Use, Risk and Revalorization of Veterinary Antibiotics: A Canadian Perspective
Abstract
1. Introduction
2. Antibiotic Mechanisms
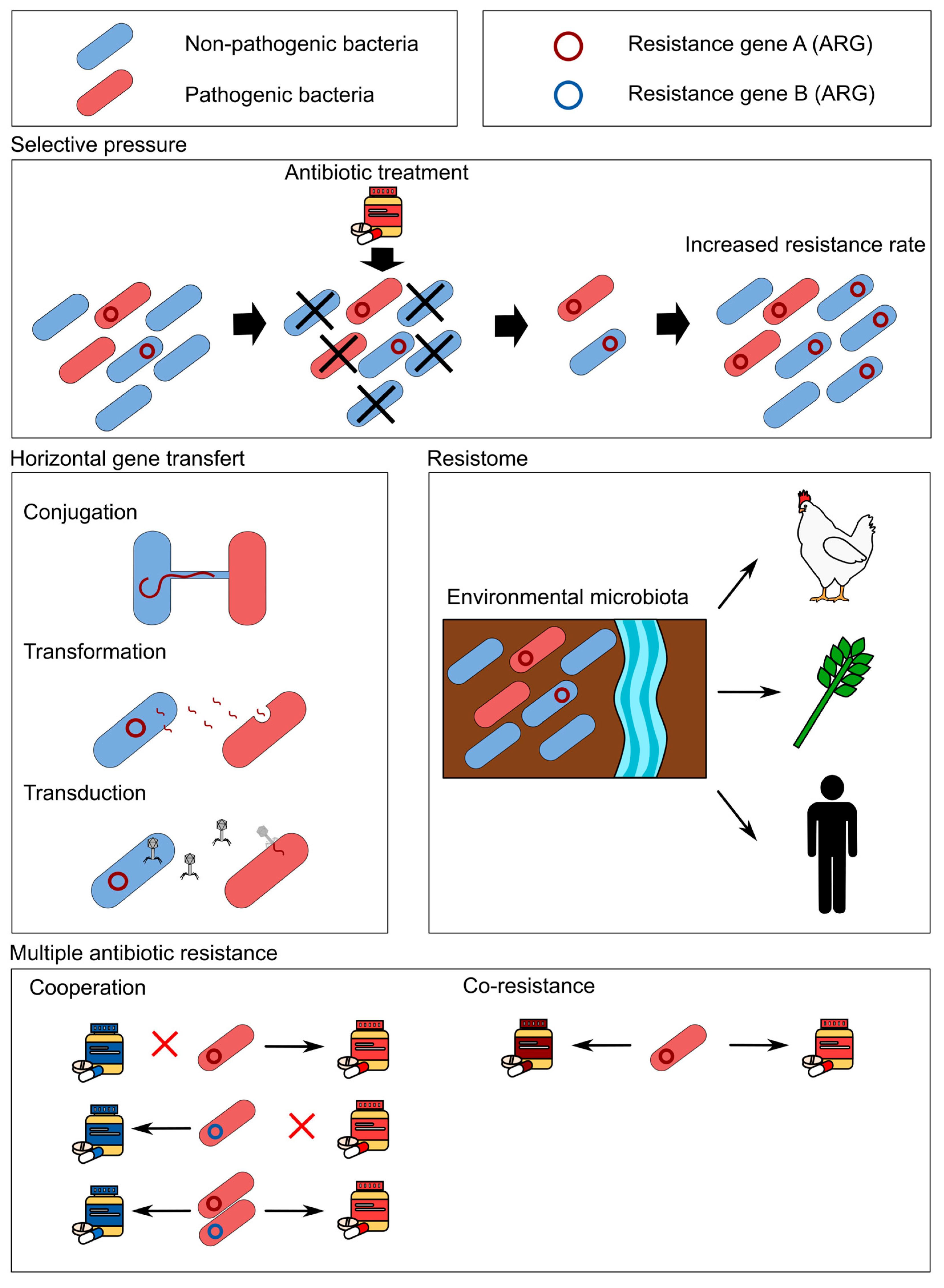
3. The Ubiquitous Use of Antibiotics and Antibiotic Resistance
4. The Canadian Regulation and Surveillance of Veterinary Antibiotics Use in Livestock
5. Discharge of ARGs and ARM from Animal Production
| Antibiotic | Active Ingredient | Animal | Dosage | Excretion (%) | References |
|---|---|---|---|---|---|
| Tetracyclines | Tetracycline | Pigs | Not reported | 25–80 | Feinman 1978 as cited by [66,67] |
| Chlortetracycline | 40 mg per kg bw | 65–75 | Veterinrermedicinsk Produktkatalog 1999, as cited by [68,69] | ||
| Oxytetracycline | 5–25 mg per kg bw | 23–65 | [70]; Veterinrermedicinsk Produktkatalog 1999, as cited by [68] | ||
| Lincosamides | Lincomycin | Not reported | 60 | Aiello 1998 as cited by [51] | |
| Macrolides | Tylosin | Pigs & cattle | 5–10 mg per kg bw | 40–100 | Feinman 1978 as cited by [66]; Veterinrermedicinsk Produktkatalog 1999 as cited by [68] |
| Sulfonamide | Metronidazole | Not reported | 40 | Kümmerer et al. 2000 as cited by [51] | |
| Sulfadiazine | Pigs | 200 mg per 15 kg bw | 90 | Veterinrermedicinsk Produktkatalog 1999 as cited by [68] | |
| Sulfatroxazole | Cattle | 200 mg per 15 kg bw | 90 | ||
| Sulfadoxine | 200 mg per 15 kg bw | 90 | |||
| Sulfapyrazole | 50–70 mg per kg bw | 90 | |||
| Chloroquine | Not reported | 70 | Goldsmith 1992 as cited by [51] | ||
| β-lactam | Penicillin G | Pigs | 10–20 mg per kg bw | 90 | Veterinrermedicinsk Produktkatalog 1999 as cited by [68] |
| Amoxicillin | Cattle | 10–30 mg per kg bw | 90 | ||
| Ampicillin | 15–30 mg per kg bw | 90 | |||
| Quinolones | Enrofloxacin | Pigs & cattle | 5–10 mg per kg bw | 35 | Veterinrermedicinsk Produktkatalog 1999 as cited by [68] |
| Norfloxacin | Not reported | 30 |
6. Fate of Antibiotic Contaminants from Animal Production
6.1. Degradation in Soil
6.2. Effect of Manure Treatment on Antibiotics
6.2.1. Composting Effect on Antibiotics
6.2.2. Anaerobic Digestion on Antibiotics
7. A Revalorization Avenue for Antibiotic Contaminated Agricultural Waste
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, E.Y.; Impalli, I.; Poleon, S.; Denoel, P.; Cipriano, M.; Van Boeckel, T.P.; Pecetta, S.; Bloom, D.E.; Nandi, A. Global Trends in Antibiotic Consumption during 2016–2023 and Future Projections through 2030. Proc. Natl. Acad. Sci. USA 2024, 121, e2411919121. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System (CARSS): 2024 Key Findings; Public Health Agency of Canada: Ottawa, ON, Canada, 2024. [Google Scholar]
- Scott, A.M.; Beller, E.; Glasziou, P.; Clark, J.; Ranakusuma, R.W.; Byambasuren, O.; Bakhit, M.; Page, S.W.; Trott, D.; Mar, C.D. Is Antimicrobial Administration to Food Animals a Direct Threat to Human Health? A Rapid Systematic Review. Int. J. Antimicrob. Agents 2018, 52, 316–323. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. In Antimicrobial Resistance in Bacteria from Livestock and Companion Animals; Schwarz, S., Cavaco, L.M., Shen, J., Eds.; ASM Press: Washington, DC, USA, 2018; pp. 521–547. ISBN 978-1-68367-052-0. [Google Scholar]
- Mercer, M. Antimicrobial Drug Factors for Animals. Available online: https://www.merckvetmanual.com/pharmacology/antimicrobials/antimicrobial-drug-factors-for-animals (accessed on 6 December 2024).
- Samuelson, J. Why Metronidazole Is Active against Both Bacteria and Parasites. Antimicrob. Agents Chemother. 1999, 43, 1533–1541. [Google Scholar] [CrossRef]
- Salam, M.; Al-Amin, M.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.G.; Oliveira, J.T.A.; Amaral, J.L.; Freitas, C.D.T.; Souza, P.F.N. Synthetic Antimicrobial Peptides: Characteristics, Design, and Potential as Alternative Molecules to Overcome Microbial Resistance. Life Sci. 2021, 278, 119647. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M. Antibiotic Action and Resistance: Updated Review of Mechanisms, Spread, Influencing Factors, and Alternative Approaches for Combating Resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef]
- Ritchie, D.J. Antimicrobial Pharmacodynamics in Theory and Clinical Practice, Second Edition: Antimicrobial Pharmacody-Namics in Theory and Clinical Practice, Second Edition. Clin. Infect. Dis. 2008, 46, 1942. [Google Scholar] [CrossRef]
- Asano, D.; Takakusa, H.; Nakai, D. Oral Absorption of Middle-to-Large Molecules and Its Improvement, with a Focus on New Modality Drugs. Pharmaceutics 2023, 16, 47. [Google Scholar] [CrossRef]
- Council of Canadian Academies. When Antibiotics Fail; The Expert Panel on the Potential Socio-Economic Impacts of Antimicrobial Resistance in Canada: Ottawa, ON, Canada, 2019; ISBN 978-1-926522-75-3. [Google Scholar]
- Burstein, R.; Henry, N.J.; Collison, M.L.; Marczak, L.B.; Sligar, A.; Watson, S.; Marquez, N.; Abbasalizad-Farhangi, M.; Abbasi, M.; Abd-Allah, F. Mapping 123 Million Neonatal, Infant and Child Deaths between 2000 and 2017. Nature 2019, 574, 353–358. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and Its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D. Restricting the Use of Antibiotics in Food-Producing Animals and Its Associations with Antibiotic Re-Sistance in Food-Producing Animals and Human Beings: A Systematic Review and Meta-Analysis. Lancet Planet. Health 2017, 1, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Saucier, L.M.S. Quality and Safety within the Context of Meat Sustainability. Meat Sci. 2016, 120, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ali Kamboh, A. (Ed.) Antibiotics and Probiotics in Animal Food: Impact and Regulation; Veterinary Medicine and Science; IntechOpen: London, UK, 2023; ISBN 978-1-80356-589-7. [Google Scholar]
- Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing Antimicrobial Use in Food Animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System Report 2021; Public Health Agency of Canada: Ottawa, ON, Canada, 2021; ISBN 2369-0712. [Google Scholar]
- Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System—2022; Public Health Agency of Canada: Ottawa, ON, Canada, 2022; ISBN 2369-0720. [Google Scholar]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic Use in Plant Agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.M. Plasmid Encoded Antibiotic Resistance: Acquisition and Transfer of Antibiotic Resistance Genes in Bacteria. Br. J. Pharmacol. 2008, 153, S347–S357. [Google Scholar] [CrossRef]
- Zhang, S.; Abbas, M.; Rehman, M.U.; Huang, Y.; Zhou, R.; Gong, S.; Yang, H.; Chen, S.; Wang, M.; Cheng, A. Dissemination of Antibiotic Resistance Genes (ARGs) via Integrons in Escherichia Coli: A Risk to Human Health. Environ. Pollut. 2020, 266, 115260. [Google Scholar] [CrossRef]
- Von Wintersdorff, C.J.H.; Penders, J.; Van Niekerk, J.M.; Mills, N.D.; Majumder, S.; Van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, 00088-17. [Google Scholar] [CrossRef]
- Aminov, R.I.; Mackie, R.I. Evolution and Ecology of Antibiotic Resistance Genes. FEMS Microbiol. Lett. 2007, 271, 147–161. [Google Scholar] [CrossRef]
- León-Sampedro, R.; DelaFuente, J.; Díaz-Agero, C.; Crellen, T.; Musicha, P.; Rodríguez-Beltrán, J.; De La Vega, C.; Hernández-García, M.; R-GNOSIS WP5 Study Group; López-Fresneña, N.; et al. Pervasive Transmission of a Carbapenem Resistance Plasmid in the Gut Microbiota of Hospitalized Patients. Nat. Microbiol. 2021, 6, 606–616. [Google Scholar] [CrossRef]
- Chang, H.-H.; Cohen, T.; Grad, Y.H.; Hanage, W.P.; O’Brien, T.F.; Lipsitch, M. Origin and Proliferation of Multiple-Drug Resistance in Bacterial Pathogens. Microbiol. Mol. Biol. Rev. J. 2015, 79, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Simjee, S.; Weese, J.S.; Singh, R.; Trott, D.J.; Essack, S.; Chuanchuen, R.; Mehrotra, S. Redefining Cross-Resistance, Co-Resistance and Co-Selection: Beyond Confusion? J. Antimicrob. Chemother. 2024, 79, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Ruiz-Garbajosa, P. Co-Resistance: An Opportunity for the Bacteria and Resistance Genes. Curr. Opin. Pharmacol. 2011, 11, 477–485. [Google Scholar] [CrossRef]
- Poole, K. Efflux Pumps as Antimicrobial Resistance Mechanisms. Ann. Med. 2007, 39, 162–176. [Google Scholar] [CrossRef]
- Jang, S. AcrAB-TolC, a Major Efflux Pump in Gram Negative Bacteria: Toward Understanding Its Operation Mechanism. BMB Rep. 2023, 56, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Périchon, B.; Courvalin, P.; Stratton, C.W. Antibiotic Resistance☆. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2015; p. B9780128012383023850. ISBN 978-0-12-801238-3. [Google Scholar]
- Frost, I.; Smith, W.P.J.; Mitri, S.; Millan, A.S.; Davit, Y.; Osborne, J.M.; Pitt-Francis, J.M.; MacLean, R.C.; Foster, K.R. Cooperation, Competition and Antibiotic Resistance in Bacterial Colonies. ISME J. 2018, 12, 1582–1593. [Google Scholar] [CrossRef]
- Yurtsev, E.A.; Chao, H.X.; Datta, M.S.; Artemova, T.; Gore, J. Bacterial Cheating Drives the Population Dynamics of Cooperative Antibiotic Resistance Plasmids. Mol. Syst. Biol. 2013, 9, 683. [Google Scholar] [CrossRef]
- Agence de santé publique du Canada. Programme intégré Canadien de Surveillance de la Résistance aux Antimicrobiens (PICRA): 2017 Figures et Tableaux; Agence de santé publique du Canada: Ottawa, ON, Canada, 2019; ISBN 978-0-660-32674-0. [Google Scholar]
- Kirchhelle, C. Pharming Animals: A Global History of Antibiotics in Food Production (1935–2017). Palgrave Commun. 2018, 4, 96. [Google Scholar] [CrossRef]
- Phillips, I. Does the Use of Antibiotics in Food Animals Pose a Risk to Human Health? A Critical Review of Published Data. J. Antimicrob. Chemother. 2003, 53, 28–52. [Google Scholar] [CrossRef]
- Monger, X.C.; Gilbert, A.-A.; Saucier, L.; Vincent, A.T. Antibiotic Resistance: From Pig to Meat. Antibiotics 2021, 10, 1209. [Google Scholar] [CrossRef]
- Garcia-Alvarez, L.; Dawson, S.; Cookson, B.; Hawkey, P. Working across the Veterinary and Human Health Sectors. J. Antimicrob. Chemother. 2012, 67, 37–49. [Google Scholar] [CrossRef]
- Gompel, L.; Luiken, R.E.C.; Hansen, R.B.; Munk, P.; Bouwknegt, M.; Heres, L.; Greve, G.D.; Scherpenisse, P.; Jongeri-us-Gortemaker, B.G.M.; Tersteeg-Zijderveld, M.H.G. Description and Determinants of the Faecal Resistome and Mi-Crobiome of Farmers and Slaughterhouse Workers: A Metagenome-Wide Cross-Sectional Study. Environ. Int. 2020, 143, 105939. [Google Scholar] [CrossRef]
- Gao, F.-Z.; He, L.-Y.; He, L.-X.; Zou, H.-Y.; Zhang, M.; Wu, D.-L.; Liu, Y.-S.; Shi, Y.-J.; Bai, H.; Ying, G.-G. Untreated Swine Wastes Changed Antibiotic Resistance and Microbial Community in the Soils and Impacted Abundances of Antibiotic Resistance Genes in the Vegetables. Sci. Total Environ. 2020, 741, 140482. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; He, L.-Y.; Wu, D.-L.; Gao, F.-Z.; Zhang, M.; Zou, H.-Y.; Yao, M.-S.; Ying, G.-G. Spread of Airborne Antibiotic Resistance from Animal Farms to the Environment: Dispersal Pattern and Exposure Risk. Environ. Int. 2022, 158, 106927. [Google Scholar] [CrossRef]
- Wang, B.; Song, L.; Li, W.; Hou, L.; Li, J.; Xu, X.; Sheng, G. Distribution and Migration of Antibiotic Resistance Genes, as Well as Their Correlation with Microbial Communities in Swine Farm and Its Surrounding Environments. Environ. Pollut. 2023, 316, 120618. [Google Scholar] [CrossRef]
- Lanoie, P.; Rivard, C.; Bellemare, J.; Beaulieu, M.; Grenier, J.; Lacouline, F. Rapport Du Vérificateur Général Du Québec à l’Assemblée Nationale Pour l’année 2019–2020: Utilisation Des Antibiotiques Chez Les Animaux Destinés à l’alimentation; Ministère de l’Agriculture, des Pêcheries et de l’Alimentation: Montreal, QC, Canada, 2019. [Google Scholar]
- Dutil, L.; Irwin, R.; Finley, R.; Ng, L.K.; Avery, B.; Boerlin, P.; Bourgault, A.-M.; Cole, L.; Daignault, D.; Desruisseau, A. Ceftiofur Resistance in Salmonella Enterica Serovar Heidelberg from Chicken Meat and Humans, Canada. Emerg. Infect. Dis. 2010, 16, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Léger, D.F.; Carson, C.A.; Gow, S.P.; Bosman, A.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial Use Surveillance in Broiler Chicken Flocks in Canada, 2013–2015. PLoS ONE 2017, 12, 0179384. [Google Scholar] [CrossRef]
- Health Canada Categorization of Antimicrobial Drugs Based on Importance in Human Medicine. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html (accessed on 20 June 2025).
- Tableau 32-10-0130-01; Nombre de Bovins, Selon la Classe et le Type D’Exploitation Agricole. Statistique Canada: Ottawa, ON, Canada, 2025.
- Burow, E.; Simoneit, C.; Tenhagen, B.-A.; Käsbohrer, A. Oral Antimicrobials Increase Antimicrobial Resistance in Porcine E Coli—A Systematic Review. Prev. Vet. Med. 2014, 113, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Gupta, S.C.; Chander, Y.; Singh, A.K. Antibiotic Use in Agriculture and Its Impact on the Terrestrial Environment. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 87, pp. 1–54. ISBN 978-0-12-000785-1. [Google Scholar]
- Bao, Y.; Zhou, Q.; Guan, L.; Wang, Y. Depletion of Chlortetracycline during Composting of Aged and Spiked Manures. Waste Manag. 2009, 29, 1416–1423. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A.M. The Analysis of Animal Faeces as a Tool to Monitor Antibiotic Usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Lu, C.; Liao, Q.; Gudda, F.O.; Ling, W. Antibiotics in Animal Manure and Manure-Based Fertilizers: Oc-Currence and Ecological Risk Assessment. Chemosphere 2020, 255, 127006. [Google Scholar] [CrossRef] [PubMed]
- Frey, L.; Tanunchai, B.; Glaser, B. Antibiotics Residues in Pig Slurry and Manure and Its Environmental Contamination Po-Tential. A Meta-Analysis. Agron. Sustain. Dev. 2022, 42, 31. [Google Scholar] [CrossRef]
- Xi, C.; Zhang, Y.; Marrs, C.F.; Ye, W.; Simon, C.; Foxman, B.; Nriagu, J. Prevalence of Antibiotic Resistance in Drinking Water Treatment and Distribution Systems. Appl. Environ. Microbiol. 2009, 75, 5714–5718. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.P.; Nachman, K.E. Managing Waste from Confined Animal Feeding Operations in the United States: The Need for Sanitary Reform. J. Water Health 2010, 8, 646–670. [Google Scholar] [CrossRef]
- Graham, J.P.; Price, L.B.; Evans, S.L.; Graczyk, T.K.; Silbergeld, E.K. Antibiotic Resistant Enterococci and Staphylococci Isolated from Flies Collected near Confined Poultry Feeding Operations. Sci. Total Environ. 2009, 407, 2701–2710. [Google Scholar] [CrossRef]
- Zhang, H.; Schroder, J. Animal Manure Production and Utilization in the US. In Applied Manure and Nutrient Chemistry for Sustainable Agriculture and Environment; He, Z., Zhang, H., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–21. ISBN 978-94-017-8806-9. [Google Scholar]
- Kümmerer, K. Antibiotics in the Environment. In Pharmaceuticals in the Environment; Kümmerer, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 75–93. ISBN 978-3-540-74663-8. [Google Scholar]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Ma, L.; Li, B.; Jiang, X.-T.; Wang, Y.-L.; Xia, Y.; Li, A.-D.; Zhang, T. Catalogue of Antibiotic Resistome and Host-Tracking in Drinking Water Deciphered by a Large Scale Survey. Microbiome 2017, 5, 154. [Google Scholar] [CrossRef]
- Marszałek, M.; Kowalski, Z.; Makara, A. The Possibility of Contamination of Water-Soil Environment as a Result of the Use of Pig Slurry. Ecol. Chem. Eng. S 2019, 26, 313–330. [Google Scholar] [CrossRef]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef]
- Nkoh, J.N.; Shang, C.; Okeke, E.S.; Ejeromedoghene, O.; Oderinde, O.; Etafo, N.O.; Mgbechidinma, C.L.; Bakare, O.C.; Meugang, E.F. Antibiotics Soil-Solution Chemistry: A Review of Environmental Behavior and Uptake and Transformation by Plants. J. Environ. Manag. 2024, 354, 120312. [Google Scholar] [CrossRef]
- Zubair, M.; Li, Z.; Zhu, R.; Wang, J.; Liu, X.; Liu, X. The Antibiotics Degradation and Its Mechanisms During the Livestock Manure Anaerobic Digestion. Molecules 2023, 28, 4090. [Google Scholar] [CrossRef] [PubMed]
- Winckler, C.; Grafe, A. Use of Veterinary Drugs in Intensive Animal Production: Evidence for Persistence of Tetracycline in Pig Slurry. J. Soils Sediments 2001, 1, 66–70. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Jensen, J.; Tjørnelund, J.; Montforts, M.H.M.M. Worst-Case Estimations of Predicted Environmental Soil Concentrations (PEC) of Selected Veterinary Antibiotics and Residues Used in Danish Agriculture. In Pharmaceuticals in the Environment; Kümmerer, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 143–157. ISBN 978-3-662-04636-4. [Google Scholar]
- Elmund, G.K.; Morrison, S.M.; Grant, D.W.; Nevins, M.P. Role of Excreted Chlortetracycline in Modifying the Decomposition Process in Feedlot Waste. Bull. Environ. Contam. Toxicol. 1971, 6, 129–132. [Google Scholar] [CrossRef]
- Arikan, O.A. Degradation and Metabolization of Chlortetracycline during the Anaerobic Digestion of Manure from Medicated Calves. J. Hazard. Mater. 2008, 158, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Søeborg, T.; Ingerslev, F.; Halling-Sørensen, B. Chemical Stability of Chlortetracycline and Chlortetracycline Degradation Products and Epimers in Soil Interstitial Water. Chemosphere 2004, 57, 1515–1524. [Google Scholar] [CrossRef]
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP Effluents and Their Solar Photodegradation in Aquatic Envi-Ronment. Chemosphere 2003, 50, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Kemper, N.; Färber, H.; Skutlarek, D.; Krieter, J. Analysis of Antibiotic Residues in Liquid Manure and Leachate of Dairy Farms in Northern Germany. Agric. Water Manag. 2008, 95, 1288–1292. [Google Scholar] [CrossRef]
- Kim, S.; Eichhorn, P.; Jensen, J.N.; Weber, A.S.; Aga, D.S. Removal of Antibiotics in Wastewater: Effect of Hydraulic and Solid Retention Times on the Fate of Tetracycline in the Activated Sludge Process. Environ. Sci. Technol. 2005, 39, 5816–5823. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, D.; Zheng, Y.; Yang, Y.; He, Y.; Luo, L.; Zhou, Y. Current Progress in the Adsorption Transport and Biodegradation of Antibiotics in Soil. J. Environ. Manag. 2019, 251, 109598. [Google Scholar] [CrossRef]
- Wen, A.; Wang, H.; Yuan, S.; Yu, H.; Guo, Y.; Yao, W. Underestimation of Tetracycline Antibiotic Residues in Chicken Meat: The Role of Protein Binding. Food Chem. 2025, 463, 141057. [Google Scholar] [CrossRef]
- Statistics Canada. Manure Storage in Canada; Statistics Canada: Ottawa, ON, Canada, 2003; Volume 1, ISBN 0-662-34832-X. [Google Scholar]
- Table 32-10-0476-01; Solid Manure Management Practices, by Farm Production Type 2023. Statistics Canada: Ottawa, ON, Canada, 2023.
- Graham, J.P.; Evans, S.L.; Price, L.B.; Silbergeld, E.K. Fate of Antimicrobial-Resistant Enterococci and Staphylococci and Re-Sistance Determinants in Stored Poultry Litter. Environ. Res. 2009, 109, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, L.-Y.; Liu, Y.-S.; Zhao, J.-L.; Liu, W.-R.; Zhang, J.-N.; Chen, J.; He, L.-K.; Zhang, Q.-Q.; Ying, G.-G. Fate of Vet-Erinary Antibiotics during Animal Manure Composting. Sci. Total Environ. 2019, 650, 1363–1370. [Google Scholar] [CrossRef]
- Storteboom, H.N.; Kim, S.; Doesken, K.C.; Carlson, K.H.; Davis, J.G.; Pruden, A. Response of Antibiotics and Resistance Genes to High-Intensity and Low-Intensity Manure Management. J. Environ. Qual. 2007, 36, 1695–1703. [Google Scholar] [CrossRef]
- Beaulieu, M.S. Manure Management in Canada; Statistics Canada: Ottawa, ON, Canada, 2004. [Google Scholar]
- Zou, Y.; Xiao, Y.; Wang, H.; Fang, T.; Dong, P. New Insight into Fates of Sulfonamide and Tetracycline Resistance Genes and Resistant Bacteria during Anaerobic Digestion of Manure at Thermophilic and Mesophilic Temperatures. J. Hazard. Mater. 2020, 384, 121433. [Google Scholar] [CrossRef] [PubMed]
- Gurmessa, B.; Pedretti, E.F.; Cocco, S.; Cardelli, V.; Corti, G. Manure Anaerobic Digestion Effects and the Role of Pre- and Post-Treatments on Veterinary Antibiotics and Antibiotic Resistance Genes Removal Efficiency. Sci. Total Environ. 2020, 721, 137532. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Schmitt, E.; Mathys, A. Sustainable Use of Hermetia illucens Insect Biomass for Feed and Food: Attributional and Consequential Life Cycle Assessment. Resour. Conserv. Recycl. 2019, 144, 285–296. [Google Scholar] [CrossRef]
- Tokwaro, R.; Semiyaga, S.; Niwagaba, C.B.; Nakagiri, A.; Sempewo, J.I.; Muoghalu, C.C.; Manga, M. Application of Black Soldier Fly Larvae in Decentralized Treatment of Faecal Sludge from Pit Latrines in Informal Settlements in Kampala City. Front. Environ. Sci. 2023, 11, 1118635. [Google Scholar] [CrossRef]
- Salam, M.; Alam, F.; Dezhi, S.; Nabi, G.; Shahzadi, A.; Hassan, S.U.; Ali, M.; Saeed, M.A.; Hassan, J.; Ali, N.; et al. Exploring the Role of Black Soldier Fly Larva Technology for Sustainable Management of Municipal Solid Waste in Developing Countries. Environ. Technol. Innov. 2021, 24, 101934. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Gadge, A.S.; Hasan, M.; Rahayu, T.; Povetkin, S.N.; Fernando, I.; Castro-Muñoz, R. Future Opportunities for Products Derived from Black Soldier Fly (BSF) Treatment as Animal Feed and Fertilizer—A Systematic Review. Environ. Dev. Sustain. 2024, 26, 30273–30354. [Google Scholar] [CrossRef]
- Anedo, E.O.; Beesigamukama, D.; Mochoge, B.; Korir, N.K.; Haukeland, S.; Cheseto, X.; Nyongesa, M.; Pwaipwai, P.; Subramanian, S.; Tenkouano, A.; et al. Unpacking the Benefits of Black Soldier Fly Frass Fertilizer towards Nematode Suppression and Potato Production. Front. Plant Sci. 2025, 16, 1509643. [Google Scholar] [CrossRef]
- Gurung, S.K.; Mickan, B.S.; Middleton, J.A.; Singh, P.K.; Jenkins, S.N.; Rengel, Z.; Siddique, K.H.M.; Solaiman, Z.M. Manure-Derived Black Soldier Fly Frass Enhanced the Growth of Chilli Plants (Capsicum annuum L.) and Altered Rhizosphere Bacterial Community. Appl. Soil Ecol. 2024, 202, 105605. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of Feedstock on Larval Development and Process Efficiency in Waste Treatment with Black Soldier Fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Bulak, P.; Polakowski, C.; Nowak, K.; Waśko, A.; Wiącek, D.; Bieganowski, A. Hermetia illucens as a New and Promising Species for Use in Entomoremediation. Sci. Total Environ. 2018, 633, 912–919. [Google Scholar] [CrossRef]
- Clark, M.; Tepper, K.; Petroll, K.; Kumar, S.; Sunna, A.; Maselko, M. Bioremediation of Industrial Pollutants by Insects Ex-Pressing a Fungal Laccase. ACS Synth. Biol. J. 2022, 11, 308–316. [Google Scholar] [CrossRef]
- Purschke, B.; Scheibelberger, R.; Axmann, S.; Adler, A.; Jäger, H. Impact of Substrate Contamination with Mycotoxins, Heavy Metals and Pesticides on the Growth Performance and Composition of Black Soldier Fly Larvae (Hermetia illucens) for Use in the Feed and Food Value Chain. Food Addit. Contam. Part A 2017, 34, 1410–1420. [Google Scholar] [CrossRef]
- Kaczor, M.; Bulak, P.; Proc-Pietrycha, K.; Kirichenko-Babko, M.; Bieganowski, A. The Variety of Applications of Hermetia illucens in Industrial and Agricultural Areas—Review. Biology 2022, 12, 25. [Google Scholar] [CrossRef]
- Diener, S.; Zurbrügg, C.; Tockner, K. Bioaccumulation of Heavy Metals in the Black Soldier Fly, Hermetia illucens and Effects on Its Life Cycle. J. Insects Food Feed 2015, 1, 261–270. [Google Scholar] [CrossRef]
- Bohm, K.; Hatley, G.A.; Robinson, B.H.; Gutiérrez-Ginés, M.J. Black Soldier Fly-Based Bioconversion of Biosolids Creates High-Value Products with Low Heavy Metal Concentrations. Resour. Conserv. Recycl. 2022, 180, 106149. [Google Scholar] [CrossRef]
- Liu, C.; Yao, H.; Chapman, S.J.; Su, J.; Wang, C. Changes in Gut Bacterial Communities and the Incidence of Antibiotic Resistance Genes During Degradation of Antibiotics by Black Soldier Fly Larvae. Environ. Int. 2020, 142, 105834. [Google Scholar] [CrossRef]
- Liu, C.; Yao, H.; Cao, Q.; Wang, T.; Wang, C. The Enhanced Degradation Behavior of Oxytetracycline by Black Soldier Fly Larvae with Tetracycline Resistance Genes in the Larval Gut: Kinetic Process and Mechanism. Environ. Res. 2022, 214, 114211. [Google Scholar] [CrossRef]
- Mei, H.; Li, C.; Li, X.; Hu, B.; Lu, L.; Tomberlin, J.K.; Hu, W. Characteristics of Tylosin and Enrofloxacin Degradation in Swine Manure Digested by Black Soldier Fly (Hermetia illucens L.) Larvae. Environ. Pollut. 2022, 293, 118495. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ma, S.; Li, F.; Zheng, L.; Tomberlin, J.K.; Yu, Z.; Zhang, J.; Yu, C.; Fan, M.; Cai, M. Characteristics and Mechanisms of Ciprofloxacin Degradation by Black Soldier Fly Larvae Combined with Associated Intestinal Microorganisms. Sci. Total Environ. 2022, 811, 151371. [Google Scholar] [CrossRef]
- Luo, X.; Yang, Q.; Lin, Y.; Tang, Z.; Tomberlin, J.K.; Liu, W.; Huang, Y. Black Soldier Fly Larvae Effectively Degrade Lincomycin from Pharmaceutical Industry Wastes. J. Environ. Manag. 2022, 307, 114539. [Google Scholar] [CrossRef]
- Hoek-van Den Hil, E.F.; Van De Schans, M.G.M.; Bor, G.; Van Der Fels-Klerx, H.J. Effects of Veterinary Drugs on Rearing and Safety of Black Soldier Fly (Hermetia illucens) Larvae. J. Insects Food Feed 2022, 8, 1097–1106. [Google Scholar] [CrossRef]
- Van Dongen, K.C.W.; De Lange, E.; Van Asseldonk, L.L.M.; Zoet, L.; Van Der Fels-Klerx, H.J. Safety and Transfer of Veterinary Drugs from Substrate to Black Soldier Fly Larvae. Animal 2024, 18, 101214. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Zhang, S.-K.; Ren, X.-B.; Su, J. Effects of Dietary Additives in Artificial Diets on Survival and Larval Development of Cnaphalocrocis medinalis (Lepidoptera: Crambidae). Fla. Entomol. 2014, 97, 1041–1048. [Google Scholar] [CrossRef]
- Galarza, J.A.; Murphy, L.; Mappes, J. Antibiotics Accelerate Growth at the Expense of Immunity. Proc. R. Soc. B Biol. Sci. 2021, 288, 20211819. [Google Scholar] [CrossRef]
- Meijer, N.; Safitri, R.A.; Tao, W.; Hoek-Van Den Hil, E.F. Review: European Union Legislation and Regulatory Framework for Edible Insect Production—Safety Issues. Animal 2025, 101468. [Google Scholar] [CrossRef]
- CFIA Registration Triggers for Fertilizers and Supplements. Available online: https://inspection.canada.ca/plant-health/fertilizers/fertilizer-or-supplement-registration/fertilizers-and-supplements/eng/1330932243713/1330933201778 (accessed on 20 June 2025).
| Antimicrobial Class | Antimicrobial Agent |
|---|---|
| Aminoglycosides | Amikacin, apramycin, dihydrostreptomycin, framycetin sulfate, gentamicin, neomycin, spectinomycin, streptomycin |
| β-Lactams/Penicillins | Amoxicillin, ampicillin, cloxacillin, penicillin, sulbactam, clavulanic acid |
| Cephalosporins | Ceftiofur, cephapirin, cefovecin, cefaclor, cefadroxil |
| Fluoroquinolones | Ciprofloxacin, danofloxacin, enrofloxacin, marbofloxacin, orbifloxacin, pradofloxacin |
| Synthetic Anticoccidials and Arsenicals | Amprolium, clopidol, decoquinate, diclazuril, narasin, nicarbazine, pyrimethamine, robenidine, toltrazuril, zoalene |
| Ionophore Anticoccidials | Lasalocid, maduramycin, monensin, salinomycin |
| Lincosamides | Clindamycin, lincomycin, pirlimycin |
| Macrolides | Erythromycin, gamithromycin, tilmicosin, tylosin, tulathromycin, tildipirosine, tylvalosin |
| Other Antimicrobials | Avilamycin, bacitracin, bambermycin, chloramphenicol, chlorhexidine gluconate, florfenicol, fusidic acid, nitarsone, nitrofurantoin, nitrofurazone, novobiocin, polymyxin, tiamulin, virginiamycin |
| Tetracyclines | Chlortetracycline, oxytetracycline, tetracycline |
| Trimethoprim and Sulfonamides | Ormetoprim, sulfabenzamide, sulfacetamide, sulfadiazine, sulfadimethoxine, sulfadoxine, sulfguanidine, sulfamerazine, sulfamethazine, sulfanilamide, sulfaquinoxaline, sulfathiazole, trimethoprim |
| Category of Importance | Antimicrobial Class |
|---|---|
| Category I: Very high importance | Carbapenems, cephalosporins (third and fourth generations), fluoroquinolones, glycopeptides, glycylcyclines, ketolides, lipopeptides, monobactams, nitroimidazoles (metronidazole), oxazolidinones, penicillin-β-lactamase inhibitor combinations, polymyxins (colistin), therapeutic agents for tuberculosis (e.g., ethambutol, isoniazid, pyrazinamide and rifampin) |
| Category II: High importance | Aminoglycosides (except topical agents), cephalosporins (first and second generations, including cephamycins), fusidic acid, lincosamides, macrolides, penicillins, quinolones (except fluoroquinolones), streptogramins, trimethoprim/sulfamethoxazole |
| Category III: Medium importance | Aminocyclitols, aminoglycosides (topical agents), bacitracins, fosfomycin, nitrofurans, phenicols, sulphonamides, tetracyclines, trimethoprim |
| Category IV: Low importance | Flavophospholipids, ionophores |
| Antibiotic | Class | MIB | Inhibition or Disruption Target | Treatment and Prevention Approved Claim(s) | Concentration in Complete Feed (mg/kg) | Approved Livestock Species | Caution |
|---|---|---|---|---|---|---|---|
| Avilamycin | Streptogramine | AVI | Protein synthesis | Necrotic enteritis | 15–30 | Broiler chickens | NA |
| Post-weaning diarrhea | 80 | Swine | |||||
| Bacitracin (methylenedisalicylate) | Polypeptide | BACN-M | Cell wall synthesis | Necrotic enteritis | 110 | Laying hens | NA |
| Necrotic enteritis | 110 | Broiler chickens | |||||
| Clostridial enteritis | 275 | Pregnant and lactating sows and gilts | |||||
| Bacitracin zinc | Polypeptide | BACN_Z | Cell wall synthesis | Reduction in early mortality | 110 | Chicks | NA |
| Necrotic enteritis | 55 | Broiler chickens | |||||
| Bacterial enteritis | 55–110 | Swine | |||||
| Bambermycin (flavomycin) | Ionophore | BAM | Cell wall synthesis | Increased rate of weight gain and improved feed efficiency | 2 | Broiler chickens | NA |
| Increased rate of weight gain | 2 | Broiler turkeys | |||||
| Chlortetracycline hydrochloride | Tetracycline | CTC | Protein synthesis | Hexamitiasis and synovitis | 55–220 | Turkeys | NA |
| Bacterial enteritis and porcine proliferative enteropathy | 55–220 | Swine | |||||
| Foot rot | 0.22 * | Beef and non-lactating dairy cattle | |||||
| Bacterial diarrhea | 55 | Calves | |||||
| Reduction in losses due to Enterotoxemia | 22 | Lambs | |||||
| Chlortetracycline hydrochloride, Sulfamethazine, and Penicillin | Tetracycline, sulfamide, penicillin | CSP | Multiaction | Bacterial enteritis | 110 CTC, 110 sulfamethazine, 55 penicillin | Swine | NA |
| Decoquinate | Hydroquinolone | DEC | Electron transport and sporozoite development | Caecal and intestinal coccidiosis | 30 | Broiler chickens | NA |
| Coccidiosis | 0.5 * | Cattle and calves | |||||
| Coccidiosis | 0.5 * | Lambs | |||||
| Florfenicol | Phénicolé | FLOR | RNA synthesis | Furunculosis | 200–2000 | Salmonids | NA |
| Lasalocide sodique | Ionophore | LAS | Ionic homeostasis, leading to osmotic lysis | Coccidiosis | 105 | Broiler chickens | Do not allow horses or other equines access to feeds containing lincomycin, as ingestion may be fatal. |
| Coccidiosis | 100 | Turkeys | |||||
| Increased rate of weight gain | 36 | Cattle | |||||
| Coccidiosis | 36 | Calves | |||||
| Coccidiosis | 36 | Lambs | |||||
| Lincomycin | Licosamide | LINC | Protein synthesis | Mycoplasmal pneumonia and porcine proliferative enteropathy, swine dysentery, and disease following treatment | 220 | Swine | Not for breeding swine. Do not allow rabbits, hamsters, guinea pigs, horses, dairy cattle, or other ruminants access to feeds containing lincomycin. |
| Monensin sodium | Ionophore | MOS | Protein synthesis | Coccidiosis | 100 | Broiler chickens and turkeys | Do not allow dogs, horses, other equines, or guinea fowl access to formulations containing monensin. Ingestion of monensin by these species has been fatal. |
| Coccidiosis and improve feed efficiency and increased rate of weight gain | 22–48 | Beef cattle | |||||
| Coccidiosis | 11–22 | Sheep | |||||
| Coccidiosis | 11–22 | Goats | |||||
| Narasin | Ionophore | NAR | Ionic homeostasis, cellular function, and metabolism | Coccidiosis | 70 | Broiler chickens | Do not allow canines, horses, or other equines access to formulations containing narasin. Ingestion of narasin by these species has been fatal. |
| Increased rate of weight gain and improved feed efficiency | 15 | Swine | |||||
| Oxytetracycline hydrochloride | Tetracycline | OTC | Protein synthesis | Infectious sinusitis and synovitis | 110 | Turkeys | Do not administer to lactating dairy cattle. |
| Bacterial enteritis and abortion caused by leptospirosis | 55–550 | Swine | |||||
| Bloat in young cattle on pasture and feedlots | 75 ** | Beef cattle | |||||
| Bacterial enteritis | 55 | Calves | |||||
| Bacterial enteritis in creep-fed suckling lambs and losses due to enterotoxemia | 22–110 | Lambs | |||||
| Ulcer disease | 75 * | Salmonids | |||||
| Penicillin G Procaine | β-lactamines | PEN | Cell wall synthesis | Necrotic enteritis | 55 | Broiler chickens | Do not feed to laying hens. |
| Salinomycin sodium | Ionophore | SAL | Signaling pathways by lowering intracellular pH | Coccidiosis | 60 | Broiler chickens | Do not allow turkeys, dogs, or horses access to this medicated feed, as it is known to be toxic to these species. |
| Increased rate of weight gain and feed efficiency | 25 | Swine | |||||
| Improvement of feed efficiency and growth rate | 100 ** | Beef cattle | |||||
| Coccidiosis and reduction in coccidian shedding | 20 | Rabbits | |||||
| Sulfadimethoxine and Ormetoprim | Sulfamide and diaminopyrimidine | SMOR | Synthesis of nucleic acids | Furunculosis | 5 g of Romet 30 Medicated Premix/100 kg of fish body weight per day | Salmonids | NA |
| Tiamulin | Pleuromutilines | TIA | Protein synthesis | Swine dysentery, porcine colonic Spirochaetosis, porcine proliferative enteropathy and enzootic pneumonia, and mortality associated with epizootic rabbit enterocolitis | 31.2–178.1 | Swine | Do not feed animals other than swine. |
| Tilmicosin | Macrolide | TIL | Protein synthesis | Swine respiratory disease (SRD), porcine polyserositis, and arthritis | 200–400 | Swine | Do not allow horses or other equines access to feeds containing tilmicosin (toxic for horses). |
| Reduction in bovine respiratory disease morbidity | 12.5 | Feedlot beef cattle | |||||
| Reduction in the severity of respiratory disease | 12.5 | Rabbits | |||||
| Tylosin | Macrolide | TYL | Protein synthesis | Necrotic enteritis | 200 | Broiler chickens | Do not use in laying hens. |
| Cyclic recurrence of swine dysentery, porcine proliferative enteropathy | 44–110 | Swine | |||||
| Liver abscesses | 11 | Beef cattle | |||||
| Tylvalosin | Macrolide | TYLV | Protein synthesis | Porcine proliferative enteropathy | 42.5 | Swine | Not for use in breeding animals. |
| Virginiamycin | Streptogramines | VMY | Protein synthesis | Necrotic enteritis | 22 | Broiler chickens | Do not feed to birds producing eggs for human consumption, pregnant or lactating females, or animals intended for breeding. |
| Swine dysentery | 55–110 | Swine | |||||
| Liver abscesses | 20 | Beef cattle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auger, L.; Saucier, L.; Gaucher, M.-L.; Vandenberg, G.W.; Vincent, A.T.; Thibodeau, A.; Deschamps, M.-H. Use, Risk and Revalorization of Veterinary Antibiotics: A Canadian Perspective. Antibiotics 2025, 14, 665. https://doi.org/10.3390/antibiotics14070665
Auger L, Saucier L, Gaucher M-L, Vandenberg GW, Vincent AT, Thibodeau A, Deschamps M-H. Use, Risk and Revalorization of Veterinary Antibiotics: A Canadian Perspective. Antibiotics. 2025; 14(7):665. https://doi.org/10.3390/antibiotics14070665
Chicago/Turabian StyleAuger, Laurence, Linda Saucier, Marie-Lou Gaucher, Grant W. Vandenberg, Antony T. Vincent, Alexandre Thibodeau, and Marie-Hélène Deschamps. 2025. "Use, Risk and Revalorization of Veterinary Antibiotics: A Canadian Perspective" Antibiotics 14, no. 7: 665. https://doi.org/10.3390/antibiotics14070665
APA StyleAuger, L., Saucier, L., Gaucher, M.-L., Vandenberg, G. W., Vincent, A. T., Thibodeau, A., & Deschamps, M.-H. (2025). Use, Risk and Revalorization of Veterinary Antibiotics: A Canadian Perspective. Antibiotics, 14(7), 665. https://doi.org/10.3390/antibiotics14070665








