Giacobbe, D.R.; Marelli, C.; Cattardico, C.; Fanelli, C.; Signori, A.; Di Meco, G.; Di Pilato, V.; Mikulska, M.; Mazzitelli, M.; Cattelan, A.M.; et al. Mortality in KPC-producing Klebsiella pneumoniae bloodstream infections: A changing landscape. J. Antimicrob. Chemother. 2023, 78, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, M.; Gregori, D.; Sasset, L.; Trevenzoli, M.; Scaglione, V.; Menzo, S.L.; Marinello, S.; Mengato, D.; Venturini, F.; Tiberio, I.; et al. Cefiderocol-Based versus Colistin-Based Regimens for Severe Carbapenem-Resistant Acinetobacter baumannii Infections: A Propensity Score-Weighted, Retrospective Cohort Study during the First Two Years of the COVID-19 Pandemic. Microorganisms. 2023, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; De Pascale, G.; Cascio, A.; De Rosa, F.G.; Cattelan, A.M.; Oliva, A.; Saracino, A.; Bassetti, M.; et al. Outcomes and Predictors of Mortality in Patients With KPC-Kp Infections Treated With Meropenem Vaborbactam: An Observational Multicenter Study. Open Forum Infect. Dis. 2024, 11, ofae273. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Labate, L.; Russo Artimagnella, C.; Marelli, C.; Signori, A.; Di Pilato, V.; Aldieri, C.; Bandera, A.; Briano, F.; Cacopardo, B.; et al. Use of Cefiderocol in Adult Patients: Descriptive Analysis from a Prospective, Multicenter, Cohort Study. Infect. Dis. Ther. 2024, 13, 1929–1948. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Strazzulla, A.; Quirino, A.; Rizzo, C.; Marano, V.; Postorino, M.C.; Mazzitelli, M.; Greco, G.; Pisani, V.; Costa, C.; et al. Patterns of multi-drug-resistant bacteria at first culture from patients admitted to a third level University hospital in Calabria from 2011 to 2014: Implications for empirical therapy and infection control. Infez. Med. 2017, 25, 98–107. [Google Scholar]
- Scaglione, V.; Reale, M.; Davoli, C.; Mazzitelli, M.; Serapide, F.; Lionello, R.; La Gamba, V.; Fusco, P.; Bruni, A.; Procopio, D.; et al. Prevalence of Antibiotic Resistance Over Time in a Third-Level University Hospital. Microb. Drug Resist. 2022, 28, 425–435. [Google Scholar] [CrossRef]

| n Patients per Discharge Period | ||||
|---|---|---|---|---|
| Features | Overall n = 3313 | 2022/2023 (Not ASP Group) n = 1591 | 2023/2024 (ASP Group) n = 1722 | p-Value * |
| Sex, n (%) | 1572 (47.4) | 779 (49) | 793 (46.1) | 0.094 |
| Age, years, median (IQR) | 79 (67–86) | 79 (67–86) | 79 (68–86) | 0.371 |
| Urgent admission, n (%) | 3185 (96.1) | 1536 (96.5) | 1649 (95.8) | 0.243 |
| Length of stay, days, median (IQR) | 7 (4–12) | 7 (5, 12) | 7 (4–12) | 0.077 |
| Need of ICU admission, yes, n (%) | 279 (8.4) | 143 (9) | 136 (7.9) | 0.259 |
| Standard admission weight, median (IQR) | 1.18 (0.91–1.49) | 1.15 (0.89–1.49) | 1.2 (0.94–1.49) | 0.651 |
| Barthel scale value at admission, median (IQR) | 25 (5–55) | 25 (5–55) | 25 (5–55) | 0.085 |
| Type of discharge | 0.201 | |||
| Death, n (%) | 330 (10) | 158 (9.9) | 172 (10) | |
| Home, n (%) | 2035 (61.4) | 997 (62.7) | 1038 (60.3) | |
| Home with need of home care, n (%) | 496 (15) | 236 (14.8) | 260 (15.1) | |
| Nursing home or hospice, n (%) | 314 (9.5) | 142 (8.9) | 172 (10) | |
| Self-discharge against doctor’s advice, n (%) | 37 (1.1) | 25 (1.6) | 12 (0.7) | |
| Transferred for care to other hospital, n (%) | 25 (0.8) | 9 (0.6) | 16 (0.9) | |
| Sent to rehabilitation institutes, n (%) | 47 (1.4) | 22 (1.4) | 25 (1.5) | |
| Other, n (%) | 29 (0.9) | 2 (0.1) | 27 (1.6) | |
| n Patients per Discharge Period | ||||
|---|---|---|---|---|
| Features | Overall n = 3313 | 2022/2023 (Not ASP Group) n = 1591 | 2023/2024 (ASP Group) n = 1722 | p-Value * |
| At least one infection, n (%) | 1624 (49) | 791 (49.7) | 833 (48.4) | 0.440 |
| At least a bacterial infection, n (%) | 1310 (39.5) | 644 (40.5) | 666 (38.7) | 0.289 |
| At least a fungal/protozoal infection, n (%) | 21 (0.6) | 9 (0.6) | 12 (0.7) | 0.635 |
| At least a viral (non-COVID-19) infection, n (%) | 123 (3.7) | 59 (3.7) | 64 (3.7) | 0.990 |
| COVID-19, n (%) | 293 (8.8) | 126 (7.9) | 167 (9.7) | 0.072 |
| Clostridioides difficile, n (%) | 26 (0.8) | 10 (0.6) | 16 (0.9) | 0.327 |
| Type of infection n (%) | ||||
| Pneumonia | 586 (17.7) | 269 (16.9) | 317 (18.4) | 0.254 |
| Bloodstream infection | 415 (12.5) | 198 (12.4) | 217 (12.6) | 0.892 |
| Septic shock | 135 (4.1) | 57 (3.6) | 78 (4.5) | 0.168 |
| Urinary tract infection/pyelonephritis | 316 (9.5) | 170 (10.7) | 146 (8.5) | 0.031 |
| Septic arthritis | 5 (0.2) | 5 (0.3) | 0 (0) | 0.025 |
| Endocarditis | 15 (0.5) | 5 (0.3) | 10 (0.6) | 0.254 |
| Intra-abdominal infection | 84 (2.5) | 45 (2.8) | 39 (2.3) | 0.303 |
| Skin structure and soft tissue infection | 87 (2.6) | 40 (2.5) | 47 (2.7) | 0.699 |
| Meningitis | 5 (0.2) | 4 (0.3) | 1 (0.1) | 0.201 |
| Prosthetic infection | 9 (0.3) | 7 (0.4) | 2 (0.1) | 0.097 |
| Prostatitis | 4 (0.1) | 2 (0.1) | 2 (0.1) | 0.99 |
| Osteomyelitis | 12 (0.4) | 8 (0.5) | 4 (0.2) | 0.195 |
| Other | 147 (4.4) | 80 (5) | 67 (3.9) | 0.112 |
| N infections/person, n (%) | 0.785 | |||
| 0 | 1689(51.0) | 800 (50.3) | 889 (51.6) | |
| 1 | 944(28.5) | 464 (29.2) | 480 (27.9) | |
| 2 | 577 (17.4 | 282 (17.7) | 295 (17.1) | |
| 3 | 94 (2.8) | 41 (2.6) | 53 (3.1) | |
| 4 | 9 (0.3) | 4 (0.3) | 5 (0.3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzitelli, M.; Mengato, D.; Barbato, G.; Lo Menzo, S.; Dalla Valle, F.; Boschetto, M.; Stano, P.; Contessa, C.; Donà, D.; Scaglione, V.; et al. Outcomes of Implementing a Multidimensional Antimicrobial Stewardship Program in a Medical Ward in a Third-Level University Hospital in Northern Italy. Antibiotics 2025, 14, 683. https://doi.org/10.3390/antibiotics14070683
Mazzitelli M, Mengato D, Barbato G, Lo Menzo S, Dalla Valle F, Boschetto M, Stano P, Contessa C, Donà D, Scaglione V, et al. Outcomes of Implementing a Multidimensional Antimicrobial Stewardship Program in a Medical Ward in a Third-Level University Hospital in Northern Italy. Antibiotics. 2025; 14(7):683. https://doi.org/10.3390/antibiotics14070683
Chicago/Turabian StyleMazzitelli, Maria, Daniele Mengato, Gianmaria Barbato, Sara Lo Menzo, Fabio Dalla Valle, Margherita Boschetto, Paola Stano, Cristina Contessa, Daniele Donà, Vincenzo Scaglione, and et al. 2025. "Outcomes of Implementing a Multidimensional Antimicrobial Stewardship Program in a Medical Ward in a Third-Level University Hospital in Northern Italy" Antibiotics 14, no. 7: 683. https://doi.org/10.3390/antibiotics14070683
APA StyleMazzitelli, M., Mengato, D., Barbato, G., Lo Menzo, S., Dalla Valle, F., Boschetto, M., Stano, P., Contessa, C., Donà, D., Scaglione, V., Berti, G., Giunco, E. M., Martello, T., Venturini, F., Castagliuolo, I., Tessarin, M., Simioni, P., & Cattelan, A. (2025). Outcomes of Implementing a Multidimensional Antimicrobial Stewardship Program in a Medical Ward in a Third-Level University Hospital in Northern Italy. Antibiotics, 14(7), 683. https://doi.org/10.3390/antibiotics14070683





