Evaluation of the Potential of Metal–Organic Compounds ZIF-8 and F300 in a Membrane Filtration–Adsorption Process for the Removal of Antibiotics from Water
Abstract
1. Introduction
| Antibiotic | MOF | Synthesis Method | References |
|---|---|---|---|
| Amoxicillin | MIL-53(Al) | Hydrothermal | [32,33] |
| Ciprofloxacin | UiO-66-NH2 | Solvothermal | [34] |
| Nalidixic Acid | PCN-224 | Solvothermal | [35] |
| Neomycin | SA-g-P3AP MOF(Fe)/Ag | Solvothermal | [32] |
| Norfloxacin | UiO-66-NH2 | Solvothermal | [34] |
| Ofloxacin | PCN-224 | Solvothermal | [35] |
| Tetracycline | MIL-101-Fe | Solvothermal | [36,37] |
| NH2-MIL-101-Fe | Solvothermal | [36] | |
| Fe/MIL-100(Fe) | Hyrothermal | [38] | |
| ZIF-8 | Precipitation | [39] | |
| ZIF-67 | Precipitation | [39] | |
| HKUST-1 | Solvothermal | [39] | |
| Fe-BTC | n/a | [39] | |
| CoFe | Hyrothermal | [40] | |
| UiO-66 | Hyrothermal | [41,42] | |
| MOF-5 | Solvothermal | [37] | |
| Cu-ZIF-8 | Solvothermal | [37] |
2. Materials and Methods
2.1. Materials
2.2. Testing of MOF Adsorption Properties
- -
- kF [(mg(1−(1/n))·(dm3)1/n)/g] is an empirical constant representing the adsorption capacity of the material, 1/n [−] is a parameter that indicates adsorption intensity
- -
- kL [dm3/g] is the Langmuir constant and qmax [dm3/mg] is the theoretical maximum adsorption capacity
- -
- kR [dm3/g] is the Redlich–Peterson constant, B [dm3/mg] is also constant, β [−] is a parameter between 0 and 1. If β = 1, the equation reduces to the Langmuir expression.
2.3. Membrane Modification
2.4. Testing Membranes
3. Results and Discussion
3.1. Evaluation of MOF Adsorption Properties
3.2. Adsorption Kinetic
3.3. Adsorption Isotherms
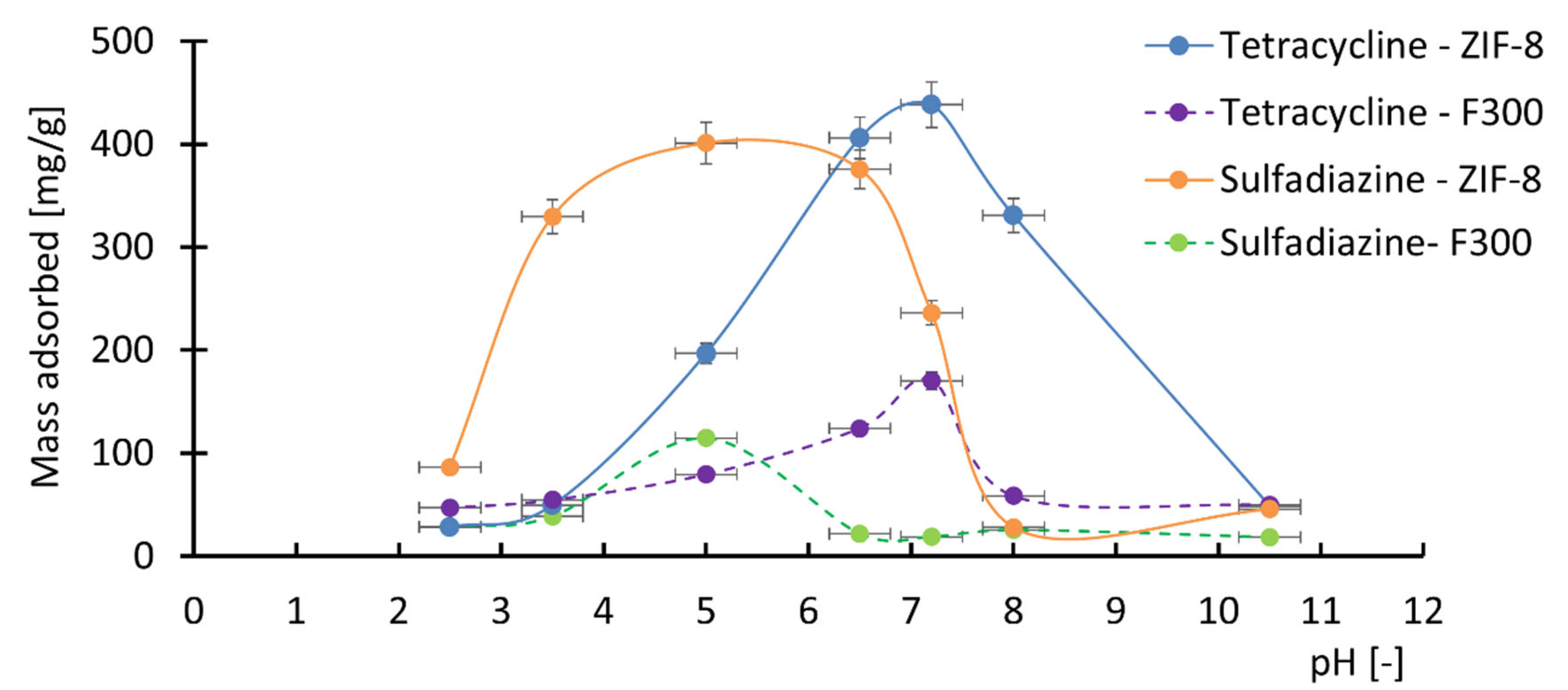
3.4. Effect of pH
3.5. Analysis of Adsorption Data
3.6. Membranes’ Properties
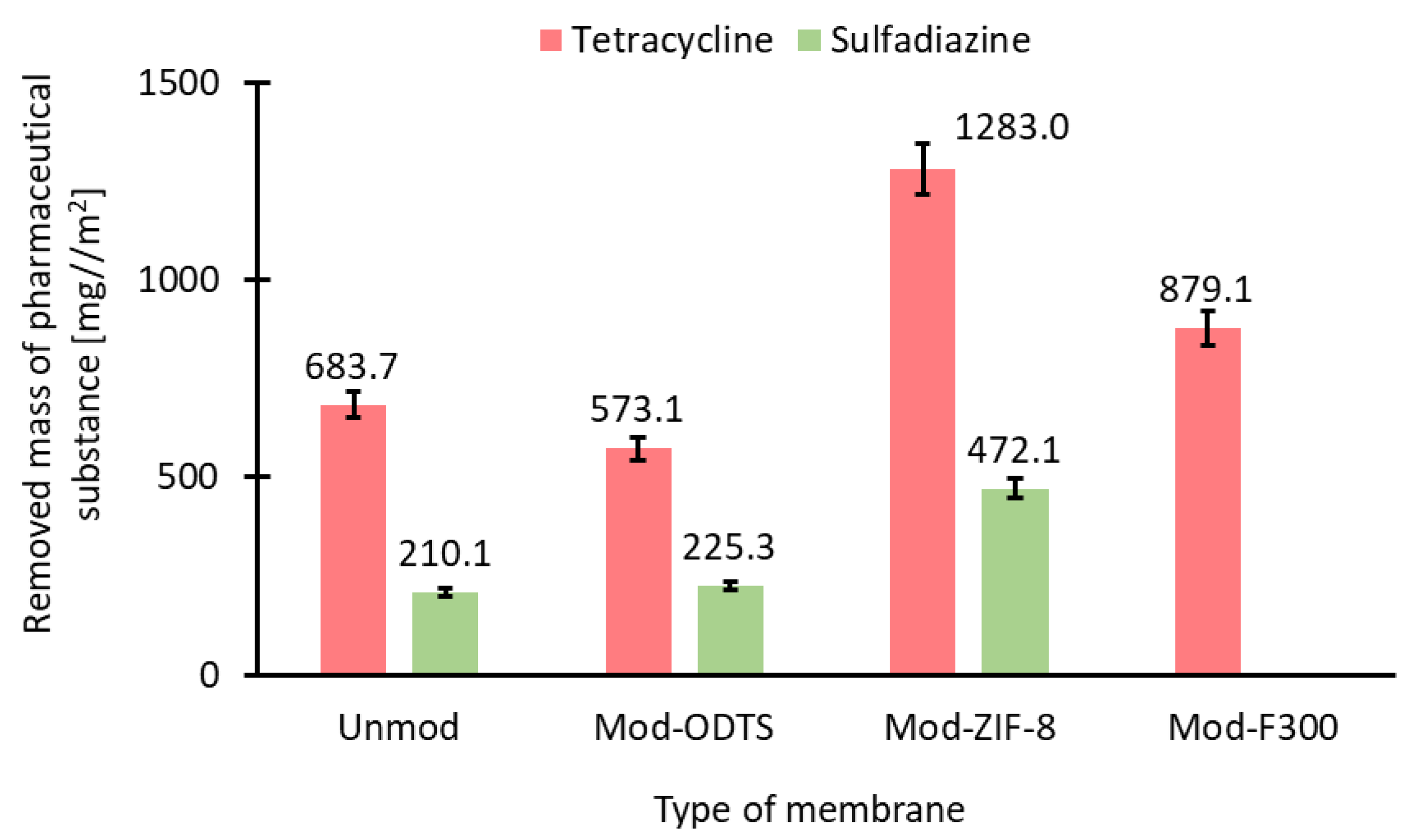
3.7. Evaluation of Filtration–Adsorption Process
| Membrane Type/Material | Membrane Process | Pharmaceutical Substance | Rejection [%] | Reference |
|---|---|---|---|---|
| Al2O3 + ZIF-8 | MF | Tetracycline | 70.6 | This work |
| Sulfadiazine | 25.9 | |||
| PVDF + PVP-TiO2-Dopamine | UF | Sufladiazine | ~70 | [125] |
| COF-LZU1 | UF | Tetracycline | 34.7–82.5 | [126] |
| Sulfadiazine | 4.3–78 | |||
| NF-90 | NF | Sulfamethoxazole | >95 | [116] |
| Carbamazepine | >95 | |||
| Ibuprofen | >95 | |||
| UF ceramic TiO2 + GO | UF | Ibuprofen | ~70 | [124] |
| Diclofenac | ~80 | |||
| Carbamazepine | ~25 | |||
| Naproxen | ~70 | |||
| PVDF | UF | Sulfadiazine | 48.62 | [120] |
| PP | 51.55 | |||
| NF-90 | NF | Carbamazepine | bd | [118] |
| Diatrizoate | 98 | |||
| NF-270 | NF | Carbamazepine | 92 | [118] |
| Diatrizoate | 97 | |||
| NF-90 | NF | Sulfadiazine | ~95 | [127] |
| NF-270 | ~90 | |||
| PSf + GO | NF | Ibuprofen | ~95 | [122] |
| SW30 | RO | Carbamazepine | ~100 | [118] |
| Diatrizoate | ~100 | |||
| TFC | FO | Tetracycline | ~99 | [119] |
| TFC + TiO2 | FO | Metoprolol | ~90 | [123] |
| Sulfamethoxazole | ~100 | |||
| Triclosan | ~100 |
3.8. Membrane Regeneration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dai, H.; Wang, W.; Peng, R.; Yu, S.; Zhang, X.; Huo, Z.-Y.; Yuan, Q.; Luo, Y. Local Electric Field-Incorporated In-Situ Copper Ions Eliminating Pathogens and Antibiotic Resistance Genes in Drinking Water. Antibiotics 2024, 13, 1161. [Google Scholar] [CrossRef] [PubMed]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S.; Show, P.L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Rogowska, J.; Gałęzowska, G.; Zimmermann, A. Challenges and Current Trends in Preventing Antimicrobial Resistance in EU Water Law Context. Antibiotics 2025, 14, 18. [Google Scholar] [CrossRef]
- García, L.; Leyva-Díaz, J.C.; Díaz, E.; Ordóñez, S. A review of the adsorption-biological hybrid processes for the abatement of emerging pollutants: Removal efficiencies, physicochemical analysis, and economic evaluation. Sci. Total Environ. 2021, 780, 146554. [Google Scholar] [CrossRef]
- Imreová, Z.; Staňová, A.V.; Zažímal, F.; Debnárová, S.; Vrána, L.; Petrovičová, N.; Tulipánová, A.; Lukáč, T.; Végh, D.; Stýskalík, A.; et al. Low-cost carbon-based sorbents for the removal of pharmaceuticals from wastewaters. J. Water Process Eng. 2024, 61, 105181. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Lütke, S.F.; Dotto, G.L.; Salau, N.P.G. Adsorption mechanisms of single and simultaneous removal of pharmaceutical compounds onto activated carbon: Isotherm and thermodynamic modeling. J. Mol. Liq. 2021, 336, 116203. [Google Scholar] [CrossRef]
- Streit, A.F.M.; Collazzo, G.C.; Druzian, S.P.; Verdi, R.S.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Adsorption of ibuprofen, ketoprofen, and paracetamol onto activated carbon prepared from effluent treatment plant sludge of the beverage industry. Chemosphere 2021, 262, 128322. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Soltys, L.; Macyk, W. Magnetic adsorbents for removal of pharmaceuticals: A review of adsorption properties. J. Mol. Liq. 2023, 384, 122174. [Google Scholar] [CrossRef]
- Ahammad, N.A.; Ahmad, M.A.; Hameed, B.H.; Mohd Din, A.T. A mini review of recent progress in the removal of emerging contaminants from pharmaceutical waste using various adsorbents. Environ. Sci. Pollut. Res. 2023, 30, 124459–124473. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Zielińska, I.; Szwast, M.; Kogut, I.; Małolepszy, A. Modification of ceramic membranes with carbon compounds for pharmaceutical substances removal from water in a filtration—Adsorption System. Membranes 2021, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of antibiotics from wastewaters by membrane technology: Limitations, successes, and future improvements. Sci. Total Environ. 2022, 838, 156010. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Leonardo, I.C.; Silva, A.F.; Crespo, J.G.; Nunes, M.; Crespo, M.T.B. Nanofiltration as an Efficient Tertiary Wastewater Treatment: Elimination of Total Bacteria and Antibiotic Resistance Genes from the Discharged Effluent of a Full-Scale Wastewater Treatment Plant. Antibiotics 2022, 11, 630. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kumar, R.; Kumar, A.; Shankar, R.; Khan, N.A.; Gupta, K.N.; Ram, M.; Arya, R.K. Pharmaceutical waste-water treatment via advanced oxidation based integrated processes: An engineering and economic perspective. J. Water Process Eng. 2023, 54, 103977. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, Y.H.; Hsu, T.H. The competitive effect of different chlorination disinfection methods and additional inorganic nitrogen on nitrosamine formation from aromatic and heterocyclic amine-containing pharmaceuticals. Chemosphere 2021, 267, 128922. [Google Scholar] [CrossRef]
- Femina Carolin, C.; Senthil Kumar, P.; Janet Joshiba, G.; Vinoth Kumar, V. Analysis and removal of pharmaceutical residues from wastewater using membrane bioreactors: A review. Environ. Chem. Lett. 2021, 19, 329–343. [Google Scholar] [CrossRef]
- Gulamhussein, M.A.; Saini, B.; Dey, A. Removal of pharmaceutical contaminants through membrane bioreactor. Mater. Today Proc. 2023, 77, 260–268. [Google Scholar] [CrossRef]
- Petala, A.; Mantzavinos, D.; Frontistis, Z. Impact of water matrix on the photocatalytic removal of pharmaceuticals by visible light active materials. Curr. Opin. Green Sustain. Chem. 2021, 28, 100445. [Google Scholar] [CrossRef]
- Ruziwa, D.T.; Oluwalana, A.E.; Mupa, M.; Meili, L.; Selvasembian, R.; Nindi, M.M.; Sillanpaa, M.; Gwenzi, W.; Chaukura, N. Pharmaceuticals in wastewater and their photocatalytic degradation using nano-enabled photocatalysts. J. Water Process Eng. 2023, 54, 103880. [Google Scholar] [CrossRef]
- Yadav, D.; Karki, S.; Ingole, P.G. Current advances and opportunities in the development of nanofiltration (NF) membranes in the area of wastewater treatment, water desalination, biotechnological and pharmaceutical applications. J. Environ. Chem. Eng. 2022, 10, 108109. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Hu, C.; Zuo, C.; Wang, P.; Chen, W.; Ao, T. Efficient removal of tetracycline by a hierarchically porous ZIF-8 metal organic framework. Environ. Res. 2021, 198, 111254. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.L.; Mohammad, A.W.; Hilal, N.; Leo, C.P. A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 2015, 363, 2–18. [Google Scholar] [CrossRef]
- Davoodbeygi, Y.; Askari, M.; Salehi, E.; Kheirieh, S. A review on hybrid membrane-adsorption systems for intensified water and wastewater treatment: Process configurations, separation targets, and materials applied. J. Environ. Manag. 2023, 335, 117577. [Google Scholar] [CrossRef]
- Khan, N.A.; Singh, S.; López-Maldonado, E.A.; Narasimhappa, P.; Méndez-Herrera, P.F.; López-López, J.R.; Baig, U.; Ramamurthy, P.C.; Mubarak, N.M.; Karri, R.R.; et al. Emerging membrane technology and hybrid treatment systems for the removal of micropollutants from wastewater. Desalination 2023, 565, 116873. [Google Scholar] [CrossRef]
- Saha, P.; Jampa, S.S.; Sinha, M.K.; Khuntia, S. Hybrid membrane process for water treatment: A short review. AQUA—Water Infrastruct. Ecosyst. Soc. 2023, 72, 608–622. [Google Scholar] [CrossRef]
- Cai, G.; Yan, P.; Zhang, L.; Zhou, H.C.; Jiang, H.L. Metal-Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications. Chem. Rev. 2021, 121, 12278–12326. [Google Scholar] [CrossRef]
- Du, R.; Wu, Y.; Yang, Y.; Zhai, T.; Zhou, T.; Shang, Q.; Zhu, L.; Shang, C.; Guo, Z. Porosity Engineering of MOF-Based Materials for Electrochemical Energy Storage. Adv. Energy Mater. 2021, 11, 2100154. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, S.; Wu, P.; Wang, D.; Lu, S.; Wang, S.; Gong, F.; Wei, X.Q.; Ye, X.; Ding, P. Recent advances in performance improvement of Metal-organic Frameworks to remove antibiotics: Mechanism and evaluation. Sci. Total Environ. 2022, 811, 152351. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Z.; Yu, G.; Wu, H.; Chen, H.; Zhou, L.; Zhang, Y.; Su, Y.; Tan, S.; Yang, L.; et al. A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis. Chemosphere 2021, 272, 129501. [Google Scholar] [CrossRef]
- Dzumbira, W.; Ali, N.; Duanmu, C.; Yang, Y.; Khan, A.; Ali, F.; Bilal, M.; Aleya, L.; Iqbal, H.M.N. Separation and remediation of environmental pollutants using metal–organic framework-based tailored materials. Environ. Sci. Pollut. Res. 2022, 29, 4822–4842. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C. Exploring the state-of-the-art in metal-organic frameworks for antibiotic adsorption: A review of performance, mechanisms, and regeneration. Environ. Toxicol. Chem. 2025, 44, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Imanipoor, J.; Mohammadi, M.; Dinari, M.; Ehsani, M.R. Adsorption and Desorption of Amoxicillin Antibiotic from Water Matrices Using an Effective and Recyclable MIL-53(Al) Metal-Organic Framework Adsorbent. J. Chem. Eng. Data 2021, 66, 389–403. [Google Scholar] [CrossRef]
- Fang, S.Y.; Gong, J.L.; Tang, L.; Cao, W.C.; Li, J.; Tan, Z.K.; Wang, Y.W.; Wang, W.B. Loosely Sandwich-Structured Membranes Decorated with UiO-66-NH2for Efficient Antibiotic Separation and Organic Solvent Resistance. ACS Appl. Mater. Interfaces 2022, 14, 38990–39003. [Google Scholar] [CrossRef]
- Cho, H.J.; Kang, E.; Kim, S.; Yang, D.C.; Nam, J.; Jin, E.; Choe, W. Impact of Zr6Node in a Metal-Organic Framework for Adsorptive Removal of Antibiotics from Water. Inorg. Chem. 2021, 60, 16966–16976. [Google Scholar] [CrossRef]
- Ma, Z.; Li, M.; Wang, X.; Wang, Q.; Li, Q.; Wang, Y.; Zhang, Z.; Gao, J.; Gao, X.; Yuan, H.; et al. Selective and high-efficient removal of tetracycline from antibiotic-containing aqueous solution via combining adsorption with membrane pre-concentration. J. Water Process Eng. 2022, 50, 103281. [Google Scholar] [CrossRef]
- Islam, I.U.; Hu, X.; Shang, J.; Ashraf, M.A.; Ali, T.; Aslam, A.A.; Li, S.; Li, D.; Nazir, M.S.; Wang, X.; et al. MOF and MOF-based membranes: Promising solutions for pharmaceutical wastewater treatment. J. Mater. Sci. 2025, 60, 3634–3662. [Google Scholar] [CrossRef]
- Li, W.; Cao, J.; Xiong, W.; Yang, Z.; Sun, S.; Jia, M.; Xu, Z. In-situ growing of metal-organic frameworks on three-dimensional iron network as an efficient adsorbent for antibiotics removal. Chem. Eng. J. 2020, 392, 124844. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, T.; Zhao, S.; Rong, H.; Tian, Y.; Zhu, G. Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water. Chem. Eng. J. 2020, 382, 122893. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, Z.; Guo, F.; Yang, W. Ultrahigh-flux two-dimensional metal organic frameworks membrane for fast antibiotics removal. J. Membr. Sci. 2023, 686, 122026. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Hu, Z.; Wang, J.; Li, F.; Yang, W. Sustainable and ultrafast antibiotics removal, self-cleaning and disinfection with electroactive metal-organic frameworks/carbon nanotubes membrane. J. Hazard. Mater. 2024, 475, 134944. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, M.; Liu, H.; Zhu, Y.; Wang, D.; Yan, M. Adsorptive removal of dye and antibiotic from water with functionalized zirconium-based metal organic framework and graphene oxide composite nanomaterial Uio-66-(OH)2/GO. Appl. Surf. Sci. 2020, 525, 146614. [Google Scholar] [CrossRef]
- Xiao, H.; Chai, M.; Abdollahzadeh, M.; Ahmadi, H.; Chen, V.; Gore, D.B.; Asadnia, M.; Razmjou, A. A lithium ion selective membrane synthesized from a double layered Zrbased metalorganic framework (MOF-on-MOF) thin film. Desalination 2022, 532, 115733. [Google Scholar] [CrossRef]
- Crivello, C.; Sevim, S.; Graniel, O.; Franco, C.; Pane, S.; Puigmarti-Luis, J.; Munoz-Rojas, D. Advanced technologies for the fabrication of MOF thin films. Mater. Horizons 2021, 8, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Kulak, H.; Thür, R.; Vankelecom, I.F.J. MOF/Polymer Mixed-Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance. Membranes 2022, 12, 425. [Google Scholar] [CrossRef]
- Carja, I.D.; Tavares, S.R.; Shekhah, O.; Ozcan, A.; Semino, R.; Kale, V.S.; Eddaoudi, M.; Maurin, G. Insights into the Enhancement of MOF/Polymer Adhesion in Mixed-Matrix Membranes via Polymer Functionalization. ACS Appl. Mater. Interfaces 2021, 13, 29041–29047. [Google Scholar] [CrossRef]
- Wang, N.; Guo, X.; Xu, J.; Kong, X.; Gao, S.; Shan, Z. Pollution characteristics and environmental risk assessment of typical veterinary antibiotics in livestock farms in Southeastern China. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2014, 49, 468–479. [Google Scholar] [CrossRef]
- Bailey, C.; Spielmeyer, A.; Hamscher, G.; Schüttrumpf, H.; Frings, R.M. The veterinary antibiotic journey: Comparing the behaviour of sulfadiazine, sulfamethazine, sulfamethoxazole and tetracycline in cow excrement and two soils. J. Soils Sediments 2016, 16, 1690–1704. [Google Scholar] [CrossRef]
- Sanusi, I.O.; Olutona, G.O.; Wawata, I.G.; Onohuean, H. Occurrence, environmental impact and fate of pharmaceuticals in groundwater and surface water: A critical review. Environ. Sci. Pollut. Res. 2023, 30, 90595–90614. [Google Scholar] [CrossRef]
- Akhter, S.; Bhat, M.A.; Ahmed, S.; Siddiqui, W.A. Antibiotic residue contamination in the aquatic environment, sources and associated potential health risks. Environ. Geochem. Health 2024, 46, 387. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Sun, Q.; Wang, W.; Lu, L.; Zhang, K.; Xu, J.; et al. A review on pollution situation and treatment methods of tetracycline in groundwater. Sep. Sci. Technol. 2020, 55, 1005–1021. [Google Scholar] [CrossRef]
- Sikder, S.; Toha, M.; Anik, A.H.; Sultan, M.B.; Alam, M.; Parvin, F.; Tareq, S.M. A comprehensive review on the fate and impact of antibiotic residues in the environment and public health: A special focus on the developing countries. Water Environ. Res. 2024, 96, e10987. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Pratap, S.G.; Raj, A. Occurrence and dissemination of antibiotics and antibiotic resistance in aquatic environment and its ecological implications: A review. Environ. Sci. Pollut. Res. 2024, 31, 47505–47529. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.C.; Gillett, E.; Danielle, N.; Dionne, B. Tetracyclines, the old and the new: A narrative review. CMI Commun. 2025, 2, 105059. [Google Scholar] [CrossRef]
- Kounatidis, D.; Dalamaga, M.; Grivakou, E.; Karampela, I.; Koufopoulos, P.; Dalopoulos, V.; Adamidis, N.; Mylona, E.; Kaziani, A.; Vallianou, N.G. Third-Generation Tetracyclines: Current Knowledge and Therapeutic Potential. Biomolecules 2024, 14, 783. [Google Scholar] [CrossRef]
- Seyedeh, M.; Mohhamadreza, Y.; Seyed, S. Histopathological investigating of brain lesions caused by Toxoplasma gondii and ketamine- induced schizophrenia in mice after treatment with sulfadiazine-trimethoprim. J. Vet. Res. 2024, 15, 219–237. [Google Scholar]
- Kumar, N.; Gupta, R.K.; Gupta, A. a Recent Advances in the Antibacterial Potential of Sulfadiazine: A Review. World J. Pharm. Res. 2023, 12, 1296. [Google Scholar]
- Lopresto, C.M.; Palmeiro, B.S.; Cole, S.D.; Xu, X.; Dietrich, J.; Stefanovski, D. Chemical stability and in vitro antimicrobial efficacy of diluted silver sulfadiazine powder and cream over a six-month period. Vet. Dermatol. 2024, 35, 704–715. [Google Scholar] [CrossRef]
- Huang, Y.; Kang, H.; Wang, Y.; Liu, K.; Wei, W.; Dai, H. One Stone Three Birds: Silver Sulfadiazine Modulates the Stability and Dynamics of Hydrogels for Infected Wound Healing. Adv. Healthc. Mater. 2024, 13, 2400242. [Google Scholar] [CrossRef]
- Khodadad, N.; Hoseininejad, S.S.; Nazeri, A. Advantage of Amnion Dressing (Biological Dressing) + Silver Sulfadiazine Cream vs. Standard Silver Sulfadiazine Cream Dressings in Acute Deep Second-Degree and Third-Degree Burn Wounds: A Single Center Experience. MAEDICA—J. Clin. Med. 2024, 19, 756–763. [Google Scholar]
- Hemati, S.; Asnaashari, O.; Sarvizadeh, M.; Motlagh, B.N.; Akbari, M.; Tajvidi, M.; Gookizadeh, A. Topical silver sulfadiazine for the prevention of acute dermatitis during irradiation for breast cancer. Support. Care Cancer 2012, 20, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, Z.; Chen, X.; Jiao, C.; Li, H.; Yu, R. Facile Synthesis of Fe-based MOFs(Fe-BTC) as Efficient Adsorbent for Water Purifications. Chem. Res. Chinese Univ. 2019, 35, 564–569. [Google Scholar] [CrossRef]
- Castañeda-Ramírez, A.A.; Rojas-García, E.; López-Medina, R.; García-Martínez, D.C.; Nicolás- Antúnez, J.; Maubert-Franco, A.M. Magnetite nanoparticles into Fe-BTC MOF as adsorbent material for the remediation of metal (Cu(II), Pb(II, As(III) and Hg(II)) ions-contaminated water. Catal. Today 2022, 394–396, 94–102. [Google Scholar] [CrossRef]
- Ramírez, A.A.C.; García, E.R.; Medina, R.L.; Larios, J.L.C.; Parra, R.S.; Franco, A.M.M. Selective Adsorption of Aqueous Diclofenac Sodium, Naproxen Sodium, and Ibuprofen Using a Stable Fe3O4–FeBTC Metal–Organic Framework. Materials 2021, 14, 2293. [Google Scholar] [CrossRef]
- Dai, H.; Yuan, X.; Jiang, L.; Wang, H.; Zhang, J.; Zhang, J.; Xiong, T. Recent advances on ZIF-8 composites for adsorption and photocatalytic wastewater pollutant removal: Fabrication, applications and perspective. Coord. Chem. Rev. 2021, 441, 213985. [Google Scholar] [CrossRef]
- Kouser, S.; Hezam, A.; Khadri, M.J.N.; Khanum, S.A. A review on zeolite imidazole frameworks: Synthesis, properties, and applications. J. Porous Mater. 2022, 29, 663–681. [Google Scholar] [CrossRef]
- Karmakar, S.; Mukherjee, M.; Bhattacharya, P.; Neogi, S.; De, S. Mechanistic insights into the antibacterial property of MIL-100 (Fe) metal-organic framework. J. Environ. Chem. Eng. 2024, 12, 113088. [Google Scholar] [CrossRef]
- Taheri, M.; Ashok, D.; Sen, T.; Enge, T.G.; Verma, N.K.; Tricoli, A.; Lowe, A.; Nisbet, D.R.; Tsuzuki, T. Stability of ZIF-8 nanopowders in bacterial culture media and its implication for antibacterial properties. Chem. Eng. J. 2021, 413, 127511. [Google Scholar] [CrossRef]
- Fu, X.; Tian, X.; Lin, J.; Wang, Q.; Gu, L.; Wang, Z.; Chi, M.; Yu, B.; Feng, Z.; Liu, W.; et al. Zeolitic Imidazolate Framework-8 Offers an Anti-Inflammatory and Antifungal Method in the Treatment of Aspergillus Fungal Keratitis in vitro and in vivo. Int. J. Nanomed. 2024, 19, 11163–11179. [Google Scholar] [CrossRef]
- Elaouni, A.; El Ouardi, M.; Zbair, M.; BaQais, A.; Saadi, M.; Ait Ahsaine, H. ZIF-8 metal organic framework materials as a superb platform for the removal and photocatalytic degradation of organic pollutants: A review. RSC Adv. 2022, 12, 31801–31817. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, L.; Liu, Y.; Zhu, M. A review on zeolitic imidazolate framework-8 based materials with special wettability for oil/water separation. J. Environ. Chem. Eng. 2023, 11, 111360. [Google Scholar] [CrossRef]
- Hu, X.; Lou, X.; Li, C.; Ning, Y.; Liao, Y.; Chen, Q.; Mananga, E.S.; Shen, M.; Hu, B. Facile synthesis of the Basolite F300-like nanoscale Fe-BTC framework and its lithium storage properties. RSC Adv. 2016, 6, 114483–114490. [Google Scholar] [CrossRef]
- Qu, Z.; Lai, C.; Zhao, G.; Knebel, A.; Fan, H.; Meng, H. Pore engineering in covalent organic framework membrane for gas separation. Adv. Membr. 2022, 2, 100037. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Qiu, G.; Jia, W.; Guo, Y.; Guo, F.; Wu, J. Synthesis of porous material from coal gasification fine slag residual carbon and its application in removal of methylene blue. Molecules 2021, 26, 6116. [Google Scholar] [CrossRef]
- Wu, F.C.; Liu, B.L.; Wu, K.T.; Tseng, R.L. A new linear form analysis of Redlich-Peterson isotherm equation for the adsorptions of dyes. Chem. Eng. J. 2010, 162, 21–27. [Google Scholar] [CrossRef]
- Tran, H.N.; Thanh Trung, N.P.; Lima, E.C.; Bollinger, J.C.; Dat, N.D.; Chao, H.P.; Juang, R.S. Revisiting the calculation of thermodynamic parameters of adsorption processes from the modified equilibrium constant of the Redlich–Peterson model. J. Chem. Technol. Biotechnol. 2023, 98, 462–472. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Aldás, M.B.; Cabrera, G.; Guerrero, V.H. Caffeine removal from synthetic wastewater using magnetic fruit peel composites: Material characterization, isotherm and kinetic studies. Environ. Chall. 2021, 5, 100343. [Google Scholar] [CrossRef]
- Gabriunaite, I.; Valincius, G.; Žilinskas, A.; Valiūnienė, A. Tethered Bilayer Membrane Formation on Silanized Fluorine Doped Tin Oxide Surface. J. Electrochem. Soc. 2022, 169, 037515. [Google Scholar] [CrossRef]
- Shayesteh, H.; Rahbar-Kelishami, A.; Norouzbeigi, R. Superhydrophobic/superoleophilic micro/nanostructure nickel particles for oil/water mixture and emulsion separation. Ceram. Int. 2022, 48, 10999–11008. [Google Scholar] [CrossRef]
- Van Tran, T.; Jalil, A.A.; Nguyen, D.T.C.; Nguyen, T.M.; Alhassan, M.; Nabgan, W.; Rajendran, S.; Firmansyah, M.L. Novel ZIF-67-derived Co@CNTs nanocomposites as effective adsorbents for removal of tetracycline and sulfadiazine antibiotics. Environ. Res. 2023, 225, 115516. [Google Scholar] [CrossRef] [PubMed]
- Gouamid, M.; Ouahrani, M.R.; Bensaci, M.B. Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using Date palm Leaves. Energy Procedia 2013, 36, 898–907. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Debord, J.; Harel, M.; Bollinger, J.C.; Chu, K.H. The Elovich isotherm equation: Back to the roots and new developments. Chem. Eng. Sci. 2022, 262, 118012. [Google Scholar] [CrossRef]
- Mohamed Nasser, S.; Abbas, M.; Trari, M. Understanding the rate-limiting step adsorption kinetics onto biomaterials for mechanism adsorption control. Prog. React. Kinet. Mech. 2024, 49, 14686783241226858. [Google Scholar] [CrossRef]
- How, C.K.; Soltani, S.; Chuah, T.G.; Phuah, E.T.; Choong, T.S.Y. Adsorption properties of β-carotene on mesoporous carbon-coated honeycomb monolith: Kinetics, thermodynamics, and regeneration studies. Korean J. Chem. Eng. 2022, 39, 3109–3120. [Google Scholar] [CrossRef]
- Ren, D.; Zhu, B.; Xiong, J.; Huang, K.; Cai, M.; Liu, C.; Bai, X.; Liu, T.; Zhang, X.; Zou, B. A novel design of copper selenide/zinc selenide/Nitrogen-doped carbon derived from MOF for sulfadiazine adsorption: Performance and mechanism. J. Colloid Interface Sci. 2024, 669, 804–815. [Google Scholar] [CrossRef]
- Demba, A.N.; Sid, M.; Kankou, A. Modeling of adsorption isotherms of pharmaceutical products onto various adsorbents: A Short Review. J. Mater. Environ. Sci 2020, 1264, 1264–1276. [Google Scholar]
- Hu, Q.; Lan, R.; He, L.; Liu, H.; Pei, X. A critical review of adsorption isotherm models for aqueous contaminants: Curve characteristics, site energy distribution and common controversies. J. Environ. Manag. 2023, 329, 117104. [Google Scholar] [CrossRef]
- Zhai, G.; Ma, Y.; Liu, H.; Jia, H.; Wang, S.; Liu, S. Adsorption of sulfadiazine from water by Pedicularis kansuensis derived biochar: Preparation and properties studies. J. Ind. Eng. Chem. 2024, 143, 581–591. [Google Scholar] [CrossRef]
- Butyrskaya, E. Understanding the mechanism of monolayer adsorption from isotherm. Adsorption 2024, 30, 1395–1406. [Google Scholar] [CrossRef]
- Cao, L.; Chen, X.; Peng, Y. The adsorption and orientation of frother surfactants on heterogeneous wetting surfaces. Appl. Surf. Sci. 2021, 548, 149225. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Metal-organic frameworks supported on nanofibers to remove heavy metals. J. Mater. Chem. A 2018, 6, 4550–4555. [Google Scholar] [CrossRef]
- Latrach, Z.; Moumen, E.; Kounbach, S.; El Hankari, S. Mixed-Ligand Strategy for the Creation of Hierarchical Porous ZIF-8 for Enhanced Adsorption of Copper Ions. ACS Omega 2022, 7, 15862–15869. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Y.; Shen, G.; Zhang, H.; Yuan, Z.; Zhang, W. Adsorption/desorption behavior and mechanisms of sulfadiazine and sulfamethoxazole in agricultural soil systems. Soil Tillage Res. 2019, 186, 233–241. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Vallés, J.M.; Baschini, M.T.; Avena, M.J. Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl. Clay Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Hooriabad Saboor, F.; Nasirpour, N.; Shahsavari, S.; Kazemian, H. The Effectiveness of MOFs for the Removal of Pharmaceuticals from Aquatic Environments: A Review Focused on Antibiotics Removal. Chem.—Asian J. 2022, 17, e202101105. [Google Scholar] [CrossRef]
- Huang, S.; Du, P.; Min, C.; Liao, Y.; Sun, H.; Jiang, Y. Poly(1-amino-5-chloroanthraquinone): Highly Selective and Ultrasensitive Fluorescent Chemosensor for Ferric Ion. J. Fluoresc. 2013, 23, 621–627. [Google Scholar] [CrossRef]
- Khan, A.; Wang, J.; Li, J.; Wang, X.; Chen, Z.; Alsaedi, A.; Hayat, T.; Chen, Y.; Wang, X. The role of graphene oxide and graphene oxide-based nanomaterials in the removal of pharmaceuticals from aqueous media: A review. Environ. Sci. Pollut. Res. 2017, 24, 7938–7958. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Zhao, F.; Fang, S.; Gao, Y.; Bi, J. Removal of aqueous pharmaceuticals by magnetically functionalized Zr-MOFs: Adsorption Kinetics, Isotherms, and regeneration. J. Colloid Interface Sci. 2022, 615, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, A.; Długoń, E. The FTIR studies of gels and thin films of Al 2O 3-TiO 2 and Al 2O 3-TiO 2-SiO 2 systems. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2012, 89, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Valente, P.A.; Mota, S.I.; Teixeira, A.; Ferreiro, E.; Sarmento, H.; Cipriano, I.; Campos, J.R.; Rama, L.; Oliveira, P.J. Fourier Transform Infrared (FTIR) Spectroscopy as a Tool to Characterize Exercise and Physical Activity: A Systematic Review. Sport. Med. 2024, 55, 459–472. [Google Scholar] [CrossRef]
- Frank, F.; Baumgartner, B.; Lendl, B. Metal-organic frameworks combined with mid-infrared spectroscopy for the trace analysis of phosphates in water. Sens. Actuators B Chem. 2024, 399, 134778. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Chiavetta, D.; Wolden, C.A. Formation of octadecyltrichlorosilane (OTS) self-assembled monolayers on amorphous alumina. Appl. Surf. Sci. 2013, 282, 291–296. [Google Scholar] [CrossRef]
- Sann, E.E.; Pan, Y.; Gao, Z.; Zhan, S.; Xia, F. Highly hydrophobic ZIF-8 particles and application for oil-water separation. Sep. Purif. Technol. 2018, 206, 186–191. [Google Scholar] [CrossRef]
- Burts, K.; Plisko, T.; Dmitrenko, M.; Zolotarev, A.; Kuzminova, A.; Bildyukevich, A.; Ermakov, S.; Penkova, A. Novel Thin Film Nanocomposite Membranes Based on Chitosan Succinate Modified with Fe-BTC for Enhanced Pervaporation Dehydration of Isopropanol. Membranes 2022, 12, 653. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, H.; Yang, F.; Gray, S. Cost and efficiency perspectives of ceramic membranes for water treatment. Water Res. 2022, 220, 118629. [Google Scholar] [CrossRef]
- Kallem, P.; Ouda, M.; Bharath, G.; Hasan, S.W.; Banat, F. Enhanced water permeability and fouling resistance properties of ultrafiltration membranes incorporated with hydroxyapatite decorated orange-peel-derived activated carbon nanocomposites. Chemosphere 2022, 286, 131799. [Google Scholar] [CrossRef]
- Miri, S.; De Girolamo, A.; Nadeem, H.; Chin, B.W.X.; Hora, Y.; Andrews, P.C.; Batchelor, W. Composite membranes of cellulose–mesoporous silica: Optimization of membrane fabrication and adsorption capacity. Cellulose 2023, 30, 339–357. [Google Scholar] [CrossRef]
- Li, Z.; Luo, X.; Li, Y. Reed Rhizome Residue-Based Activated Carbon Adsorption Ultrafiltration Membranes for Enhanced MB Removal. ACS Omega 2022, 7, 43829–43838. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Xiong, Z.H.; Li, C.; Zhang, J.M. Zeolitic imidazolate metal organic framework ZIF-8 with ultra-high adsorption capacity bound tetracycline in aqueous solution. RSC Adv. 2015, 5, 82127–82137. [Google Scholar] [CrossRef]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Schäfer, A.I.; Elimelech, M. Pharmaceutical retention mechanisms by nanofiltration membranes. Environ. Sci. Technol. 2005, 39, 7698–7705. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Firoozpour, L.; Vanneste, J.; Luis, P.; Degrève, J.; Van der Bruggen, B. Performance of solvent resistant nanofiltration membranes for purification of residual solvent in the pharmaceutical industry: Experiments and simulation. Green Chem. 2011, 13, 3476–3483. [Google Scholar] [CrossRef]
- Gur-Reznik, S.; Koren-Menashe, I.; Heller-Grossman, L.; Rufel, O.; Dosoretz, C.G. Influence of seasonal and operating conditions on the rejection of pharmaceutical active compounds by RO and NF membranes. Desalination 2011, 277, 250–256. [Google Scholar] [CrossRef]
- Pan, S.-F.; Zhu, M.-P.; Chen, J.P.; Yuan, Z.-H.; Zhong, L.-B.; Zheng, Y.-M. Separation of tetracycline from wastewater using forward osmosis process with thin film composite membrane—Implications for antibiotics recovery. Sep. Purif. Technol. 2015, 153, 76–83. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, Y.; Sun, S.; Xin, X.; Wang, M.; Zhao, Q.; Zhu, H.; Jia, R. Removal of sulfadiazine in a modified ultrafiltration membrane (PVDF-PVP-TiO2-FeCl3) filtration-photocatalysis system: Parameters optimizing and interferences of drinking water. Environ. Sci. Pollut. Res. 2020, 27, 45605–45617. [Google Scholar] [CrossRef]
- Arminini Neto, A.; Januário, E.F.D.; Vidovix, T.B.; Beluci, N.D.C.L.; Bergamasco, R.; Vieira, A.M.S. The role of membrane technology in addressing pharmaceutical pollution in water. Chem. Eng. Process.—Process Intensif. 2024, 202, 109837. [Google Scholar] [CrossRef]
- Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; León, G.; Miguel, B.; Gago, I.; Martínez, P.M. Ibuprofen Removal by Graphene Oxide and Reduced Graphene Oxide Coated Polysulfone Nanofiltration Membranes. Membranes 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Chen, Y.; Huang, C.H.; Sun, P.; Crittenden, J. Rejection and adsorption of trace pharmaceuticals by coating a forward osmosis membrane with TiO2. Chem. Eng. J. 2015, 279, 904–911. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Yang, C.; Li, S. Systematic evaluation of TiO2-GO-modified ceramic membranes for water treatment: Retention properties and fouling mechanisms. Chem. Eng. J. 2019, 378, 122138. [Google Scholar] [CrossRef]
- Zhou, A.; Jia, R.; Wang, Y.; Sun, S.; Xin, X.; Wang, M.; Zhao, Q.; Zhu, H. Abatement of sulfadiazine in water under a modified ultrafiltration membrane (PVDF-PVP-TiO2-dopamine) filtration-photocatalysis system. Sep. Purif. Technol. 2020, 234, 116099. [Google Scholar] [CrossRef]
- Kong, F.-X.; Zhang, S.-Y.; Zhang, D.-W.; Xia, P.; Chen, J.-F. The influence of the preheated PSf substrate on COF-LZU1 layer formation and the performance for salt and pharmaceutical rejection. Desalination 2024, 579, 117514. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, B.; Yang, F. Removal of sulfadiazine by polyamide nanofiltration membranes: Measurement, modeling, and mechanisms. Membranes 2021, 11, 104. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kim, S.; Shin, J.; Rho, H.; Kim, Y.M.; Cho, K.H.; Eom, H.; Oh, S.E.; Cho, J.; Chon, K. Sequential effects of cleaning protocols on desorption of reverse osmosis membrane foulants: Autopsy results from a full-scale desalination plant. Desalination 2021, 500, 114830. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Lovitt, R.W. Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 2007, 303, 4–28. [Google Scholar] [CrossRef]

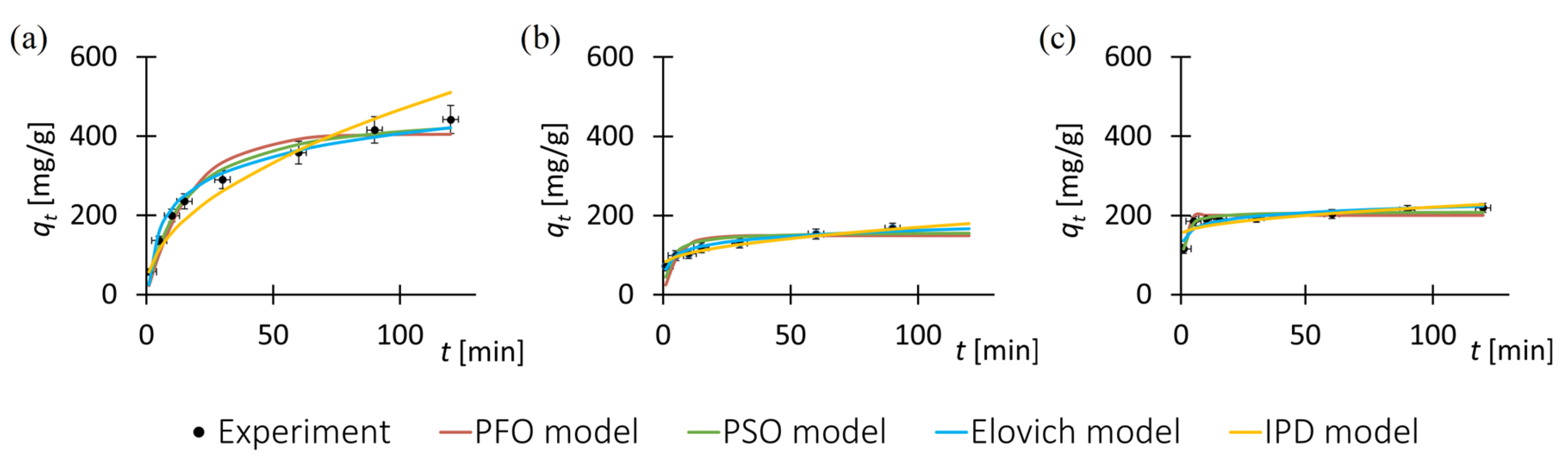



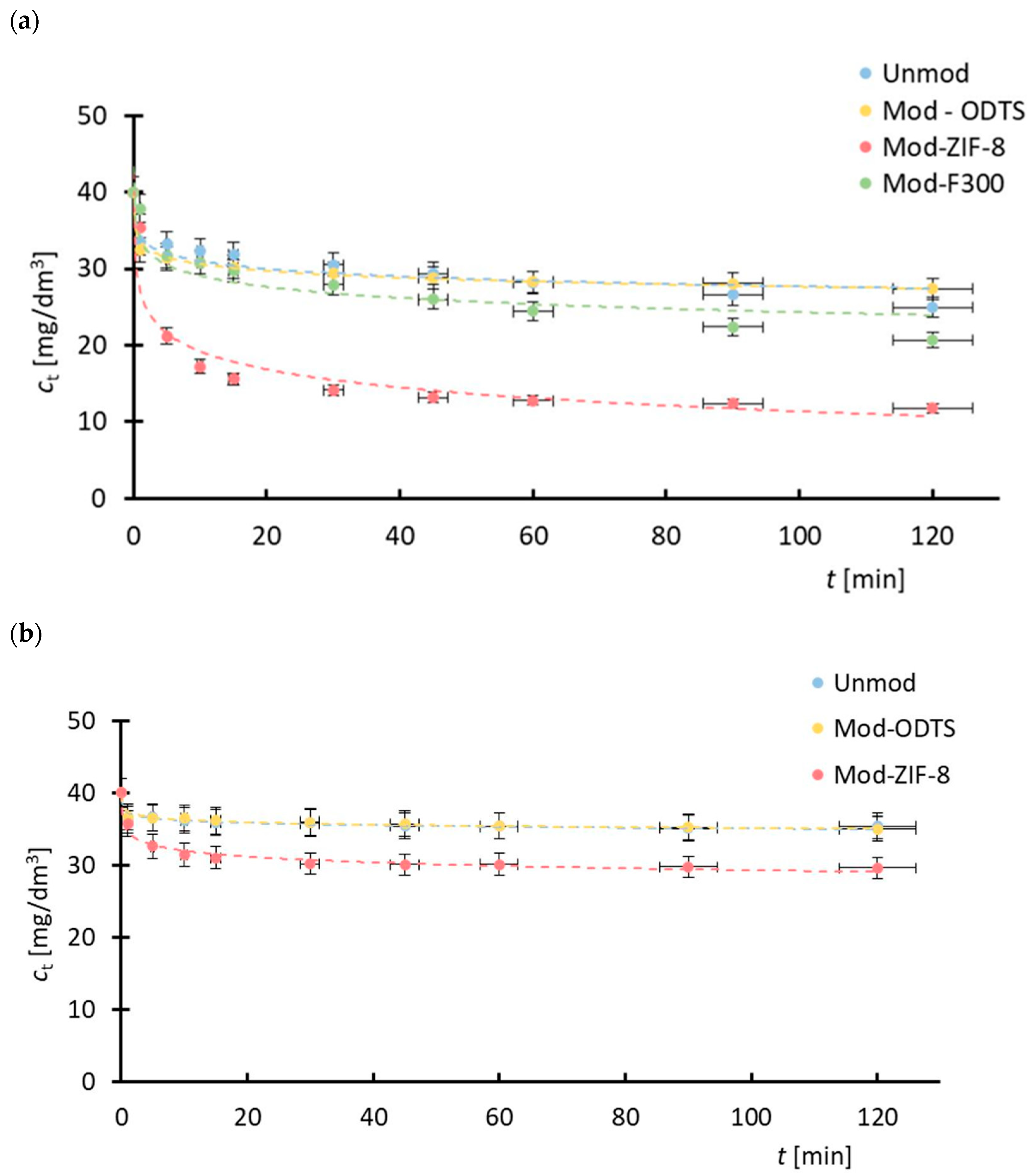



| MOF | Pharmaceutical Substance | q120 (exp) [mg/g] | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|---|
| qe [mg/g] | k1 [1/min] | R2 | qe [mg/g] | k2 [mg/(g·min)] | R2 | |||
| ZIF-8 | Tetracycline | 442.2 | 405.6 | 0.06 | 0.94 | 472.5 | 32.12 | 0.98 |
| Sulfadiazine | 219.3 | 200.5 | 0.83 | 0.87 | 209.5 | 256.88 | 0.95 | |
| F300 | Tetracycline | 170.4 | 149.3 | 0.18 | 0.97 | 158.5 | 62.41 | 0.98 |
| MOF | Pharmaceutical Substance | Redlich–Peterson | |||||
|---|---|---|---|---|---|---|---|
| kR | B | β | R2 | SSE | p | ||
| ZIF-8 | Tetracycline | 13.1 | 0.001 | 0.84 | 0.99 | 908,750 | 0.41 |
| Sulfadiazine | 9.4 | 0.027 | 1.15 | 0.98 | 70,711 | 0.41 | |
| F300 | Tetracycline | 7.4 | 0.006 | 0.83 | 1.00 | 86,203 | 0.45 |
| Sips | |||||||
| kL | qmax | 1/n | R2 | SSE | p | ||
| ZIF-8 | Tetracycline | 0.004 | 3145 | 0.99 | 0.99 | 1,899,596 | 0.39 |
| Sulfadiazine | 0.023 | 488 | 1.10 | 0.99 | 192,995 | 0.39 | |
| F300 | Tetracycline | 0.014 | 507 | 0.98 | 0.99 | 86,027 | 0.47 |
| Langmuir | |||||||
| kL | qmax | R2 | SSE | p | |||
| ZIF-8 | Tetracycline | 0.004 | 3065 | 0.99 | 4478 | 0.41 | |
| Sulfadiazine | 0.015 | 595 | 0.98 | 1680 | 0.41 | ||
| F300 | Tetracycline | 0.014 | 499 | 1.00 | 112 | 0.45 | |
| Freundlich | |||||||
| kF | 1/n | R2 | SSE | p | |||
| ZIF-8 | Tetracycline | 21.6 | 0.81 | 0.99 | 895,036 | 0.33 | |
| Sulfadiazine | 17.4 | 0.69 | 0.97 | 70,602 | 0.33 | ||
| F300 | Tetracycline | 18.3 | 0.61 | 0.99 | 83,481 | 0.36 | |
| Type of Membrane | ||||
|---|---|---|---|---|
| Unmod | Mod-ODTS | Mod-ZIF-8 | Mod-F300 | |
| Contact angle [°] | <10 | 84.3 | 118.6 | 99.1 |
| Desorption Agents | Desorption Agents Volume [cm3] | Antibiotics Amount in Desorption Agents | |||
|---|---|---|---|---|---|
| Tetracycline Concentration [mg/dm3] | Tetracycline Mass [g] | Sulfadiazine Concentration [mg/dm3] | Sulfadiazine Mass [g] | ||
| EtOH | 200 | 12.32 | 2.46 | 13.64 | 2.73 |
| 200 | 4.08 | 0.82 | 2.98 | 0.60 | |
| 200 | 2.36 | 0.47 | 0.36 | 0.07 | |
| NaOH | 1500 | 3.87 | 5.80 | 0.73 | 1.10 |
| Sum | 9.55 | 4.50 | |||
| Recovery | 68% | 87% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polak, D.; Kamocki, S.; Szwast, M. Evaluation of the Potential of Metal–Organic Compounds ZIF-8 and F300 in a Membrane Filtration–Adsorption Process for the Removal of Antibiotics from Water. Antibiotics 2025, 14, 619. https://doi.org/10.3390/antibiotics14060619
Polak D, Kamocki S, Szwast M. Evaluation of the Potential of Metal–Organic Compounds ZIF-8 and F300 in a Membrane Filtration–Adsorption Process for the Removal of Antibiotics from Water. Antibiotics. 2025; 14(6):619. https://doi.org/10.3390/antibiotics14060619
Chicago/Turabian StylePolak, Daniel, Szymon Kamocki, and Maciej Szwast. 2025. "Evaluation of the Potential of Metal–Organic Compounds ZIF-8 and F300 in a Membrane Filtration–Adsorption Process for the Removal of Antibiotics from Water" Antibiotics 14, no. 6: 619. https://doi.org/10.3390/antibiotics14060619
APA StylePolak, D., Kamocki, S., & Szwast, M. (2025). Evaluation of the Potential of Metal–Organic Compounds ZIF-8 and F300 in a Membrane Filtration–Adsorption Process for the Removal of Antibiotics from Water. Antibiotics, 14(6), 619. https://doi.org/10.3390/antibiotics14060619








