Cellulose Nanocrystal/Zinc Oxide Bio-Nanocomposite Activity on Planktonic and Biofilm Producing Pan Drug-Resistant Clostridium perfringens Isolated from Chickens and Turkeys
Abstract
1. Introduction
2. Results
2.1. Prevalence of C. Perfringens in Poultry Samples
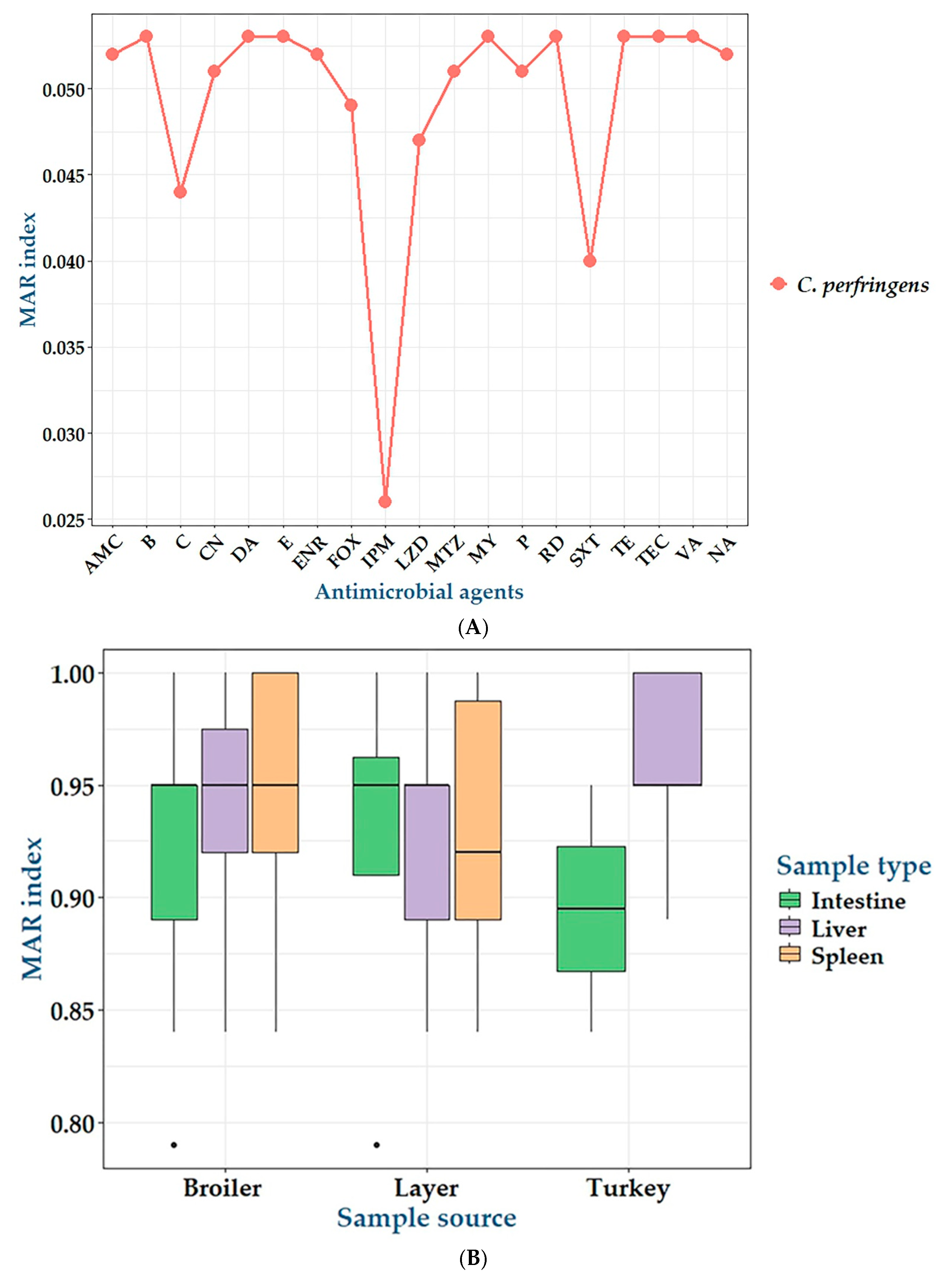
2.2. Antibiogram of C. perfringens Isolates
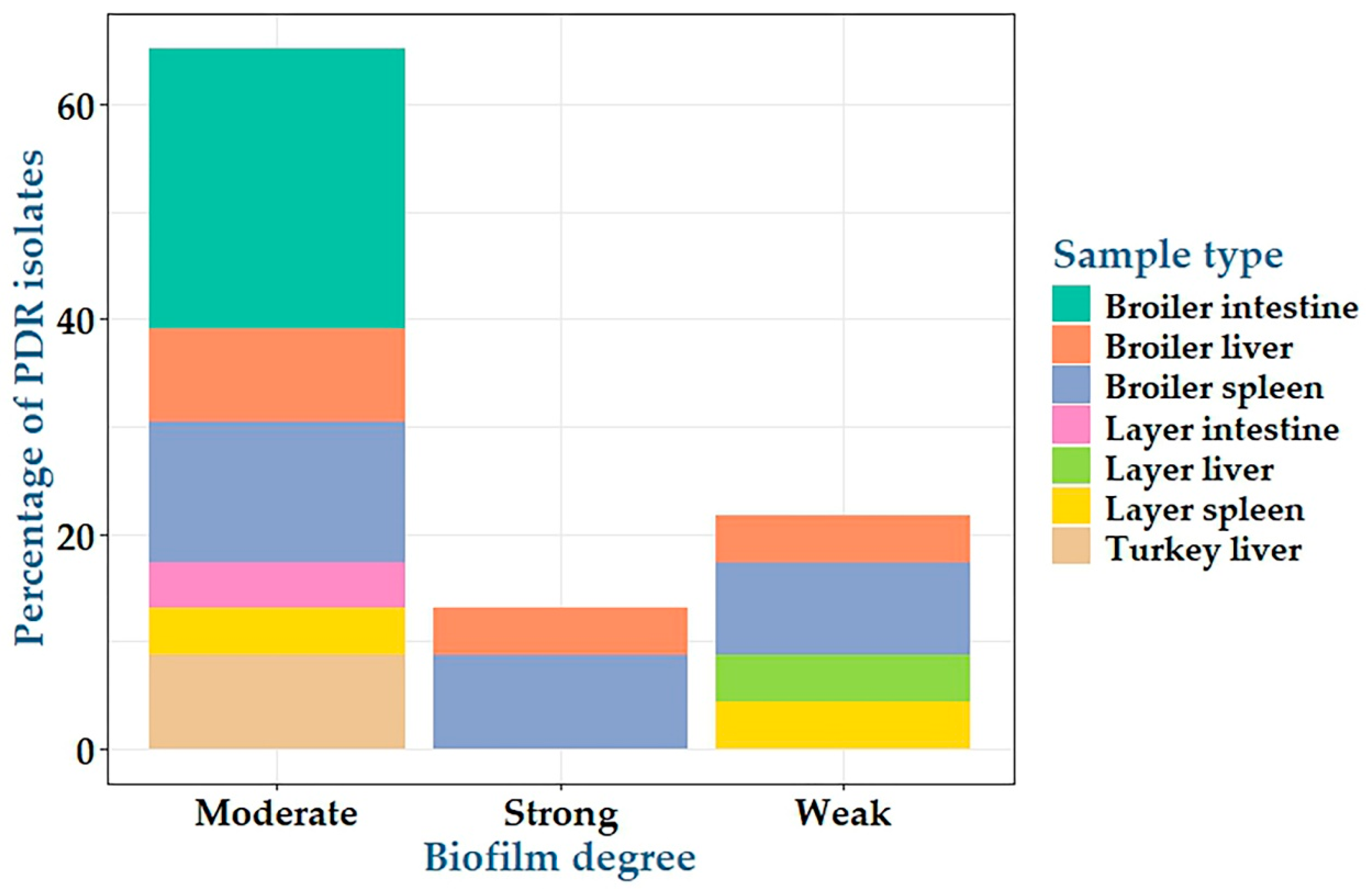
2.3. Biofilm Formation by C. perfringens Isolates
2.4. Antimicrobial Activity of CNCs/ZnO Bio-Nanocomposite Against PDR C. perfringens Isolates
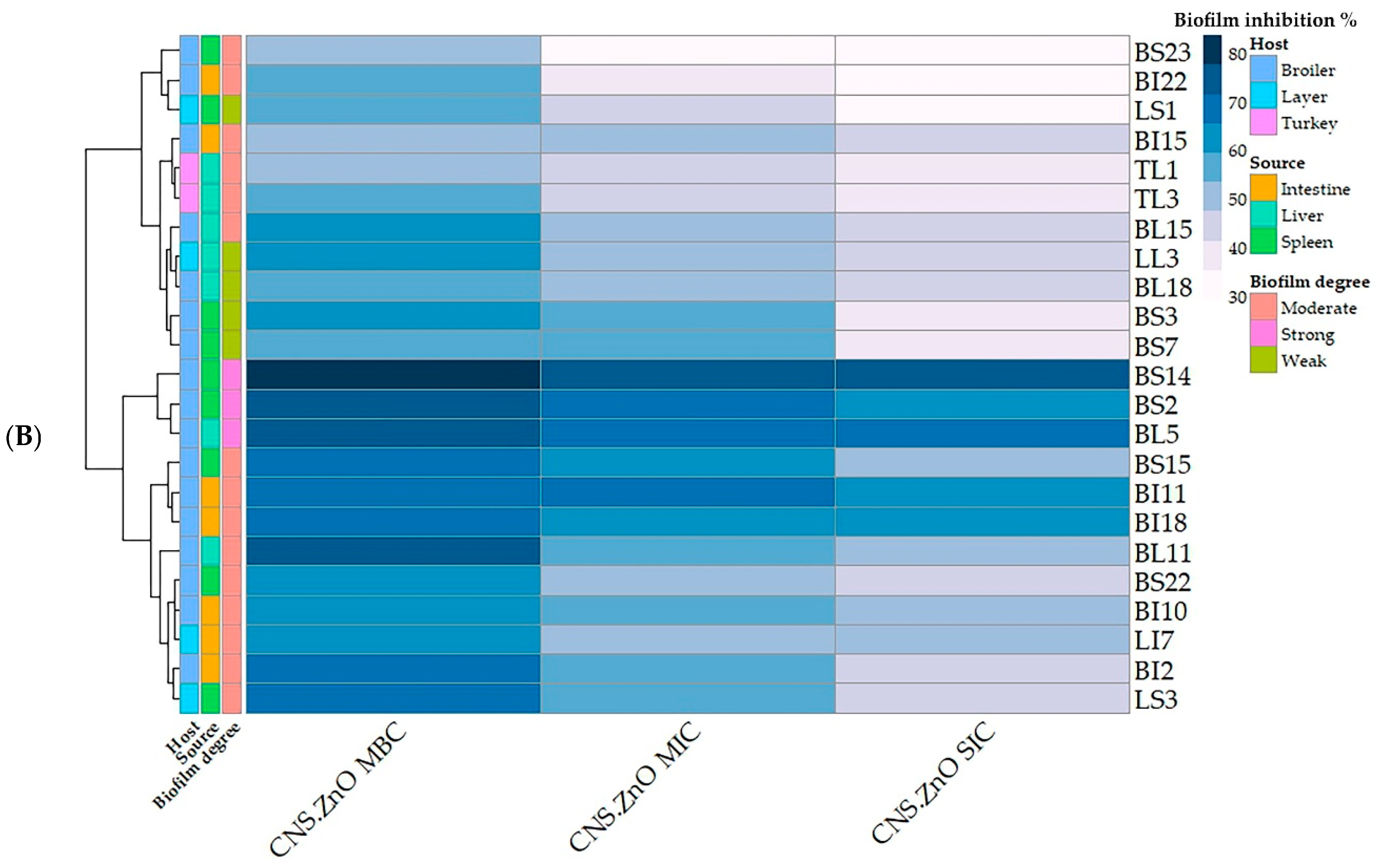
2.5. Antibiofilm Activity of CNCs/ZnO Bio-Nanocomposite Against PDR C. perfringens
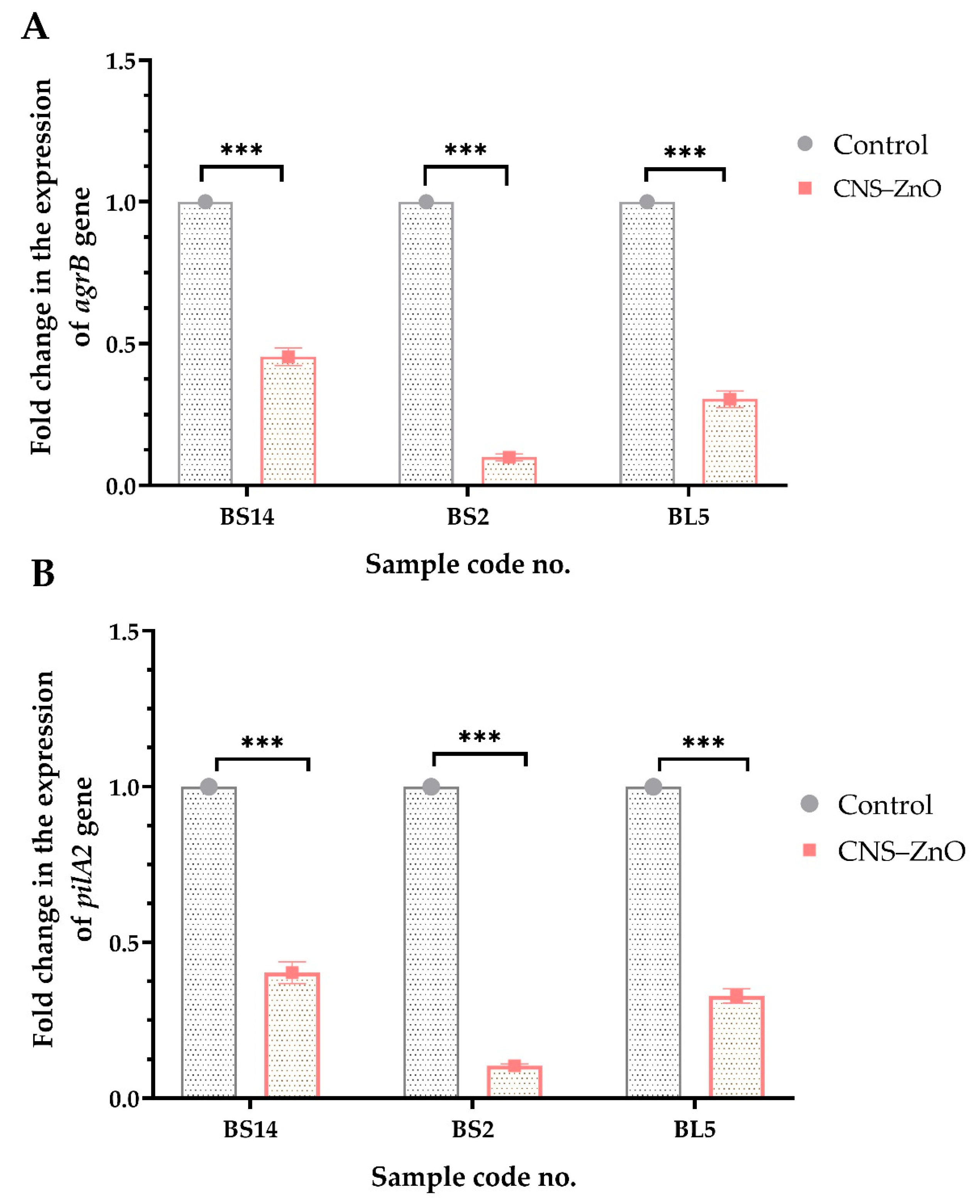
2.6. Expression Analysis of CNCs/ZnO Bio-Nanocomposite Against PDR C. perfringens Using RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Samples and Ethical Approval
4.2. Bacteriological Analysis and Molecular Identification
4.3. Antimicrobial Susceptibility Testing
4.4. Biofilm Growth and Quantification
4.4.1. Qualitative Congo Red Agar Method
4.4.2. Quantitative Microtiter Plate Assay
4.5. Synthesis of CNCs/ZnO Bio-Nanocomposite
4.6. Antimicrobial Activities of CNCs/ZnO Against PDR C. perfringens
4.7. Antibiofilm Activities of CNCs/ZnO Bio-Nanocomposite Against Biofilm Producing C. perfringens
4.8. Gene Expression of Biofilm Biosynthesis Genes by Real-Time Quantitative PCR (RT-qPCR)
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mottet, A.; Tempio, G. Global poultry production: Current state and future outlook and challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.K.; Songer, J.G.; Uzal, F.A. Diagnosing clostridial enteric disease in poultry. J. Vet. Diagn. Investig. 2013, 25, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Fathima, S.; Hakeem, W.G.A.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic enteritis in broiler chickens: A review on the pathogen, pathogenesis, and prevention. Microorganisms 2022, 10, 1958. [Google Scholar] [CrossRef]
- Gharib-Naseri, K.; Kheravii, S.; Keerqin, C.; Morgan, N.; Swick, R.; Choct, M.; Wu, S.-B. Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poult. Sci. 2019, 98, 6422–6432. [Google Scholar] [CrossRef]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef]
- Sattar, M.M.K.; Anjum, A.A.; Chang, Y.F.; Yaqub, T.; Aslam, A.; Ali, T. Molecular characterization and toxins optimization of indigenous Clostridium perfringens toxinotype B isolated from lamb dysentery clinical cases. Kafkas Univ. Vet. Fak. Derg. 2023, 29, 79. [Google Scholar]
- de Souza, M.; Baptista, A.A.S.; Menck-Costa, M.F.; Justino, L.; da Glória, E.M.; Shimizu, G.D.; Ferraz, C.R.; Verri, W.A.; Van Immerseel, F.; Bracarense, A. Modulation of broiler intestinal changes induced by Clostridium perfringens and deoxynivalenol through probiotic, paraprobiotic, and postbiotic supplementation. Toxins 2024, 16, 46. [Google Scholar] [CrossRef]
- Aqeel, M.; Mirani, A.H.; Khoso, P.A.; Sahito, J.K.; Bhutto, A.L.; Leghari, R.A.; Rahimoon, M.M.; Ali, K.; Ali, N. A review on the study of immunomodulators and herbal remedies: A natural approach to treating necrotic enteritis. Pure Appl. Biol. (PAB) 2024, 13, 275–302. [Google Scholar] [CrossRef]
- El-Aziz, N.K.A.; Tartor, Y.H.; Gharieb, R.M.A.; Erfan, A.M.; Khalifa, E.; Said, M.A.; Ammar, A.M.; Samir, M. Extensive drug-resistant Salmonella enterica Isolated from poultry and humans: Prevalence and molecular determinants behind the co-resistance to ciprofloxacin and tigecycline. Front. Microbiol. 2021, 12, 738784. [Google Scholar] [CrossRef]
- Góchez, D.; Raicek, M.; Pinto Ferreira, J.; Jeannin, M.; Moulin, G.; Erlacher-Vindel, E. OIE annual report on antimicrobial agents intended for use in animals: Methods used. Front. Vet. Sci. 2019, 6, 317. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Löffler, S.; Richter-Dahlfors, A. High-resolution large-area image analysis deciphers the distribution of Salmonella cells and ECM components in biofilms formed on charged PEDOT: PSS surfaces. Adv. Sci. 2024, 11, 2307322. [Google Scholar] [CrossRef] [PubMed]
- Pantaléon, V.; Bouttier, S.; Soavelomandroso, A.P.; Janoir, C.; Candela, T. Biofilms of Clostridium species. Anaerobe 2014, 30, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.M.; Lanigan, N.; O’Neill, I.J.; Bottacini, F.; Lugli, G.A.; Viappiani, A.; Turroni, F.; Ventura, M.; van Sinderen, D. Bifidobacterial biofilm formation is a multifactorial adaptive phenomenon in response to bile exposure. Sci. Rep. 2020, 10, 11598. [Google Scholar] [CrossRef]
- Yu, S.; Su, T.; Wu, H.; Liu, S.; Wang, D.; Zhao, T.; Jin, Z.; Du, W.; Zhu, M.-J.; Chua, S.L. PslG, a self-produced glycosyl hydrolase, triggers biofilm disassembly by disrupting exopolysaccharide matrix. Cell Res. 2015, 25, 1352–1367. [Google Scholar] [CrossRef]
- Czuban, M.; Srinivasan, S.; Yee, N.A.; Agustin, E.; Koliszak, A.; Miller, E.; Khan, I.; Quinones, I.; Noory, H.; Motola, C. Bio-orthogonal chemistry and reloadable biomaterial enable local activation of antibiotic prodrugs and enhance treatments against Staphylococcus aureus infections. ACS Cent. Sci. 2018, 4, 1624–1632. [Google Scholar] [CrossRef]
- De Oliveira, A.; Cataneli Pereira, V.; Pinheiro, L.; Moraes Riboli, D.F.; Benini Martins, K.; Ribeiro de Souza da Cunha, M.d.L. Antimicrobial resistance profile of planktonic and biofilm cells of Staphylococcus aureus and coagulase-negative staphylococci. Int. J. Mol. Sci. 2016, 17, 1423. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial biofilm formation on biomaterials and approaches to its treatment and prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef]
- Seukep, A.J.; Mbuntcha, H.G.; Kuete, V.; Chu, Y.; Fan, E.; Guo, M.-Q. What Approaches to thwart bacterial efflux pumps-mediated resistance? Antibiotics 2022, 11, 1287. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Dovnar, R.; Smotryn, S.; Anufrik, S.; Anuchin, S.; Dovnar, I.; Iaskevich, N. Copper and selenium nanoparticles as a new tool against antibiotic-resistant pathogenic microorganisms. Surg. East. Eur. 2022, 11, 315–328. [Google Scholar]
- Hashem, A.H.; Al-Askar, A.A.; Haponiuk, J.; Abd-Elsalam, K.A.; Hasanin, M.S. Biosynthesis, characterization, and antifungal activity of novel trimetallic copper oxide–selenium–zinc oxide nanoparticles against some mucorales fungi. Microorganisms 2023, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Raja, F.N.; Worthington, T.; Martin, R.A. The antimicrobial efficacy of copper, cobalt, zinc and silver nanoparticles: Alone and in combination. Biomed. Mater. 2023, 18, 045003. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Khan, S.A.; Walsh, L.J.; Seneviratne, C.J.; Ziora, Z.M. Characteristics of metallic nanoparticles (especially silver nanoparticles) as anti-biofilm agents. Antibiotics 2024, 13, 819. [Google Scholar] [CrossRef] [PubMed]
- Terea, H.; Rebiai, A.; Selloum, D.; Tedjani, M.L. Cellulose/ZnO nanoparticles (CNC/ZnO NPs): Synthesis, characterization, and evaluation of their antibacterial and antifungal activities. Cellulose 2024, 31, 5027–5042. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO nanostructures in active antibacterial food packaging: Preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Dawwam, G.E.; Al-Shemy, M.T.; El-Demerdash, A.S. Green synthesis of cellulose nanocrystal/ZnO bio-nanocomposites exerting antibacterial activity and downregulating virulence toxigenic genes of food-poisoning bacteria. Sci. Rep. 2022, 12, 16848. [Google Scholar] [CrossRef]
- García-Vela, S.; Martínez-Sancho, A.; Said, L.B.; Torres, C.; Fliss, I. Pathogenicity and antibiotic resistance diversity in Clostridium perfringens isolates from poultry affected by necrotic enteritis in Canada. Pathogens 2023, 12, 905. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Eman, M.; Sharaf, D.M. Comparing the effect of nitazoxanide and tylosin against necrotic enteritis in broilers. J. Adv. Vet. Res. 2024, 14, 8–16. [Google Scholar]
- Gomaa, N.H.; El-Aziz, N.K.A.; El-Naenaeey, E.-s.Y.; Abdelaziz, W.S.; Sewid, A.H. Antimicrobial potential of myricetin-coated zinc oxide nanocomposite against drug-resistant Clostridium perfringens. BMC Microbiol. 2023, 23, 79. [Google Scholar] [CrossRef] [PubMed]
- Immerseel, F.V.; Buck, J.D.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H.; El Khashab, E.; Shakal, M.; Morsy, E.A. Unveiling antibiotic resistance, virulence, and molecular detection of enteric bacterial infections in broilers: A Study in Egypt. Egypt. J. Vet. Sci. 2024, 55, 1537–1551. [Google Scholar] [CrossRef]
- Helal, S.S.; Khalaf, N.M.; El Menisy, A.A.; Lebdah, M.A. Clostridium perfringens type A causing necrotic enteritis outbreaks among chickens in Egypt. Zagazig Vet. J. 2019, 47, 398–407. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Ai, D.; Zhang, R.; Lu, Q.; Luo, Q.; Shao, H. Prevalence and characterization of Clostridium perfringens in broiler chickens and retail chicken meat in central China. Anaerobe 2018, 54, 100–103. [Google Scholar] [CrossRef]
- Haider, Z.; Ali, T.; Ullah, A.; Basit, A.; Tahir, H.; Tariq, H.; Ilyas, S.Z.; Hayat, Z.; Rehman, S.-u. Isolation, toxinotyping and antimicrobial susceptibility testing of Clostridium perfringens isolated from Pakistan poultry. Anaerobe 2022, 73, 102499. [Google Scholar] [CrossRef]
- Rana, E.A.; Nizami, T.A.; Islam, M.S.; Barua, H.; Islam, M.Z. Phenotypical identification and toxinotyping of Clostridium perfringens isolates from healthy and enteric disease-affected chickens. Vet. Med. Int. 2023, 2023, 2584171. [Google Scholar] [CrossRef]
- Yadav, J.P.; Kaur, S.; Dhaka, P.; Vijay, D.; Bedi, J.S. Prevalence, molecular characterization, and antimicrobial resistance profile of Clostridium perfringens from India: A scoping review. Anaerobe 2022, 77, 102639. [Google Scholar] [CrossRef]
- El-Gaos, M.; Khalil, M.; Abdelrahman, M.; Ramadan, A. Molecular characterization of Clostridium perfringens isolated from turkeys. Assiut Vet. Med. J. 2020, 66, 103–110. [Google Scholar]
- Salem, S.M.; Mustafa, D.I.; Hamed, R.I.; El-Azzouny, M.M.; Anwar, N. Assessment of pathological changes of mixed infection of coccidiosis and necrotic enteritis in turkey. J. Egypt. Vet. Med. Assoc. 2020, 80, 55–84. [Google Scholar]
- Yudiarti, T.; Sugiharto, S.; Widiastuti, E.; Wahyuni, H.; Sartono, T.; Nasution, M. Physiological parameters, intestinal microbial population and internal organ weight of broilers supplemented with the fungus monascus purpureus. Adv. Anim. Vet. Sci. 2024, 12, 85–91. [Google Scholar] [CrossRef]
- Soromou, L.W.; Leno, P.F.; Kamano, A.; Souare, M.L.; Camara, A.O.D.; Camara, K. Current practices in the veterinary use of antibiotics in poultry laying hens in Friguiagbé (Guinea). J. Drug Deliv. Ther. 2024, 14, 35–40. [Google Scholar] [CrossRef]
- Miyakawa, M.E.F.; Casanova, N.A.; Kogut, M.H. How did antibiotic growth promoters increase growth and feed efficiency in poultry? Poult. Sci. 2024, 103, 103278. [Google Scholar] [CrossRef]
- Schuetz, A.N. Antimicrobial resistance and susceptibility testing of anaerobic bacteria. Clin. Infect. Dis. 2014, 59, 698–705. [Google Scholar] [CrossRef]
- Gharieb, R.; Saad, M.; Abdallah, K.; Khedr, M.; Farag, E.; Abd El-Fattah, A. Insights on toxin genotyping, virulence, antibiogram profiling, biofilm formation and efficacy of disinfectants on biofilms of Clostridium perfringens isolated from poultry, animals and humans. J. Appl. Microbiol. 2021, 130, 819–831. [Google Scholar] [CrossRef]
- Slavić, Đ.; Boerlin, P.; Fabri, M.; Klotins, K.C.; Zoethout, J.K.; Weir, P.E.; Bateman, D. Antimicrobial susceptibility of Clostridium perfringens isolates of bovine, chicken, porcine, and turkey origin from Ontario. Can. J. Vet. Res. 2011, 75, 89–97. [Google Scholar]
- Semenyuk, E.G.; Laning, M.L.; Foley, J.; Johnston, P.F.; Knight, K.L.; Gerding, D.N.; Driks, A. Spore formation and toxin production in Clostridium difficile biofilms. PLoS ONE 2014, 9, e87757. [Google Scholar] [CrossRef]
- Lu, R.; Liu, B.; Wu, L.; Bao, H.; García, P.; Wang, Y.; Zhou, Y.; Zhang, H. A broad-spectrum phage endolysin (LysCP28) able to remove biofilms and inactivate Clostridium perfringens strains. Foods 2023, 12, 411. [Google Scholar] [CrossRef]
- Bai, X.; Nakatsu, C.H.; Bhunia, A.K. Bacterial biofilms and their implications in pathogenesis and food safety. Foods 2021, 10, 2117. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Chen, Z.; Wu, W.; Lu, Y. Engineered organic nanoparticles to combat biofilms. Drug Discov. Today 2023, 28, 103455. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; El Bayomi, R.M.; Hamed, R.I.; Mohsen, R.A.; El-Gohary, F.A.; Hefny, A.A.; Elkhawaga, E.; Tolba, H.M. Genetic relatedness, antibiotic resistance, and effect of silver nanoparticle on biofilm formation by Clostridium perfringens isolated from chickens, pigeons, camels, and human consumers. Vet. Sci. 2022, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Torky, H.A.; Khalil, S.A.; Elkassas, F.A.; Rezk, M.S.; Tawfik, R.G. Effect of Silver nanoparticles on biofilm formation by Clostridium perfringens isolated from poultry and molecular typing of strains by ERIC-PCR. J. Adv. Vet. Res. 2023, 13, 877–885. [Google Scholar]
- Abdalkarim, S.Y.H.; Yu, H.Y.; Song, M.L.; Zhou, Y.; Yao, J.; Ni, Q.Q. In vitro degradation and possible hydrolytic mechanism of PHBV nanocomposites by incorporating cellulose nanocrystal-ZnO nanohybrids. Carbohydr. Polym. 2017, 176, 38–49. [Google Scholar] [CrossRef]
- Ikram, M.; Imran, M.; Hayat, S.; Shahzadi, A.; Haider, A.; Naz, S.; Ul-Hamid, A.; Nabgan, W.; Fazal, I.; Ali, S. MoS2/cellulose-doped ZnO nanorods for catalytic, antibacterial and molecular docking studies. Nanoscale Adv. 2022, 4, 211–225. [Google Scholar] [CrossRef]
- Worku, L.A.; Tadesse, M.G.; Bachheti, R.K.; Bachheti, A.; Husen, A. Synthesis of carboxylated cellulose nanocrystal/ZnO nanohybrids using Oxytenanthera abyssinica cellulose and zinc nitrate hexahydrate for radical scavenging, photocatalytic, and antibacterial activities. Int. J. Biol. Macromol. 2024, 267, 131228. [Google Scholar] [CrossRef]
- Baldelli, A.; Etayash, H.; Oguzlu, H.; Mandal, R.; Jiang, F.; Hancock, R.E.; Pratap-Singh, A. Antimicrobial properties of spray-dried cellulose nanocrystals and metal oxide-based nanoparticles-in-microspheres. Chem. Eng. J. Adv. 2022, 10, 100273. [Google Scholar] [CrossRef]
- Smith, L.D.; Holdeman, L.V. The Pathogenic Anaerobic Bacteria; CABI Databases: Springfield, IL, USA, 1968. [Google Scholar]
- Quinn, P.; Markey, B.K.; Carter, M.; Donnelly, W.; Leonard, F. Veterinary Microbiology and Microbial Disease; Blackwell Science: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Yoo, H.S.; Lee, S.U.; Park, K.Y.; Park, Y.H. Molecular typing and epidemiological survey of prevalence of Clostridium perfringens types by multiplex PCR. J. Clin. Microbiol. 1997, 35, 228–232. [Google Scholar] [CrossRef]
- M100-S27; Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Seventh Informational Supplement. CLSI: Malvern, PA, USA, 2017.
- Hu, W.-S.; Kim, H.; Koo, O.K. Molecular genotyping, biofilm formation and antibiotic resistance of enterotoxigenic Clostridium perfringens isolated from meat supplied to school cafeterias in South Korea. Anaerobe 2018, 52, 115–121. [Google Scholar] [CrossRef]
- Andrews, J. BSAC Methods for Antimicrobial Susceptibility Testing, 12th ed.; British Society for Antimicrobial Chemotherapy: Birmingham, UK, 2013. [Google Scholar]
- Tambekar, D.; Dhanorkar, D.; Gulhane, S.; Khandelwal, V.; Dudhane, M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr. J. Biotechnol. 2006, 5, 1562–1565. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.; Giske, C.G.; Harbarth, S.; Hindler, J.; Kahlmeter, G.; Olsson-Liljequist, B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Freeman, D.; Falkiner, F.; Keane, C. New method for detecting slime production by coagulase negative Staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Tendolkar, P.M.; Baghdayan, A.S.; Gilmore, M.S.; Shankar, N. Enterococcal surface protein, Esp, enhances biofilm formation by Enterococcus faecalis. Infect. Immun. 2004, 72, 6032–6039. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Choi, O.; Cho, S.K.; Kim, J.; Park, C.G.; Kim, J. In vitro antibacterial activity and major bioactive components of Cinnamomum verum essential oils against cariogenic bacteria, Streptococcus mutans and Streptococcus sobrinus. Asian Pac. J. Trop. Biomed. 2016, 6, 308–314. [Google Scholar] [CrossRef]
- Dalli, M.; Azizi, S.-e.; Benouda, H.; Azghar, A.; Tahri, M.; Bouammali, B.; Maleb, A.; Gseyra, N. Molecular composition and antibacterial effect of five essential oils extracted from Nigella sativa L. seeds against multidrug-resistant bacteria: A Comparative study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6643765. [Google Scholar] [CrossRef]
- Teh, C.H.; Nazni, W.A.; Nurulhusna, A.H.; Norazah, A.; Lee, H.L. Determination of antibacterial activity and minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay. BMC Microbiol. 2017, 17, 36. [Google Scholar] [CrossRef]
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Arora, D.S.; Shah, B.A.; Taneja, S.C. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiol. 2011, 11, 54. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Papa, R.; Selan, L.; Parrilli, E.; Tilotta, M.; Sannino, F.; Feller, G.; Tutino, M.L.; Artini, M. Anti-biofilm activities from marine cold adapted bacteria against Staphylococci and Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 1333. [Google Scholar] [CrossRef] [PubMed]
- Yasugi, M.; Okuzaki, D.; Kuwana, R.; Takamatsu, H.; Fujita, M.; Sarker, M.R.; Miyake, M. Transcriptional profile during deoxycholate-induced sporulation in a Clostridium perfringens isolate causing foodborne illness. Appl. Environ. Microbiol. 2016, 82, 2929–2942. [Google Scholar] [CrossRef] [PubMed]
- Soncini, S.R.; Hartman, A.H.; Gallagher, T.M.; Camper, G.J.; Jensen, R.V.; Melville, S.B. Changes in the expression of genes encoding type IV pili-associated proteins are seen when Clostridium perfringens is grown in liquid or on surfaces. BMC Genom. 2020, 21, 45. [Google Scholar] [CrossRef]
- Wu, S.-B.; Rodgers, N.; Choct, M. Real-time PCR assay for Clostridium perfringens in broiler chickens in a challenge model of necrotic enteritis. Appl. Environ. Microbiol. 2011, 77, 1135–1139. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; The R Foundation: Vienna, Austria, 2007. [Google Scholar]
- Kolde, R. Pheatmap: Pretty Heatmaps; The R Foundation: Vienna, Austria, 2010. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; The R Foundation: Vienna, Austria, 2016. [Google Scholar]




| Source (No.) | Prevalence of C. perfringens No. (%) a | p-Value b |
|---|---|---|
| Broiler chicken (124) | 55 (44.35) | |
| Liver | 15 (12.1) | 0.122 |
| Spleen | 15 (12.1) | |
| Intestine | 25 (20.16) | |
| Layer (36) | 15 (41.67) | |
| Liver | 5 (13.89) | 1 |
| Spleen | 6 (16.67) | |
| Intestine | 4 (11.11) | |
| Turkey (12) | 7 (58.33) | |
| Liver | 5 (41.67) | 0.046 |
| Spleen | 0 (0.0) | |
| Intestine | 2 (16.67) | |
| p-value c | - | 0.608 |
| Total (172) | 77 (44.77) | - |
| Antimicrobial Class | AMA | No. of Resistant Isolates (%) | MAR Index | p-Value |
|---|---|---|---|---|
| Penicillin | Penicillin G benzylpenicillin | 74 (96.1) | 0.051 | <0.0001 *** |
| Penicillin combinations | Amoxycillin-clavulanic acid | 76 (98.7) | 0.052 | <0.0001 *** |
| Cephalosporins | Cefoxitin | 71 (92.2) | 0.049 | <0.0001 *** |
| Carbapenems | Imipenem | 39 (50.65) | 0.026 | 0.374 |
| Aminoglycosides | Gentamicin | 75 (97.1) | 0.051 | <0.0001 *** |
| Macrolides | Erythromycin | 77 (100) | 0.053 | NA |
| Quinolones | Nalidixic acid | 76 (98.7) | 0.052 | <0.0001 *** |
| Fluoroquinolone | Enrofloxacin | 76 (98.7) | 0.052 | <0.0001 *** |
| Sulfonamides | Sulfamethoxazole-trimethoprim | 58 (75.32) | 0.040 | <0.0001 *** |
| Amphenicols | Chloramphenicol | 64 (83.12) | 0.044 | <0.0001 *** |
| Polypeptides | Bacitracin | 77 (100) | 0.053 | NA |
| Oxazolidones | Linezolid | 69 (89.61) | 0.047 | <0.0001 *** |
| Lincosamides | Clindamycin | 77 (100) | 0.053 | NA |
| Lincomycin | 77 (100) | 0.053 | NA | |
| Tetracyclines | Tetracycline | 77 (100) | 0.053 | NA |
| Glycopeptides | Vancomycin | 77 (100) | 0.053 | NA |
| Teicoplanin | 77 (100) | 0.053 | NA | |
| Nitroimidazole | Metronidazole | 74 (96.1) | 0.051 | <0.0001 *** |
| Antimycobacterials | Rifampin | 77 (100) | 0.053 | NA |
| MAR Index | Resistance to AMA (n = 19) | Resistance to AMC (n = 17) | No. of Resistant Isolates (%) | p-Value | Total No. of Isolates (%) (n = 77) | Resistance Category | ||
|---|---|---|---|---|---|---|---|---|
| Broiler (n = 55) | Layer (n = 15) | Turkey (n = 7) | ||||||
| 0.79 | 15 | 13 | 2 (3.63) | 1 (6.67) | 0 | 1 | 4 (5.19) | MDR |
| 0.84 | 16 | 14 | 6 (10.91) | 2 (13.33) | 1 (14.29) | 1 | 8 (10.39) | |
| 0.89 | 17 | 15 | 10 (18.18) | 3 (20) | 1 (14.29) | 1 | 14 (18.18) | XDR |
| 0.95 | 18 | 16 | 20 (36.36) | 5 (33.33) | 3 (42.86) | 1 | 28 (36.36) | |
| 1 | 19 | 17 | 17 (30.91) | 4 (26.67) | 2 (28.57) | 1 | 23 (29.87) | PDR |
| Code No. | PDR Isolate No. | Source | OD570 | Biofilm Degree | CNCs/ZnO Activity (Zone Diameter, mm) | p-Value | Biofilm Inhibition % at | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100% | 50% | 25% | MBC | MIC | SIC | |||||||
| LL3 | 1 | Layer liver | 0.352 | Weak | 38 ± 1.15 a | 30 ± 0.58 b | 21 ± 0.58 c | <0.0001 *** | 59.37 ± 1.95 a | 47.51 ± 1.45 b | 41.98 ± 1.14 b | 0.001 ** |
| BI15 | 2 | Broiler intestine | 0.542 | Moderate | 37 ± 1.73 a | 28 ± 1.15 b | 20 ± 1.15 c | <0.0001 *** | 52.95 ± 1.7 a | 48.70 ± 2.14 a b | 42.98 ± 1.72 b | 0.025 * |
| BI22 | 3 | Broiler intestine | 0.449 | Moderate | 36 ± 2.31 a | 28 ± 0.58 b | 21 ± 1.15 c | 0.001 ** | 57.68 ± 1.55 a | 36.08 ± 0.62 b | 34.07 ± 1.2 b | <0.0001 *** |
| BS23 | 4 | Broiler spleen | 0.422 | Moderate | 40 ± 1.73 a | 31 ± 1.15 b | 22 ± 0.58 c | <0.0001 *** | 53.08 ± 1.78 a | 30.09 ± 1.15 b | 29.14 ± 0.66 b | <0.0001 *** |
| TL1 | 5 | Turkey liver | 0.416 | Moderate | 41 ± 0.58 a | 32 ± 1.75 b | 23 ± 1.15 c | <0.0001 *** | 52.40 ± 1.39 a | 44.71 ± 2.14 b | 39.90 ± 1.1 b | 0.004 ** |
| TL3 | 6 | Turkey liver | 0.410 | Moderate | 36 ± 0.58 a | 28 ± 0.58 b | 20 ± 0.33 c | <0.0001 *** | 54.39 ± 1.38 a | 46.34 ± 0.77 b | 38.29 ± 1.32 c | <0.0001 *** |
| BL18 | 7 | Broiler liver | 0.330 | Weak | 37 ± 1.15 a | 29 ± 0.58 b | 21 ± 0.58 c | <0.0001 *** | 57.57 ± 1.48 a | 47.57 ± 1.48 b | 42.42 ± 1.4 b | 0.001 ** |
| BI11 | 8 | Broiler intestine | 0.706 | Moderate | 38 ± 1.73 a | 29 ± 1.15 b | 20 ± 1.15 c | <0.0001 *** | 70.11 ± 1.15 a | 66.43 ± 0.83 a | 60.19 ± 1.73 b | 0.005 ** |
| BS14 | 9 | Broiler spleen | 0.851 | Strong | 39 ± 0.58 a | 30 ± 1.15 b | 22 ± 1.15 c | <0.0001 *** | 83.43 ± 1.98 a | 76.96 ± 1.71 a b | 74.38 ± 1.73 b | 0.03 * |
| BI10 | 10 | Broiler intestine | 0.608 | Moderate | 41 ± 0.58 a | 31 ± 1.73 b | 24 ± 0.58 c | <0.0001 *** | 62.66 ± 1.15 a | 53.94 ± 1.73 b | 47.36 ± 1.15 c | 0.001 |
| BL11 | 11 | Broiler liver | 0.551 | Moderate | 38 ± 1.73 a | 30 ± 1.15 b | 24 ± 1.15 b | 0.002 ** | 76.04 ± 0.6 a | 55.17 ± 0.68 b | 50.63 ± 1.37 c | <0.0001 *** |
| BI2 | 12 | Broiler intestine | 0.401 | Moderate | 39 ± 1.15 a | 30 ± 1 b | 23 ± 1.73 c | <0.0001 *** | 66.58 ± 1.15 a | 55.11 ± 1.22 b | 44.38 ± 1.73 c | <0.0001 *** |
| BS3 | 13 | Broiler spleen | 0.321 | Weak | 37 ± 1.73 a | 28 ± 0.58 b | 21 ± 1.15 c | <0.0001 *** | 60.74 ± 1.58 a | 55.76 ± 1.59 a | 38.94 ± 1.15 b | <0.0001 *** |
| BS22 | 14 | Broiler spleen | 0.429 | Moderate | 39 ± 1.73 a | 30 ± 1.15 b | 22 ± 1.15 c | <0.0001 *** | 63.63 ± 1.73 a | 51.04 ± 0.6 b | 46.38 ± 1.73 b | <0.0001 *** |
| BS7 | 15 | Broiler spleen | 0.290 | Weak | 38 ± 1.15 a | 29 ± 0.58 b | 21 ± 1.73 b | <0.0001 *** | 55.17 ± 1.15 a | 53.79 ± 1.73 a | 39.65 ± 0.95 b | <0.0001 *** |
| BL15 | 16 | Broiler liver | 0.473 | Moderate | 39 ± 0.58 a | 29 ± 0.58 b | 20 ± 0.58 c | <0.0001 *** | 61.94 ± 1.12 a | 51.37 ± 0.79 b | 42.91 ± 1.15 c | <0.0001 *** |
| LI7 | 17 | Layer intestine | 0.447 | Moderate | 39 ± 0.58 a | 32 ± 1.73 b | 23 ± 1.15 c | <0.0001 *** | 65.10 ± 1.21 a | 53.02 ± 1.73 b | 47.87 ± 1.66 b | 0.001 ** |
| LS3 | 18 | Layer spleen | 0.655 | Moderate | 39 ± 0.58 a | 31 ± 0.15 b | 24 ± 0.58 c | <0.0001 *** | 69.46 ± 1.42 a | 57.25 ± 1.3 b | 46.10 ± 0.64 c | <0.0001 *** |
| BS2 | 19 | Broiler spleen | 0.739 | Strong | 40 ± 1.15 a | 32 ± 1.15 b | 25 ± 1.15 c | <0.0001 *** | 75.64 ± 1.73 a | 68.74 ± 1.58 a b | 63.05 ± 1.73 b | 0.005 ** |
| BI18 | 20 | Broiler intestine | 0.528 | Moderate | 40 ± 0.58 a | 30 ± 1.15 b | 22 ± 1.15 c | <0.0001 *** | 66.85 ± 1.12 | 62.87 ± 1.66 | 60.60 ± 1.5 | 0.086 |
| BL5 | 21 | Broiler liver | 0.780 | Strong | 38 ± 1.15 a | 31 ± 0.58 b | 20 ± 0.58 c | <0.0001 *** | 73.07 ± 1.73 | 70.76 ± 1.59 | 67.94 ± 1.7 | 0.176 |
| LS1 | 22 | Layer spleen | 0.280 | Weak | 39 ± 0.58 a | 30 ± 1.73 b | 23 ± 1.15 c | <0.0001 *** | 53.57 ± 2.06 a | 42.85 ± 1.65 b | 33.57 ± 1.48 c | 0.001 ** |
| BS15 | 23 | Broiler spleen | 0.403 | Moderate | 38 ± 1.15 a | 29 ± 1.58 b | 21 ± 0.58 c | <0.0001 *** | 66.74 ± 1.58 a | 64.01 ± 1.15 a | 52.85 ± 1.12 b | <0.0001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amin, I.; Abdelkhalek, A.; El-Demerdash, A.S.; Pet, I.; Ahmadi, M.; Abd El-Aziz, N.K. Cellulose Nanocrystal/Zinc Oxide Bio-Nanocomposite Activity on Planktonic and Biofilm Producing Pan Drug-Resistant Clostridium perfringens Isolated from Chickens and Turkeys. Antibiotics 2025, 14, 575. https://doi.org/10.3390/antibiotics14060575
Amin I, Abdelkhalek A, El-Demerdash AS, Pet I, Ahmadi M, Abd El-Aziz NK. Cellulose Nanocrystal/Zinc Oxide Bio-Nanocomposite Activity on Planktonic and Biofilm Producing Pan Drug-Resistant Clostridium perfringens Isolated from Chickens and Turkeys. Antibiotics. 2025; 14(6):575. https://doi.org/10.3390/antibiotics14060575
Chicago/Turabian StyleAmin, Ismail, Adel Abdelkhalek, Azza S. El-Demerdash, Ioan Pet, Mirela Ahmadi, and Norhan K. Abd El-Aziz. 2025. "Cellulose Nanocrystal/Zinc Oxide Bio-Nanocomposite Activity on Planktonic and Biofilm Producing Pan Drug-Resistant Clostridium perfringens Isolated from Chickens and Turkeys" Antibiotics 14, no. 6: 575. https://doi.org/10.3390/antibiotics14060575
APA StyleAmin, I., Abdelkhalek, A., El-Demerdash, A. S., Pet, I., Ahmadi, M., & Abd El-Aziz, N. K. (2025). Cellulose Nanocrystal/Zinc Oxide Bio-Nanocomposite Activity on Planktonic and Biofilm Producing Pan Drug-Resistant Clostridium perfringens Isolated from Chickens and Turkeys. Antibiotics, 14(6), 575. https://doi.org/10.3390/antibiotics14060575









