In Vitro Activity of Novel β-Lactam/β-Lactamase Inhibitors Against Carbapenem-Resistant Pseudomonas aeruginosa and Enterobacterales in Korea
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Distribution of Carbapenemases
2.3. Antimicrobial Susceptibility Profiles
3. Discussion
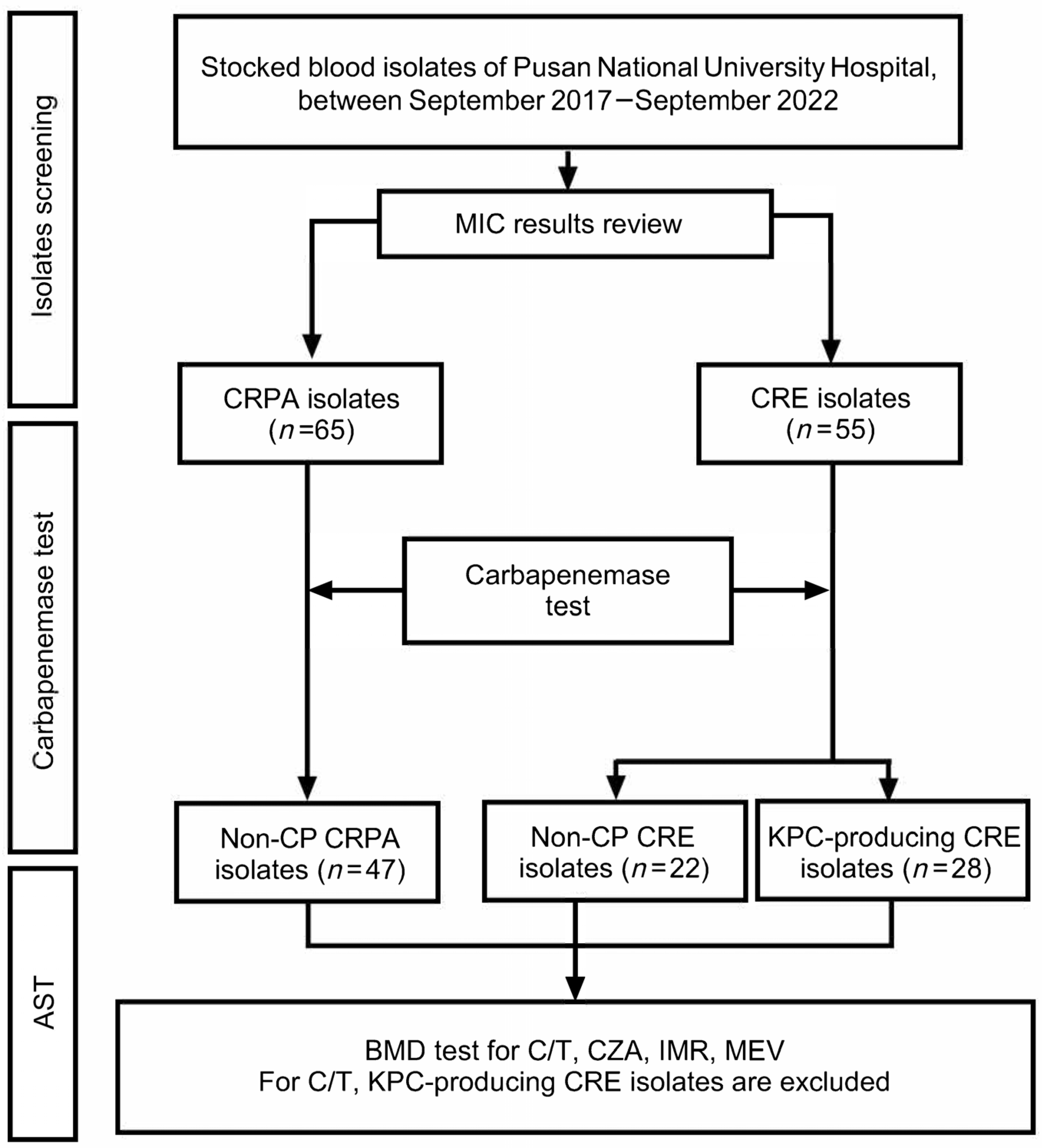
4. Materials and Methods
4.1. Study Population and Study Design
4.2. Bacterial Isolates
4.3. Carbapenemase Detection
4.4. Antimicrobial Susceptibility Testing
4.5. Data Analysis and Ethics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AmpC | AmpC β-lactamases |
| AST | Antimicrobial susceptibility testing |
| BMD | Broth microdilution |
| C/T | Ceftolozane/tazobactam |
| CZA | Ceftazidime/avibactam |
| CP | Carbapenemase-producing |
| COPD | Chronic obstructive pulmonary disease |
| CRE | Carbapenem-resistant Enterobacterales |
| CROs | Carbapenem-resistant organisms |
| CRPA | Carbapenem-resistant Pseudomonas aeruginosa |
| CLSI | Clinical and Laboratory Standards Institute |
| ESBL | Extended-spectrum β-lactamase |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| GES | Guiana-extended-spectrum |
| HIV/AIDS | Human immunodeficiency virus/acquired immunodeficiency syndrome |
| IMP | Imipenem-hydrolysing β-lactamase |
| IMR | Imipenem/cilastatin/relebactam |
| IRB | Institutional Review Board |
| KPC | Klebsiella pneumoniae carbapenemase |
| mCIM | Modified carbapenem inactivation method |
| MBLs | Metallo-β-lactamases |
| MDR | Multidrug-resistant |
| MDROs | Multidrug-resistant organisms |
| MEV | Meropenem/vaborbactam |
| MIC | Minimum inhibitory concentration |
| MRAB | Multidrug-resistant Acinetobacter baumannii |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NDM | New Delhi metallo-β-lactamase |
| NGS | Next-generation sequencing |
| Non-CP | Non-carbapenemase-producing |
| OXA | Oxacillinase |
| PCR | Polymerase chain reaction |
| VIM | Verona integron-encoded metallo-β-lactamase |
| VRE | Vancomycin-resistant Enterococcus |
References
- Cerceo, E.; Deitelzweig, S.B.; Sherman, B.M.; Amin, A.N. Multidrug-resistant Gram-negative bacterial infections in the hospital setting: Overview, implications for clinical practice, and emerging treatment options. Microb. Drug Resist. 2016, 22, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L. Epidemiology and diagnostics of carbapenem resistance in Gram-negative bacteria. Clin. Infect. Dis. 2019, 69, S521–S528. [Google Scholar] [CrossRef] [PubMed]
- Potter, R.F.; D’Souza, A.W.; Dantas, G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist. Updat. 2016, 29, 30–46. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Facility Guidance for Control of Carbapenem-Resistant Enterobacteriaceae (CRE): November 2015 Update—CRE Toolkit. Available online: https://www.cdc.gov/hai/pdfs/cre/cre-guidance-508.pdf (accessed on 1 June 2025).
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Bush, K. The ABCD’s of β-lactamase nomenclature. J. Infect. Chemother. 2013, 19, 549–559. [Google Scholar] [CrossRef]
- Van Duin, D.; Doi, Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017, 8, 460–469. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Tracking Carbapenem-Resistant Pseudomonas aeruginosa (CRPA). Available online: https://www.cdc.gov/hai/organisms/pseudomonas/tracking.html (accessed on 23 March 2024).
- Centers for Disease Control and Prevention (CDC). COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. Available online: https://www.cdc.gov/drugresistance/covid19.html (accessed on 23 March 2024).
- Jeon, K.; Jeong, S.; Lee, N.; Park, M.J.; Song, W.; Kim, H.S.; Kim, H.S.; Kim, J.-S. Impact of COVID-19 on antimicrobial consumption and spread of multidrug-resistance in bacterial infections. Antibiotics 2022, 11, 535. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. Kor-GLASS Antibiotic Resistance Statistics. Korea Disease Control and Prevention Agency, 2025. Available online: https://nih.go.kr/nohas/statistics/selectARStatisticsMainTab.do?systemName=Kor_GLASS/ (accessed on 2 June 2025).
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin. Infect. Dis. 2023, ciae403. [Google Scholar] [CrossRef]
- Wi, Y.M.; Greenwood-Quaintance, K.E.; Schuetz, A.N.; Ko, K.S.; Peck, K.R.; Song, J.H.; Patel, R. Activity of ceftolozane-tazobactam against carbapenem-resistant, non-carbapenemase-producing Pseudomonas aeruginosa and associated resistance mechanisms. Antimicrob. Agents Chemother. 2018, 62, e01970-17. [Google Scholar] [CrossRef]
- Lee, C.M.; Kim, Y.J.; Jung, S.I.; Kim, S.E.; Park, W.B.; Choe, P.G.; Kim, E.S.; Kim, C.J.; Choi, H.J.; Lee, S.; et al. Different clinical characteristics and impact of carbapenem-resistance on outcomes between Acinetobacter baumannii and Pseudomonas aeruginosa bacteraemia: A prospective observational study. Sci. Rep. 2022, 12, 8527. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Moon, C.; Mun, S.J.; Lee, J.E.; Lee, S.O.; Lee, S.; Lee, S.H. Antimicrobial susceptibility trends and risk factors for antimicrobial resistance in Pseudomonas aeruginosa bacteremia: 12-year experience in a tertiary hospital in Korea. J. Korean Med. Sci. 2021, 36, e273. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Ahn, S.M.; Kim, J.H.; Jeong, S.J.; Ku, N.S.; Choi, J.Y.; Yeom, J.S.; Song, J.E. Clinical characteristics and associated factors for mortality in patients with carbapenem-resistant Enterobacteriaceae bloodstream infection. Microorganisms 2023, 11, 1121. [Google Scholar] [CrossRef]
- Falagas, M.E.; Tansarli, G.S.; Karageorgopoulos, D.E.; Vardakas, K.Z. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg. Infect. Dis. 2014, 20, 1170–1175. [Google Scholar] [CrossRef]
- van Duin, D.; Bonomo, R.A. Ceftazidime/avibactam and ceftolozane/tazobactam: Second-generation β-lactam/β-lactamase inhibitor combinations. Clin. Infect. Dis. 2016, 63, 234–241. [Google Scholar] [CrossRef]
- Reyes, J.; Komarow, L.; Chen, L.; Ge, L.; Hanson, B.M.; Cober, E.; Herc, E.; Alenazi, T.; Kaye, K.S.; Garcia-Diaz, J.; et al. Global epidemiology and clinical outcomes of carbapenem-resistant Pseudomonas aeruginosa and associated carbapenemases (POP): A prospective cohort study. Lancet Microbe 2023, 4, e159–e170. [Google Scholar] [CrossRef]
- van Duin, D.; Arias, C.A.; Komarow, L.; Chen, L.; Hanson, B.M.; Weston, G.; Cober, E.; Garner, O.B.; Jacob, J.T.; Satlin, M.J.; et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): A prospective cohort study. Lancet Infect. Dis. 2020, 20, 731–741. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, J.; Ouyang, P.; Jin, C.; Wang, R.; Zhang, Y.; Jin, L.; Chen, H.; Wang, Z.; et al. Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: Data from a longitudinal large-scale CRE study in China (2012–2016). Clin. Infect. Dis. 2018, 67, S196–S205. [Google Scholar] [CrossRef]
- Oka, K.; Matsumoto, A.; Tetsuka, N.; Morioka, H.; Iguchi, M.; Ishiguro, N.; Nagamori, T.; Takahashi, S.; Saito, N.; Tokuda, K.; et al. Clinical characteristics and treatment outcomes of carbapenem-resistant Enterobacterales infections in Japan. J. Glob. Antimicrob. Resist. 2022, 29, 247–252. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.S.; Kim, H.S.; Yu, J.K.; Han, S.H.; Kang, M.J.; Hong, C.K.; Lee, S.M.; Oh, Y.H. Prevalence of carbapenem-resistant Enterobacteriaceae in Seoul, Korea. Korean Soc. Microbiol. 2020, 50, 107–116. [Google Scholar] [CrossRef]
- Kim, B.W.; Jo, Y.K.; Kim, G.N.; Hwang, J.Y.; Hong, M.Y.; Seo, W.D.; Hwang, S.N. Incidence of carbapenem-resistant Enterobacteriaceae and Carbapenemase-producing Enterobacteriaceae gene distribution in Ulsan, Korea, 2018~2021. J. Bacteriol. Virol. 2022, 52, 28–38. [Google Scholar] [CrossRef]
- Kang, J.S.; Yi, J.; Ko, M.K.; Lee, S.O.; Lee, J.E.; Kim, K.H. Prevalence and risk factors of carbapenem-resistant Enterobacteriaceae acquisition in an emergency intensive care unit in a tertiary hospital in Korea: A case-control study. J. Korean Med. Sci. 2019, 34, e140. [Google Scholar] [CrossRef] [PubMed]
- Pierce, V.M.; Simner, P.J.; Lonsway, D.R.; Roe-Carpenter, D.E.; Johnson, J.K.; Brasso, W.B.; Bobenchik, A.M.; Lockett, Z.C.; Charnot-Katsikas, A.; Ferraro, M.J.; et al. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J. Clin. Microbiol. 2017, 55, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Simner, P.J.; Johnson, J.K.; Brasso, W.B.; Anderson, K.; Lonsway, D.R.; Pierce, V.M.; Bobenchik, A.M.; Lockett, Z.C.; Charnot-Katsikas, A.; Westblade, L.F.; et al. Multicenter evaluation of the modified carbapenem inactivation method and the Carba NP for detection of carbapenemase-producing Pseudomonas aeruginosa and Acinetobacter baumannii. J. Clin. Microbiol. 2017, 56, e01369-e17. [Google Scholar] [CrossRef]
- Lob, S.H.; Kazmierczak, K.M.; Chen, W.T.; Siddiqui, F.; DeRyke, C.A.; Young, K.; Motyl, M.R.; Sahm, D.F. In Vitro activity of ceftolozane/tazobactam against Gram-negative isolates collected from ICU patients with lower respiratory tract infections in seven Asian countries—SMART 2017–2019. J. Glob. Antimicrob. Resist. 2022, 29, 527–533. [Google Scholar] [CrossRef]
- Lin, C.K.; Page, A.; Lohsen, S.; Haider, A.A.; Waggoner, J.; Smith, G.; Babiker, A.; Jacob, J.T.; Howard-Anderson, J.; Satola, S.W. Rates of resistance and heteroresistance to newer β-lactam/β-lactamase inhibitors for carbapenem-resistant Enterobacterales. JAC Antimicrob. Resist. 2024, 6, dlae048. [Google Scholar] [CrossRef]
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2023.
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2024.
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. Available online: http://www.eucast.org (accessed on 28 March 2024).
- Kim, K.H.; Moon, S.; Ko, M.K.; Yi, J. Ceftolozane/Tazobactam Susceptibility of Carbapenem-Resistant Pseudomonas aeruginosa and Carbapenem-Resistant Enterobacterales Isolates in a Tertiary Hospital in Korea. Open Forum Infect. Dis. 2023, 10 (Suppl. S2), ofad500.657. [Google Scholar] [CrossRef]
| Characteristics | CRPA (n = 65) | CRE (n = 55) | p a |
|---|---|---|---|
| Age, years | 65 (37–88) b | 55 (21–88) b | 0.008 c |
| Sex, male | 39 (60.0) | 37 (67.3) | 0.451 |
| Comorbidities | |||
| Cardiovascular disease | 3 (4.6) | 5 (9.1) | 0.467 |
| Hypertension | 19 (29.2) | 16 (29.1) | 1.000 |
| Heart failure | 5 (7.7) | 10 (18.2) | 0.101 |
| Chronic lung disease | 0 (0.0) | 4 (7.3) | 0.041 |
| Cerebrovascular accident | 12 (18.5) | 4 (7.3) | 0.105 |
| Dementia | 1 (1.5) | 1 (1.8) | 1.000 |
| Diabetes | 19 (29.2) | 15 (27.3) | 0.842 |
| Liver disease | 10 (15.4) | 9 (16.4) | 1.000 |
| Chronic kidney disease | 9 (13.8) | 6 (10.9) | 0.783 |
| Solid tumor | 25 (38.5) | 26 (47.3) | 0.359 |
| Hematologic malignancy | 3 (4.6) | 14 (25.5) | <0.001 |
| Immunosuppressive treatment | 10 (15.4) | 33 (60.0) | <0.001 |
| HIV/AIDS | 1 (1.5) | 0 (0.0) | 1.000 |
| Characteristics | CRPA (n = 65) | CRE (n = 55) | p a |
|---|---|---|---|
| Primary origin of bacteremia | |||
| Intraabdominal infection | 25 (38.5) | 18 (32.7) | 0.569 |
| Urinary tract infection | 16 (24.6) | 1 (1.8) | <0.001 |
| Catheter-related infection | 10 (15.4) | 13 (23.6) | 0.352 |
| Pneumonia | 5 (7.7) | 8 (14.5) | 0.253 |
| Primary bacteremia or neutropenic fever | 4 (6.2) | 13 (23.6) | 0.008 |
| Soft tissue, musculoskeletal infection | 3 (4.6) | 2 (3.6) | 1.000 |
| Others | 2 (3.1) | 0 (0.0) | 0.499 |
| Devices present at onset of infection | |||
| Central venous catheter | 23 (35.4) | 39 (70.9) | <0.001 |
| Mechanical ventilator | 4 (6.2) | 16 (29.1) | 0.001 |
| Indwelling urinary catheter | 25 (38.5) | 27 (49.1) | 0.271 |
| Nasogastric tube | 13 (20.0) | 21 (38.2) | 0.041 |
| Characteristics | CRPA (n = 65) | CRE (n = 55) | p a |
|---|---|---|---|
| Previous colonization with MDROs | |||
| MRSA | 9 (13.8) | 9 (16.4) | 0.799 |
| MRAB | 10 (15.4) | 6 (10.9) | 0.593 |
| CRPA | 25 (38.5) | 6 (10.9) | <0.001 |
| CRE | 15 (23.1) | 23 (41.8) | 0.032 |
| VRE | 7 (10.8) | 11 (20.0) | 0.202 |
| ESBL-producing bacteria | 16 (24.6) | 15 (27.3) | 0.835 |
| Antimicrobial therapy for CRPA or CRE | |||
| Colistin alone | 18 (27.7) | 11 (20.0) | 0.394 |
| Colistin + carbapenem | 1 (1.5) | 10 (18.2) | 0.003 |
| Cephalosporin alone | 17 (26.2) | 6 (10.9) | 0.039 |
| Others | 5 (7.7) | 5 (9.1) | 1.000 |
| Tigecycline | 0 (0.0) | 2 (3.6) | 0.208 |
| None for CRPA or CRE | 20 (30.8) | 21 (38.2) | 0.713 |
| In-hospital mortality | 12 (18.5) | 36 (63.6) | <0.001 |
| Carbapenemase Types and Species | No. (%) |
|---|---|
| CRPA (n = 65) | |
| Non-CP CRPA | 47 (72.3) |
| CP CRPA | 18 (27.7) |
| NDM | 14 (21.5) |
| VIM | 2 (3.1) |
| IMP | 2 (3.1) |
| OXA-48-like | 0 (0.0) |
| KPC | 0 (0.0) |
| CRE (n = 55) | |
| Non-CP CRE | 22 (40.0) |
| Klebsiella pneumoniae | 7 (12.7) |
| Escherichia coli | 7 (12.7) |
| Enterobacter cloacae | 5 (9.1) |
| Serratia marcescens | 3 (5.5) |
| CP CRE | 33 (60.0) |
| KPC | |
| Klebsiella pneumoniae | 25 (45.5) |
| Klebsiella oxytoca | 1 (1.8) |
| Escherichia coli | 1 (1.8) |
| Serratia marcescens | 1 (1.8) |
| NDM | |
| Enterobacter cloacae | 3 (5.5) |
| Escherichia coli | 1 (1.8) |
| OXA-48-like | |
| Escherichia coli | 1 (1.8) |
| Isolate Type | Antimicrobial Agent | MIC Range (µg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | Susceptible (%) | Intermediate (%) | Resistant (%) |
|---|---|---|---|---|---|---|---|
| Non-CP CRPA (n = 47) | C/T | 0.25–>8 | 1 | >8 | 83.0 | 6.4 | 10.6 |
| CZA | 0.25–>32 | 4 | 32 | 70.2 | – | 29.8 | |
| IMR | 0.25–>16 | 2 | 16 | 63.8 | 17.0 | 19.1 | |
| MEV | 0.25–>16 | 8 | >16 | 66.0 | – | 34.0 | |
| Non-CP CRE (n = 22) | C/T | 0.5–>8 | >8 | >8 | 18.2 | 13.6 | 68.2 |
| CZA | 0.25–8 | 1 | 4 | 100 | – | 0 | |
| IMR | ≤0.06–2 | 0.25 | 2 | 81.8 | 18.2 | 0 | |
| MEV | ≤0.03–16 | 0.25 | 2 | 95.5 | – | 4.5 | |
| KPC-producing CRE (n = 28) | CZA | 0.25–>32 | 1 | 4 | 92.9 | – | 7.1 |
| IMR | 0.125–16 | 0.125 | 2 | 82.1 | 10.7 | 7.1 | |
| MEV | ≤0.015–8 | 0.03 | 4 | 96.4 | 3.6 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Yi, J.; Ko, M.K.; Sim, Y.K.; Kim, K.-H. In Vitro Activity of Novel β-Lactam/β-Lactamase Inhibitors Against Carbapenem-Resistant Pseudomonas aeruginosa and Enterobacterales in Korea. Antibiotics 2025, 14, 649. https://doi.org/10.3390/antibiotics14070649
Moon S, Yi J, Ko MK, Sim YK, Kim K-H. In Vitro Activity of Novel β-Lactam/β-Lactamase Inhibitors Against Carbapenem-Resistant Pseudomonas aeruginosa and Enterobacterales in Korea. Antibiotics. 2025; 14(7):649. https://doi.org/10.3390/antibiotics14070649
Chicago/Turabian StyleMoon, Seulgi, Jongyoun Yi, Mee Kyung Ko, Yong Ki Sim, and Kye-Hyung Kim. 2025. "In Vitro Activity of Novel β-Lactam/β-Lactamase Inhibitors Against Carbapenem-Resistant Pseudomonas aeruginosa and Enterobacterales in Korea" Antibiotics 14, no. 7: 649. https://doi.org/10.3390/antibiotics14070649
APA StyleMoon, S., Yi, J., Ko, M. K., Sim, Y. K., & Kim, K.-H. (2025). In Vitro Activity of Novel β-Lactam/β-Lactamase Inhibitors Against Carbapenem-Resistant Pseudomonas aeruginosa and Enterobacterales in Korea. Antibiotics, 14(7), 649. https://doi.org/10.3390/antibiotics14070649






