Additive Manufacturing, Thermoplastics, CAD Technology, and Reverse Engineering in Orthopedics and Neurosurgery–Applications to Preventions and Treatment of Infections
Abstract
1. Introduction
2. Epidemiology of Orthopedical Implant Infections and Cranioplasty
3. Current Landscape of Orthopedic Implants
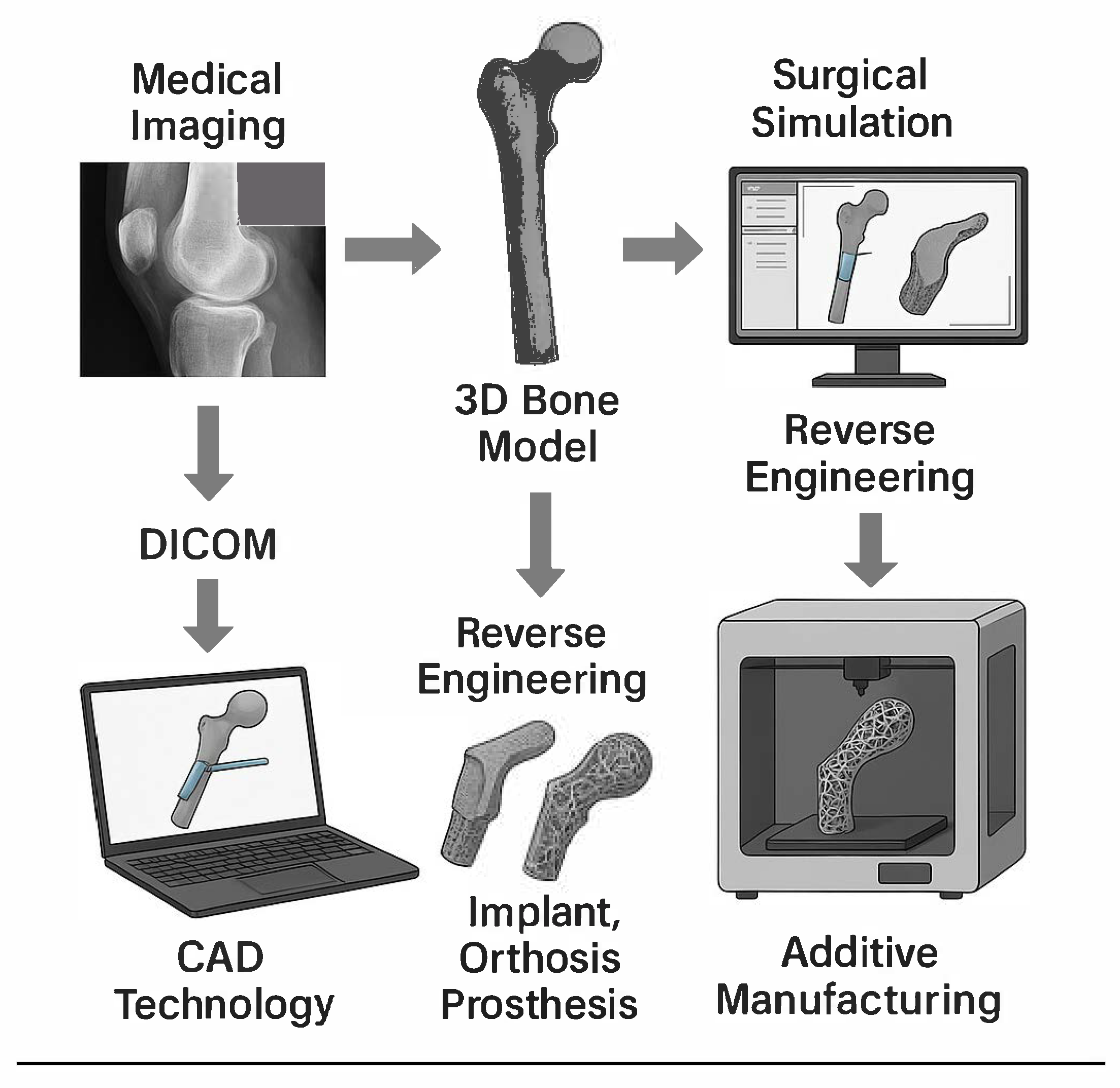
4. CAD Technology
5. The Use of Additive Manufacturing for 3D Printing in Medicine
6. Thermoplastics Used in the Healthcare Industry
7. Polymers and Antimicrobial Action in Medicine
8. Application in Cranioplasty and Infection Prevent
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, M.C.; Oliveira, J.C.P.; Zidan, F.F.; Franciozi, C.; Luzo, M.V.M.; Abdalla, R.J. Total knee and hip arthroplasty: The reality of assistance in Brazilian public health care. Rev. Bras. Ortop. 2018, 53, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef]
- Carteri, R.B.K.; Silva, R.A.D. Traumatic brain injury hospital incidence in Brazil: An analysis of the past 10 years. Rev. Bras. Ter. Intensiva 2021, 33, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Lamagni, T. Epidemiology and burden of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. S1), i5–i10. [Google Scholar] [CrossRef]
- Tuon, F.F.; Cieslinski, J.; Ono, A.F.M.; Goto, F.L.; Machinski, J.M.; Mantovani, L.K.; Kosop, L.R.; Namba, M.S.; Rocha, J.L. Microbiological profile and susceptibility pattern of surgical site infections related to orthopaedic trauma. Int. Orthop. 2019, 43, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Rollo, G.; Logroscino, G.; Stomeo, D.; Cioffi, R.; Calvisi, V.; Meccariello, L. Comparing the use of preformed vs hand-made antibiotic spacer cement in two stages revision of hip periprosthetic infection. J. Clin. Orthop. Trauma 2020, 11, S772–S778. [Google Scholar] [CrossRef]
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Costa-Pinto, A.R.; Lemos, A.L.; Tavaria, F.K.; Pintado, M. Chitosan and Hydroxyapatite Based Biomaterials to Circumvent Periprosthetic Joint Infections. Materials 2021, 14, 804. [Google Scholar] [CrossRef]
- Shohat, N.; Goh, G.S.; Harrer, S.L.; Brown, S. Dilute Povidone-Iodine Irrigation Reduces the Rate of Periprosthetic Joint Infection Following Hip and Knee Arthroplasty: An Analysis of 31,331 Cases. J. Arthroplast. 2022, 37, 226–231.e1. [Google Scholar] [CrossRef]
- Kaye, K.S.; Anderson, D.J.; Sloane, R.; Chen, L.F.; Choi, Y.; Link, K.; Sexton, D.J.; Schmader, K.E. The effect of surgical site infection on older operative patients. J. Am. Geriatr. Soc. 2009, 57, 46–54. [Google Scholar] [CrossRef]
- Porretto, M.; Parente, F.; Del Puente, F.; Parisini, A.; Tigano, S.; Nelli, M.; Mazzola, C.; Damiani, G.; Adriano, G.; Sartini, M.; et al. Surveillance of surgical site infections in orthopedic prosthetic surgery: A tool for identifying risk factors and improving clinical practice. J. Prev. Med. Hyg. 2024, 65, E273–E277. [Google Scholar] [CrossRef]
- Cieslinski, J.; Ribeiro, V.S.T.; Lima, C.K.; Kraft, L.; Suss, P.H.; Tuon, F.F. Sonication as a tool for disrupting biofilms and recovering microorganisms in bladder catheters. J. Bras. Nefrol. 2023, 45, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Cieslinski, J.; Ribeiro, V.S.T.; Kraft, L.; Suss, P.H.; Rosa, E.; Morello, L.G.; Pillonetto, M.; Tuon, F.F. Direct detection of microorganisms in sonicated orthopedic devices after in vitro biofilm production and different processing conditions. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.S.T.; Cieslinski, J.; Bertol, J.; Schumacher, A.L.; Telles, J.P.; Tuon, F.F. Detection of Microorganisms in Clinical Sonicated Orthopedic Devices Using Conventional Culture and qPCR. Rev. Bras. Ortop. 2022, 57, 689–696. [Google Scholar] [CrossRef]
- Choi, S.R.; Kwon, J.W.; Suk, K.S.; Kim, H.S.; Moon, S.H.; Park, S.Y.; Lee, B.H. The Clinical Use of Osteobiologic and Metallic Biomaterials in Orthopedic Surgery: The Present and the Future. Materials 2023, 16, 3633. [Google Scholar] [CrossRef]
- Szczesny, G.; Kopec, M.; Politis, D.J.; Kowalewski, Z.L.; Lazarski, A.; Szolc, T. A Review on Biomaterials for Orthopaedic Surgery and Traumatology: From Past to Present. Materials 2022, 15, 3622. [Google Scholar] [CrossRef]
- Richards, R.G.; Moriarty, T.F.; Miclau, T.; McClellan, R.T.; Grainger, D.W. Advances in biomaterials and surface technologies. J. Orthop. Trauma 2012, 26, 703–707. [Google Scholar] [CrossRef]
- Kirkpatrick, J.S.; Cornell, C.N.; Hoang, B.H.; Hsu, W.; Watson, J.T.; Watters, W.C., 3rd; Turkelson, C.M.; Wies, J.L.; Anderson, S. Bone void fillers. J. Am. Acad. Orthop. Surg. 2010, 18, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Evaniew, N.; Tan, V.; Parasu, N.; Jurriaans, E.; Finlay, K.; Deheshi, B.; Ghert, M. Use of a calcium sulfate-calcium phosphate synthetic bone graft composite in the surgical management of primary bone tumors. Orthopedics 2013, 36, e216–e222. [Google Scholar] [CrossRef]
- Borges, N.H.; Suss, P.H.; Ortis, G.B.; Dantas, L.R.; Tuon, F.F. Synergistic Activity of Vancomycin and Gentamicin Against Staphylococcus aureus Biofilms on Polyurethane Surface. Microorganisms 2025, 13, 1119. [Google Scholar] [CrossRef]
- Jennings, J.A.; Carpenter, D.P.; Troxel, K.S.; Beenken, K.E.; Smeltzer, M.S.; Courtney, H.S.; Haggard, W.O. Novel Antibiotic-loaded Point-of-care Implant Coating Inhibits Biofilm. Clin. Orthop. Relat. Res. 2015, 473, 2270–2282. [Google Scholar] [CrossRef]
- da Rocha, L.; Ribeiro, V.S.T.; de Andrade, A.P.; Goncalves, G.A.; Kraft, L.; Cieslinski, J.; Suss, P.H.; Tuon, F.F. Evaluation of Staphylococcus aureus and Candida albicans biofilms adherence to PEEK and titanium-alloy prosthetic spine devices. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Soni, J.F.; Ribeiro, V.S.T.; Cieslinski, J.; de Andrade, A.P.; Dantas, L.R.; Pereira, B.Z.; de Almeida, B.; Suss, P.H.; Tuon, F.F. Evaluation of silver nanoparticle-impregnated PMMA loaded with vancomycin or gentamicin against bacterial biofilm formation. Injury 2023, 54 (Suppl. S6), 110649. [Google Scholar] [CrossRef]
- Pedroni, M.A.; Ribeiro, V.S.T.; Cieslinski, J.; Lopes, A.P.A.; Kraft, L.; Suss, P.H.; Tuon, F.F. Different concentrations of vancomycin with gentamicin loaded PMMA to inhibit biofilm formation of Staphylococcus aureus and their implications. J. Orthop. Sci. 2024, 29, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Marro, A.; Bandukwala, T.; Mak, W. Three-Dimensional Printing and Medical Imaging: A Review of the Methods and Applications. Curr. Probl. Diagn. Radiol. 2016, 45, 2–9. [Google Scholar] [CrossRef]
- van Eijnatten, M.; van Dijk, R.; Dobbe, J.; Streekstra, G.; Koivisto, J.; Wolff, J. CT image segmentation methods for bone used in medical additive manufacturing. Med. Eng. Phys. 2018, 51, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Banks, J. Adding value in additive manufacturing: Researchers in the United Kingdom and Europe look to 3D printing for customization. IEEE Pulse 2013, 4, 22–26. [Google Scholar] [CrossRef]
- Serra, T.; Mateos-Timoneda, M.A.; Planell, J.A.; Navarro, M. 3D printed PLA-based scaffolds: A versatile tool in regenerative medicine. Organogenesis 2013, 9, 239–244. [Google Scholar] [CrossRef]
- Mendonça, C.J.A.; Dantas, L.R.; Soni, J.F.; Tuon, F.F. Antimicrobial Action of a Biodegradable Thermoplastic Impregnated with Vancomycin for Use in 3D Printing Technology. Braz. Arch. Biol. Technol. 2024, 67, 13. [Google Scholar] [CrossRef]
- Tilton, M.; Lewis, G.S.; Hast, M.W.; Fox, E.; Manogharan, G. Additively manufactured patient-specific prosthesis for tumor reconstruction: Design, process, and properties. PLoS ONE 2021, 16, e0253786. [Google Scholar] [CrossRef]
- Mendonca, C.J.A.; Guimaraes, R.; Pontim, C.E.; Gasoto, S.C.; Setti, J.A.P.; Soni, J.F.; Schneider, B., Jr. An Overview of 3D Anatomical Model Printing in Orthopedic Trauma Surgery. J. Multidiscip. Healthc. 2023, 16, 875–887. [Google Scholar] [CrossRef]
- Chae, M.P.; Rozen, W.M.; McMenamin, P.G.; Findlay, M.W.; Spychal, R.T.; Hunter-Smith, D.J. Emerging Applications of Bedside 3D Printing in Plastic Surgery. Front. Surg. 2015, 2, 25. [Google Scholar] [CrossRef]
- Jackson, K.C.; Clancey, E.; Call, D.R.; Lofgren, E. 3D Printers in Hospitals: Bacterial Contamination of Common and Antimicrobial 3D-Printed Material. bioRxiv 2024. [Google Scholar] [CrossRef]
- Alavi, M.S.; Memarpour, S.; Pazhohan-Nezhad, H.; Salimi Asl, A.; Moghbeli, M.; Shadmanfar, S.; Saburi, E. Applications of poly(lactic acid) in bone tissue engineering: A review article. Artif. Organs 2023, 47, 1423–1430. [Google Scholar] [CrossRef]
- Yoda, I.; Koseki, H.; Tomita, M.; Shida, T.; Horiuchi, H.; Sakoda, H.; Osaki, M. Effect of surface roughness of biomaterials on Staphylococcus epidermidis adhesion. BMC Microbiol. 2014, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; de Campos, M.R.; Sahm, B.D.; da Costa Valente, M.L.; Marcondes Agnelli, J.A.; Dos Reis, A.C. Influence of post-processing on the adhesion of dual-species biofilm on polylactic acid obtained by additive manufacturing. Saudi Dent. J. 2024, 36, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, I.G.; Cheon, G.H.; Jang, T.S.; Kim, H.E.; Jung, H.D.; Kang, M.H. Enhanced Bioactivity of Micropatterned Hydroxyapatite Embedded Poly(L-lactic) Acid for a Load-Bearing Implant. Polymers 2020, 12, 2390. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Omar, A.M.; Daskalakis, E.; Hou, Y.; Huang, B.; Strashnov, I.; Grieve, B.D.; Bartolo, P. The Potential of Polyethylene Terephthalate Glycol as Biomaterial for Bone Tissue Engineering. Polymers 2020, 12, 3045. [Google Scholar] [CrossRef]
- Moradinezhad, M.; Abbasi Montazeri, E.; Hashemi Ashtiani, A.; Pourlotfi, R.; Rakhshan, V. Biofilm formation of Streptococcus mutans, Streptococcus sanguinis, Staphylococcus epidermidis, Staphylococcus aureus, Lactobacillus casei, and Candida Albicans on 5 thermoform and 3D printed orthodontic clear aligner and retainer materials at 3 time points: An in vitro study. BMC Oral Health 2024, 24, 1107. [Google Scholar] [CrossRef]
- Tektas, S.; Thurnheer, T.; Eliades, T.; Attin, T.; Karygianni, L. Initial Bacterial Adhesion and Biofilm Formation on Aligner Materials. Antibiotics 2020, 9, 908. [Google Scholar] [CrossRef]
- Pu, F.; Yu, Y.; Zhang, Z.; Wu, W.; Shao, Z.; Li, C.; Feng, J.; Xue, L.; Chen, F. Research and Application of Medical Polyetheretherketone as Bone Repair Material. Macromol. Biosci. 2023, 23, e2300032. [Google Scholar] [CrossRef] [PubMed]
- Ishihama, H.; Ishii, K.; Nagai, S.; Kakinuma, H.; Sasaki, A.; Yoshioka, K.; Kuramoto, T.; Shiono, Y.; Funao, H.; Isogai, N.; et al. An antibacterial coated polymer prevents biofilm formation and implant-associated infection. Sci. Rep. 2021, 11, 3602. [Google Scholar] [CrossRef] [PubMed]
- Wiessner, A.; Wassmann, T.; Wiessner, J.M.; Schubert, A.; Wiechens, B.; Hampe, T.; Burgers, R. In Vivo Biofilm Formation on Novel PEEK, Titanium, and Zirconia Implant Abutment Materials. Int. J. Mol. Sci. 2023, 24, 1779. [Google Scholar] [CrossRef] [PubMed]
- Brum, R.S.; Labes, L.G.; Volpato, C.A.M.; Benfatti, C.A.M.; Pimenta, A.L. Strategies to Reduce Biofilm Formation in PEEK Materials Applied to Implant Dentistry—A Comprehensive Review. Antibiotics 2020, 9, 609. [Google Scholar] [CrossRef]
- Griffin, M.; Castro, N.; Bas, O.; Saifzadeh, S.; Butler, P.; Hutmacher, D.W. The Current Versatility of Polyurethane Three-Dimensional Printing for Biomedical Applications. Tissue Eng. Part B Rev. 2020, 26, 272–283. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J.; Zhu, Y. Properties of shape memory polyurethane used as a low-temperature thermoplastic biomedical orthotic material: Influence of hard segment content. J. Biomater. Sci. Polym. Ed. 2008, 19, 1437–1454. [Google Scholar] [CrossRef]
- Restivo, E.; Peluso, E.; Bloise, N.; Bello, G.L.; Bruni, G.; Giannaccari, M.; Raiteri, R.; Fassina, L.; Visai, L. Surface Properties of a Biocompatible Thermoplastic Polyurethane and Its Anti-Adhesive Effect against E. coli and S. aureus. J. Funct. Biomater. 2024, 15, 24. [Google Scholar] [CrossRef]
- Al Bataineh, M.T.; Alazzam, A. Transforming medical device biofilm control with surface treatment using microfabrication techniques. PLoS ONE 2023, 18, e0292647. [Google Scholar] [CrossRef]
- Kuwada, N.; Fujii, Y.; Nakatani, T.; Ousaka, D.; Tsuji, T.; Imai, Y.; Kobayashi, Y.; Oozawa, S.; Kasahara, S.; Tanemoto, K. Diamond-like carbon coating to inner surface of polyurethane tube reduces Staphylococcus aureus bacterial adhesion and biofilm formation. J. Artif. Organs 2024, 27, 108–116. [Google Scholar] [CrossRef]
- Yoon, H.K.; Yoo, J.H.; Oh, H.C.; Ha, J.W.; Park, S.H. The Incidence Rate, Microbiological Etiology, and Results of Treatments of Prosthetic Joint Infection following Total Knee Arthroplasty. J. Clin. Med. 2023, 12, 5908. [Google Scholar] [CrossRef]
- Lachiewicz, P.F.; Wellman, S.S.; Peterson, J.R. Antibiotic Cement Spacers for Infected Total Knee Arthroplasties. J. Am. Acad. Orthop. Surg. 2020, 28, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Wojasinski, M.; Jaroszewicz, J.; Kopec, K.; Ciach, T. Controlled formation of highly porous polylactic acid-calcium phosphate granules with defined structure. Biomater. Adv. 2023, 144, 213195. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Thamvasupong, P.; Viravaidya-Pasuwat, K. Controlled Release Mechanism of Vancomycin from Double-Layer Poly-L-Lactic Acid-Coated Implants for Prevention of Bacterial Infection. Polymers 2022, 14, 3493. [Google Scholar] [CrossRef] [PubMed]
- El Habnouni, S.; Lavigne, J.P.; Darcos, V.; Porsio, B.; Garric, X.; Coudane, J.; Nottelet, B. Toward potent antibiofilm degradable medical devices: A generic method for the antibacterial surface modification of polylactide. Acta Biomater. 2013, 9, 7709–7718. [Google Scholar] [CrossRef]
- Dorm, B.C.; Bastos, A.C.; Nossa, T.S.; Neto, B.D.; Iemma, M.R.C.; Carvalho, A.J.F.; Trovatti, E. Lysine grafted poly(lactic acid): An intrinsically antimicrobial polymer. Int. J. Biol. Macromol. 2024, 273, 133181. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. ISO: Geneva, Switzerland, 2018.
- ISO 14971:2019; Medical Devices—Application of Risk Management to Medical Devices. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/72704.html (accessed on 28 May 2025).
- Thurman, D.J.; Alverson, C.; Dunn, K.A.; Guerrero, J.; Sniezek, J.E. Traumatic brain injury in the United States: A public health perspective. J. Head. Trauma. Rehabil. 1999, 14, 602–615. [Google Scholar] [CrossRef]
- Capitelli-McMahon, H.; Kahlar, N.; Rahman, S. Titanium Versus Autologous Bone-Based Cranioplasty: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e39516. [Google Scholar] [CrossRef]
| Material | Composition/Main Characteristics | Advantages | Disadvantages | Common Applications |
|---|---|---|---|---|
| Stainless Steels (ASI316L) | Alloy of steel with high corrosion resistance | Durability, corrosion resistance | Lower wear resistance compared to other materials | Long-term implants, such as orthopedic screws and plates |
| Chromium–Cobalt–Molybdenum Alloys (CCM) | Alloys containing chromium, cobalt, and molybdenum | Excellent wear resistance | May be less biocompatible compared to titanium, higher density (heavier) | Joint implants, such as hip and knee prostheses |
| Titanium Alloys (Ti6Al4V) | Titanium alloy with aluminum and vanadium, known for its light weight and biocompatibility | Light, biocompatible, corrosion-resistant, less prone to immune reactions | Lower wear resistance, higher cost compared to other metals | Bone and joint implants, such as hip, knee, skull prostheses, and orthopedic screws |
| Ceramics | Inorganic materials with high compressive strength and chemical stability | High compressive strength, chemical inertness, durability in biological environments | Brittle (prone to fractures under impact or shock loads) | Hip implants, bone substitutes, joint components |
| Polyethylene | Plastic with low friction coefficient, common in joint prostheses | Low friction, wear resistance, durability | Can degrade over time under prolonged use conditions | Components of joint prostheses (e.g., acetabular components in hip prostheses) |
| Polymethylmethacrylate (PMMA) | Plastic material used for prosthesis fixation, with rapid polymerization | Good bone adhesion, easy to mold, fast curing | Can generate thermal reactions during curing, lacks elasticity and strength compared to other materials | Bone cement, fixation of orthopedic implants, such as in hip and knee prostheses |
| Material | Key Properties | Medical Applications | Antimicrobial Activity |
|---|---|---|---|
| PLA (Polylactic Acid) | Biodegradable, biocompatible, mechanically durable | Sutures, orthopedic implants, temporary devices | Surface roughness and hydrophobicity increase bacterial adhesion and biofilm development. Lacks intrinsic antimicrobial properties. |
| PLLA (Poly(L-lactic acid)) | High mechanical strength, biodegradable | Fracture fixation, tissue engineering | Similar to PLA, it exhibits no inherent antimicrobial activity. Limited bioactivity may permit bacterial colonization. |
| PLGA (Poly(glycolide-co-lactide)) | Biocompatible, tunable degradation rate | Orthopedic implants, bone regeneration | Lacks inherent antimicrobial properties; susceptible to biofilm formation unless surface-modified. |
| PETG (Polyethylene Terephthalate Glycol) | Good mechanical properties, biocompatible, printable | Dental and medical devices | Supports adhesion of various microorganisms (e.g., S. mutans, C. albicans). Surface modifications, like polishing or antimicrobial coatings, are under investigation. |
| PEEK (Polyetheretherketone) | High strength, elastic modulus similar to bone, biocompatible | Orthopedic and dental implants | Inert surface facilitates bacterial colonization. Biofilms may lead to peri-implantitis. Antimicrobial surface modifications (e.g., sulfonation, bioactive agents) are being explored. |
| TPU (Thermoplastic Polyurethane) | Flexible, shape-memory behavior, biocompatible, low-temperature processable | Custom orthotic devices, 3D-printed implants | S. aureus and S. epidermidis readily form biofilms on TPU. Surface treatments, such as diamond-like carbon coatings, are being investigated to mitigate microbial adhesion. |
| PCL (Polycaprolactone) | Biodegradable, tissue-compatible, easily processable | Bone tissue engineering, scaffolds | Does not exhibit inherent antimicrobial activity. Prolonged presence may facilitate biofilm formation over time. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortis, G.B.; Zapparoli, F.C.; Dantas, L.R.; Suss, P.H.; Soni, J.F.; Mendonça, C.J.A.; Loesch, G.H.; Loesch, M.d.M.O.N.; Tuon, F.F. Additive Manufacturing, Thermoplastics, CAD Technology, and Reverse Engineering in Orthopedics and Neurosurgery–Applications to Preventions and Treatment of Infections. Antibiotics 2025, 14, 565. https://doi.org/10.3390/antibiotics14060565
Ortis GB, Zapparoli FC, Dantas LR, Suss PH, Soni JF, Mendonça CJA, Loesch GH, Loesch MdMON, Tuon FF. Additive Manufacturing, Thermoplastics, CAD Technology, and Reverse Engineering in Orthopedics and Neurosurgery–Applications to Preventions and Treatment of Infections. Antibiotics. 2025; 14(6):565. https://doi.org/10.3390/antibiotics14060565
Chicago/Turabian StyleOrtis, Gabriel Burato, Franco Camargo Zapparoli, Leticia Ramos Dantas, Paula Hansen Suss, Jamil Faissal Soni, Celso Júnio Aguiar Mendonça, Gustavo Henrique Loesch, Maíra de Mayo Oliveira Nogueira Loesch, and Felipe Francisco Tuon. 2025. "Additive Manufacturing, Thermoplastics, CAD Technology, and Reverse Engineering in Orthopedics and Neurosurgery–Applications to Preventions and Treatment of Infections" Antibiotics 14, no. 6: 565. https://doi.org/10.3390/antibiotics14060565
APA StyleOrtis, G. B., Zapparoli, F. C., Dantas, L. R., Suss, P. H., Soni, J. F., Mendonça, C. J. A., Loesch, G. H., Loesch, M. d. M. O. N., & Tuon, F. F. (2025). Additive Manufacturing, Thermoplastics, CAD Technology, and Reverse Engineering in Orthopedics and Neurosurgery–Applications to Preventions and Treatment of Infections. Antibiotics, 14(6), 565. https://doi.org/10.3390/antibiotics14060565






