Prevalence of Extended-Spectrum β-Lactamase-Producing Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae in Wastewater Effluent in Blantyre, Malawi
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of ESBL Organisms in WWTPs
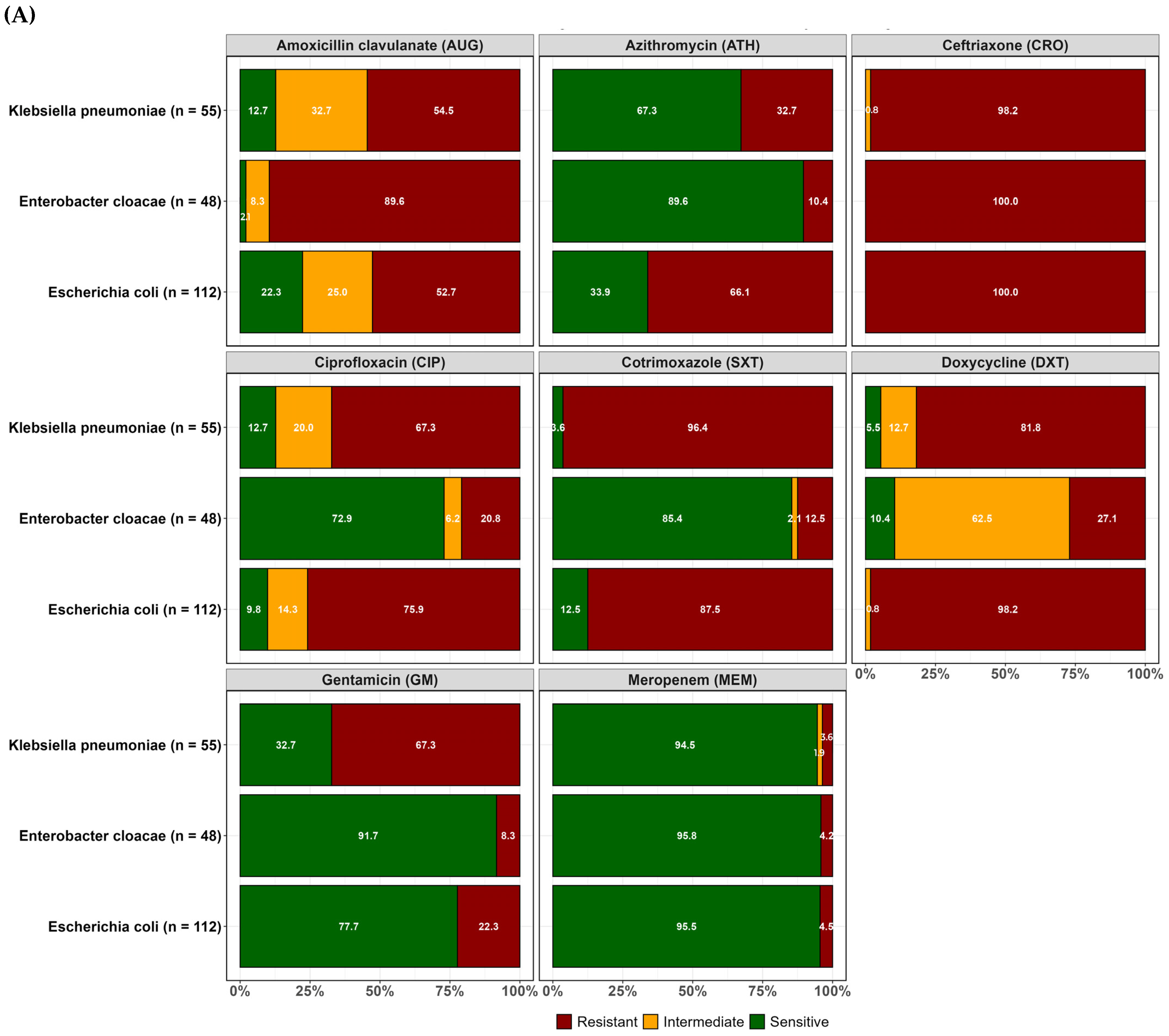
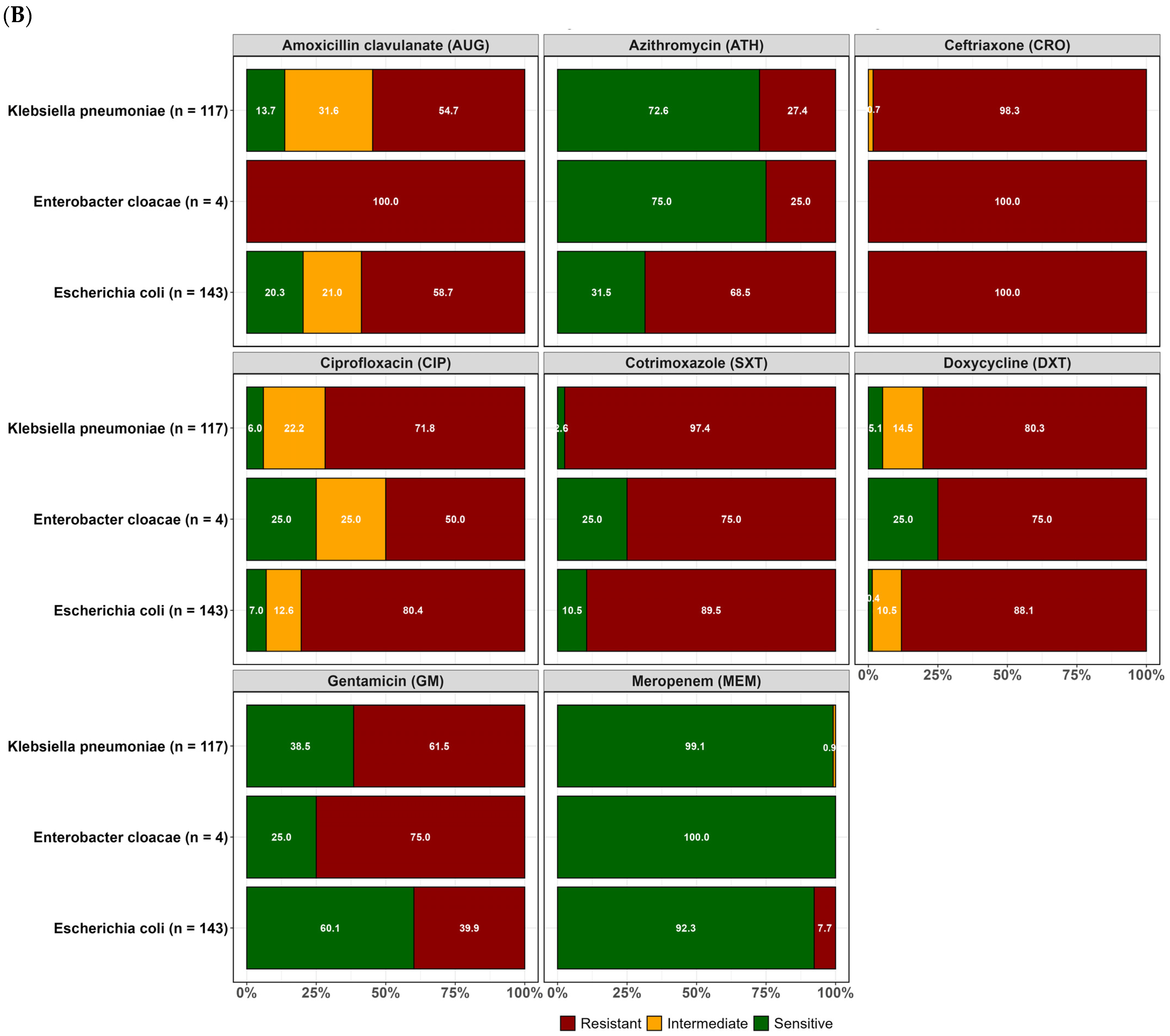
2.2. Resistance Profiles of Isolated ESBL-Producing E. coli, K. pneumoniae, and Enterobacter Cloacae
2.3. Prevalence of Multiple Antibiotic-Resistance Phenotype and Multiple Antibiotic Resistance Index ESBL E. coli, ESBL K. pneumoniae and ESBL E. cloacae
2.4. Effect of Temperature and Rainfall on the Prevalence of Resistant ESBL Organisms
3. Discussion
4. Materials and Methods
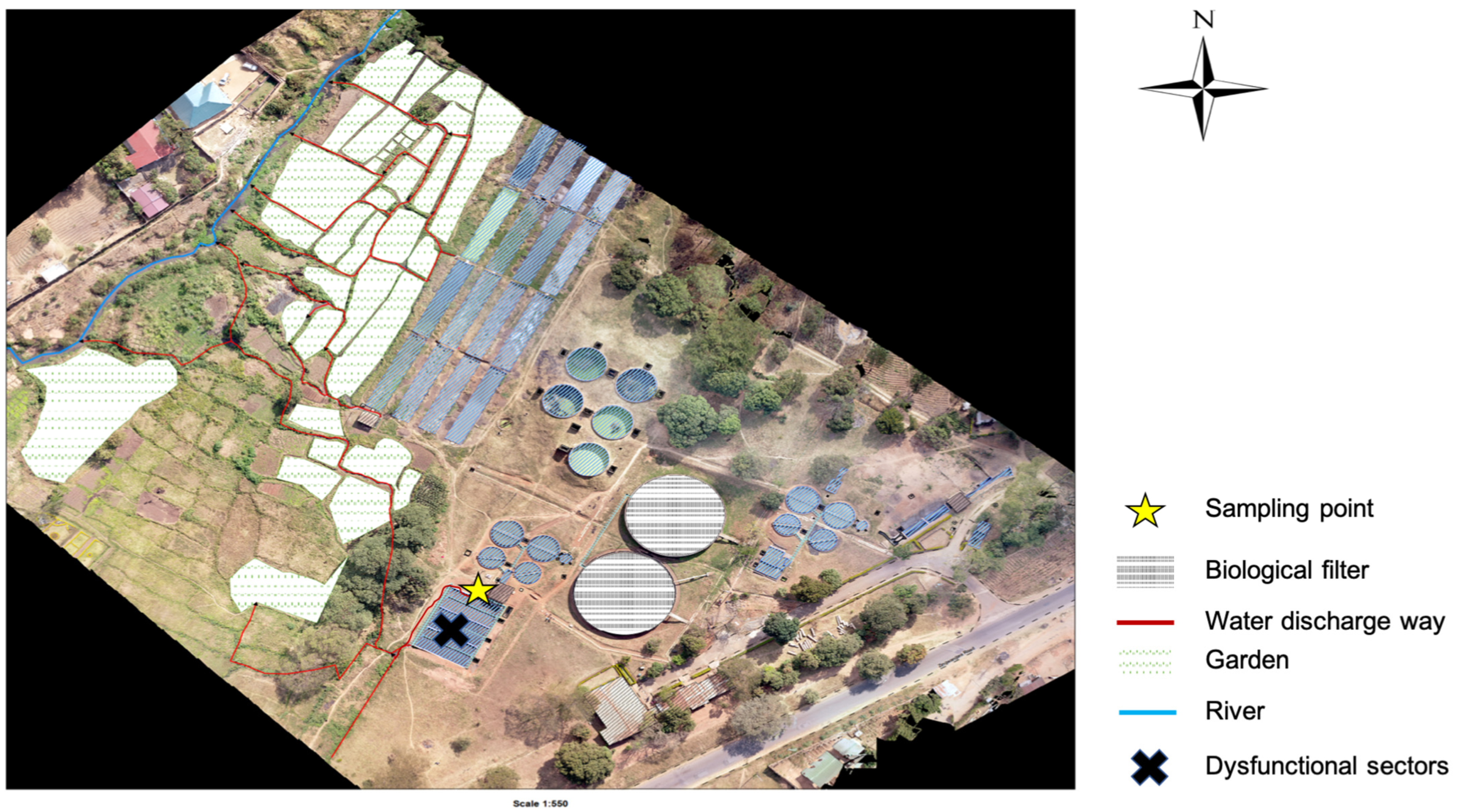
4.1. Study Sites
4.2. Sample Collection
4.3. Sample Processing, Culture, and Bacterial Identification
4.4. Antimicrobial Susceptibility Testing
4.5. Multiple Antibiotic-Resistant Phenotype and Multiple Antibiotic Resistance Index
4.6. Temperature and Rainfall Data
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A.; Balcazar, J.L. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, S.; Majid, M.; Sardar, S. Environmental and public health effects of antibiotics and AMR/ARGs. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–291. [Google Scholar] [CrossRef]
- Thai, P.K.; Xuan, L.; Ngan, V.; Hong, P.; Thi, P.; Quang, N.; Dang, N.T.T.; Kieu, N.; Tam, B.; Thi, N.; et al. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi, Vietnam. Sci. Total Environ. 2018, 645, 393–400. [Google Scholar] [CrossRef]
- Chitescu, C.L.; Lupoae, M.; Elisei, A.M. Pharmaceutical residues in the environment—New european integrated programs required. Rev. Chim. 2016, 67, 1008–1013. [Google Scholar]
- Papajov, I.; Gregov, G.; Papaj, J.; Szab, T.; Danč, N.; Schusterov, I.; Sušinkov, J.; Rakov, J. Effect of wastewater treatment on bacterial community, antibiotic-resistant bacteria and endoparasites. Int. J. Environ. Res. Public Health 2022, 19, 2750. [Google Scholar] [CrossRef] [PubMed]
- Mbanga, J.; Luther, A.; Abia, K.; Amoako, D.G.; Essack, S. Longitudinal surveillance of antibiotic resistance in Escherichia coli and Enterococcus spp. from a wastewater treatment plant and its associated waters in KwaZulu-Natal, South Africa. Microb. Drug Resist. 2021, 27, 904–918. [Google Scholar] [CrossRef]
- Moges, F.; Endris, M.; Belyhun, Y.; Worku, W. Isolation and characterization of multiple drug resistance bacterial pathogens from waste water in hospital and non-hospital environments, northwest Ethiopia. BMC Res. Notes 2014, 7, 215. [Google Scholar] [CrossRef] [PubMed]
- Milakovi, M.; Vestergaard, G.; González-plaza, J.J.; Petri, I.; Ana, Š. Antibiotic-manufacturing sites are hot-spots for the release and spread of antibiotic resistance genes and mobile genetic elements in receiving aquatic environments pollution from azithromycin-manufacturing promotes macrolide-resistance gene propagation a. Environ. Int. 2019, 123, 501–511. [Google Scholar] [CrossRef]
- Max, M. Antibiotics, antibiotic resistance and environment. Encycl. Environ. 2019. Available online: https://www.encyclopedie-environnement.org/en/health/antibiotics-antibiotic-resistance-and-environment-2/ (accessed on 15 April 2024).
- Rodríguez-molina, D.; Mang, P.; Schmitt, H.; Chifiriuc, M.C.; Radon, K.; Wengenroth, L. Do wastewater treatment plants increase antibiotic resistant bacteria or genes in the environment? protocol for a systematic review. Syst. Rev. 2019, 8, 304. [Google Scholar] [CrossRef]
- Collaborators, A.R. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Tam, P.I.; Musicha, P.; Kawaza, K.; Cornick, J.; Denis, B.; Freyne, B.; Everett, D.; Dube, Q.; French, N.; Feasey, N.; et al. Emerging resistance to empiric antimicrobial regimens for pediatric bloodstream infections in Malawi (1998–2017). Clin. Infect. Dis. 2019, 69, 61–68. [Google Scholar]
- Lester, R.; Musicha, P.; Kawaza, K.; Langton, J.; Mango, J.; Mangochi, H.; Bakali, W.; Pearse, O.; Mallewa, J.; Denis, B.; et al. Articles Effect of resistance to third-generation cephalosporins on morbidity and mortality from bloodstream infections in Blantyre, Malawi: A prospective cohort study. Lancet Microbe 2022, 3, e922–e930. [Google Scholar] [CrossRef]
- Kayambankadzanja, R.K.; Lihaka, M.; Barratt-due, A.; Kachingwe, M.; Kumwenda, W.; Lester, R.; Bilima, S.; Eriksen, J.; Baker, T. The use of antibiotics in the intensive care unit of a tertiary hospital in Malawi. BMC Infect. Dis. 2020, 20, 776. [Google Scholar] [CrossRef]
- Mankhomwa, J.; Tolhurst, R.; M’biya, E.; Chikowe, I.; Banda, P.; Mussa, J.; Mwasikakata, H.; Simpson, V.; Feasey, N.; MacPherson, E.E. A qualitative study of antibiotic use practices in intensive small-scale farming in urban and peri-urban Blantyre, Malawi: Implications for antimicrobial resistance. Front. Vet. Sci. 2022, 9, 876513. [Google Scholar] [CrossRef]
- Cocker, D.; Chidziwisano, K.; Mphasa, M.; Mwapasa, T.; Lewis, J.M.; Rowlingson, B.; Sammarro, M.; Bakali, W.; Salifu, C.; Zuza, A.; et al. Articles investigating one health risks for human colonisation with extended spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Malawian households: A longitudinal cohort study. Lancet Microbe 2023, 4, 534–543. [Google Scholar] [CrossRef]
- Tegha, G.; Ciccone, E.J.; Krysiak, R.; Kaphatika, J.; Chikaonda, T.; Ndhlovu, I.; Van, D.; Hoffman, I.; Juliano, J.J.; Wang, J. Genomic epidemiology of Escherichia coli isolates from a tertiary referral center in Lilongwe, Malawi. Microb. Genomics 2021, 7, 000490. [Google Scholar] [CrossRef]
- Nzima, B.; Adegoke, A.A.; Ofon, U.A.; Saki, M.; Inyang, C.U. Resistotyping and extended-spectrum beta-lactamase genes among Escherichia coli from wastewater treatment plants and recipient surface water for reuse in South Africa. New Microbes New Infect. 2020, 38, 100803. [Google Scholar] [CrossRef]
- Mutuku, C.; Melegh, S.; Kovacs, K.; Urban, P.; Virág, E.; Heninger, R.; Herczeg, R.; Sonnevend, A.; Gyenesei, A.; Fekete, C.; et al. Characterization of β -lactamases and multidrug resistance mechanisms in enterobacterales from hospital effluents and wastewater treatment plant. Antibiotics 2022, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Verburg, I.; Garc, S.; Hern, L.; Waar, K.; Friedrich, A.W.; Schmitt, H. Abundance and antimicrobial resistance of three bacterial species along a complete wastewater pathway. Microorganisms 2019, 7, 312. [Google Scholar] [CrossRef] [PubMed]
- Sekizuka, T.; Tanaka, R.; Hashino, M.; Yatsu, K.; Kuroda, M. Comprehensive genome and plasmidome analysis of antimicrobial resistant bacteria in wastewater treatment plant effluent of Tokyo. Antibiotics 2022, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Alouache, S.; Estepa, V.; Messai, Y.; Ruiz, E.; Torres, C.; Bakour, R. Characterization of esbls and associated quinolone resistance in Escherichia coli and Klebsiella pneumoniae isolates from an urban wastewater plant in Algeria. Microb. Drug Resist. 2013, 20, 30–38. [Google Scholar] [CrossRef]
- Mwapasa, T.; Mphasa, M.; Cocker, D.; Chidziwisano, K.; Feasey, N.; Morse, T. Community exposure assessment to anti-microbial resistance (AMR); case study of Malawi. In Proceedings of the UNC Water and Health, University of Strathclyde (Virtual), Glasgow, Scotland, 4–8 October 2021. [Google Scholar]
- Ministry of Health (Mw). Malawi Standard Treatment Guidelines, 6th ed.; Ministry of Health: Wellington, New Zealand, 2023; Available online: https://www.differentiatedservicedelivery.org/wp-content/uploads/MSTG-6th-Edition-2023-Final-Draft-CC-gn-2-edditi_230719_133059.pdf (accessed on 15 April 2024).
- Afunwa, R.A.; Ezeanyinka, J.; Afunwa, E.C.; Udeh, A.S.; Oli, A.N.; Unachukwu, M. Multiple antibiotic resistant index of gram-negative bacteria from bird droppings in two commercial poultries in Enugu, Nigeria. Open J. Med. Microbiol. 2020, 10, 171–181. [Google Scholar] [CrossRef]
- Sibande, G.T.; Banda, N.P.K.; Moya, T.; Siwinda, S.; Lester, R. Antibiotic guideline adherence by Clinicians in medical wards at Queen Elizabeth Central Hospital (QECH), Blantyre Malawi. Malawi Med. J. 2022, 34, 3–8. [Google Scholar] [CrossRef]
- Macpherson, E.E.; Mankhomwa, J.; Dixon, J.; Pongolani, R.; Phiri, M.; Feasey, N.; Byrne, T.O.; Tolhurst, R.; MacPherson, P. Household antibiotic use in Malawi: A cross-sectional survey from urban and peri-urban Blantyre. PLoS Glob. Public Health 2023, 3, e0001946. [Google Scholar] [CrossRef] [PubMed]
- Musicha, P.; Feasey, N.A.; Cain, A.K.; Kallonen, T.; Chaguza, C.; Peno, C.; Khonga, M.; Thompson, S.; Gray, K.J.; Mather, A.E.; et al. Genomic landscape of extended-spectrum β-lactamase resistance in Escherichia coli from an urban African setting. J. Antimicrob. Chemother. 2017, 72, 1602–1609. [Google Scholar] [CrossRef]
- Musicha, P.; Msefula, C.L.; Mather, A.E.; Chaguza, C.; Cain, A.K.; Peno, C.; Kallonen, T.; Khonga, M.; Denis, B.; Gray, K.J. Genomic analysis of Klebsiella pneumoniae isolates from Malawi reveals acquisition of multiple ESBL determinants across diverse lineages. J. Antimicrob. Chemother. 2019, 74, 1223–1232. [Google Scholar] [CrossRef]
- Raven, K.E.; Ludden, C.; Gouliouris, T.; Blane, B.; Naydenova, P.; Brown, N.M. Genomic surveillance of Escherichia coli in municipal wastewater treatment plants as an indicator of clinically relevant pathogens and their resistance genes. Microbiol. Soc. 2019, 5, e000267. [Google Scholar] [CrossRef]
- World Health Organization. Malawi National Action Plan on Antimicrobial Resistance: Review of Progress in the Human Health Sector; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Conforti, S.; Holschneider, A.; Sylvestre, É.; Julian, T.R. Monitoring ESBL- Escherichia coli in Swiss wastewater between November 2021 and November 2022: Insights into population carriage. mSphere 2024. Epub ahead of print. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Chang. 2018, 8, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Kraslawski, A.; Avramenko, Y. Comparison of pollutant levels in effluent from wastewater treatment plants in Blantyre, Malawi. Int. J. Water Resour. Environ. Eng. 2010, 2, 79–86. [Google Scholar]
- Feasey, N.A.; Gaskell, K.; Wong, V.; Msefula, C. Rapid emergence of multidrug resistant, H58-lineage salmonella typhi in Blantyre. PLoS Negl. Trop. Dis. 2015, 9, e0003748. [Google Scholar] [CrossRef] [PubMed]
- Teshome, A.; Alemayehu, T.; Deriba, W.; Ayele, Y. Antibiotic resistance profile of bacteria isolated from wastewater systems in eastern Ethiopia. J. Environ. Public Health 2020, 2020, 2796365. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; ISBN 9781684400669. [Google Scholar]
- Krumperman, P.H. Multiple Antibiotic Resistance Indexing of Escherichia coli to Identify High-Risk Sources of Fecal Contamination of Foodst. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef]



| Species of ESBL Organisms Isolated from Each Sample | Number of Samples Per Site | ||
|---|---|---|---|
| Blantyre, N = 138 | Soche, N = 143 | Overall, N = 281 | |
| E. coli only | 4/138 | 3/143 | 7/281 |
| (2.90%) | (2.10%) | (2.50%) | |
| Enterobacter cloacae only | 16/138 | 0/143 | 16/281 |
| (11.60%) | (0.00%) | (5.70%) | |
| Enterobacter asberiae only | 1/138 | 0/143 | 1/281 |
| (0.70%) | (0.00%) | (0.40%) | |
| E. coli and Enterobacter cloacae | 32/138 | 4/143 | 36/281 |
| (23.20%) | (2.80%) | (12.80%) | |
| E. coli and K. pneumoniae pneumoniae | 55/138 | 117/143 | 172/281 |
| (39.90%) | (81.80%) | (61.20%) | |
| E. coli and K. pneumoniae ozaenae | 3/138 | 2/143 | 5/281 |
| (2.20%) | (1.40%) | (1.80%) | |
| Proteus vulgaris | 1/138 | 0/143 | 1/281 |
| (0.70%) | (0.00%) | (0.40%) | |
| E. coli and Rahnella aqualitis | 0/138 | 1/143 | 1/281 |
| (0.00%) | (0.70%) | (0.40%) | |
| E. coli and Raoultella ornithinolytica | 0/138 | 2/143 | 2/281 |
| 0.00% | (1.40%) | (0.70%) | |
| E. coli and Low discrimination profiles (Aeromonas, other Klebsiella pneumoniae subspp, Pantoea, and Vibrio species) | 19/138 | 14/143 | 33/281 |
| (13.80%) | (9.80%) | (11.70%) | |
| Other ESBL growth, but no pink or blue colonies | 8/138 | 0/143 | 8/281 |
| (5.80%) | (0.00%) | (2.80%) | |
| Soche | Blantyre | |||||||
|---|---|---|---|---|---|---|---|---|
| MAR Phenotype | E. coli (143) | K. pneumoniae (117) | E. cloacae (4) | E. coli (112) | K. pneumoniae (55) | E. cloacae (48) | Total | MAR Index |
| AUG, MEM, SXT, DXT, CIP, GM, ATH, CRO | 6 | 0 | 0 | 0 | 0 | 0 | 6 | 1 |
| AUG, SXT, DXT, CIP, GM, ATH, CRO | 34 | 6 | 1 | 11 | 1 | 2 | 55 | 0.9 |
| AUG, SXT, DXT, GM, ATH, CRO | 0 | 1 | 0 | 1 | 3 | 0 | 5 | 0.8 |
| AUG, SXT, GM, ATH, CRO | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.6 |
| SXT, GM, ATH, CRO | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| SXT, ATH, CRO | 1 | 1 | 0 | 0 | 0 | 1 | 3 | 0.4 |
| SXT, CIP, GM, CRO | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| SXT, CIP, GM, ATH, CRO | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0.6 |
| SXT, DXT, CIP, GM, ATH, CRO | 9 | 5 | 0 | 5 | 4 | 0 | 23 | 0.8 |
| SXT, CIP, ATH, CRO | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| SXT, CIP, CRO | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0.4 |
| SXT, DXT, CRO | 3 | 6 | 0 | 6 | 0 | 0 | 15 | 0.4 |
| SXT, DXT, CIP, CRO | 9 | 7 | 0 | 7 | 4 | 1 | 28 | 0.5 |
| AUG, SXT, DXT, CIP, CRO | 4 | 5 | 0 | 6 | 3 | 0 | 18 | 0.6 |
| AUG, SXT, DXT, CRO | 5 | 2 | 0 | 2 | 0 | 1 | 10 | 0.5 |
| AUG, SXT, CRO | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.4 |
| AUG, MEM, SXT, DXT, ATH, CRO | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.8 |
| AUG, SXT, DXT, CIP, GM, CRO | 2 | 30 | 1 | 1 | 10 | 1 | 45 | 0.8 |
| AUG, SXT, DXT, GM, CRO | 0 | 4 | 1 | 0 | 3 | 0 | 8 | 0.6 |
| DXT, CIP, GM, CRO | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 0.5 |
| SXT, DXT, GM, CRO | 1 | 5 | 0 | 0 | 2 | 0 | 8 | 0.5 |
| SXT, DXT, CIP, GM, CRO | 1 | 6 | 0 | 5 | 4 | 0 | 16 | 0.6 |
| AUG, MEM, SXT, DXT, CIP, ATH, CRO | 3 | 0 | 0 | 3 | 0 | 0 | 6 | 0.9 |
| SXT, DXT, GM, ATH, CRO | 0 | 1 | 0 | 1 | 1 | 0 | 3 | 0.6 |
| AUG, SXT, CIP, ATH, CRO | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0.6 |
| AUG, MEM, SXT, CIP, GM, CRO | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0.8 |
| AUG, MEM, DXT, CIP, ATH, CRO | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.8 |
| AUG, MEM, DXT, CIP, CRO | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.6 |
| AUG, CIP, CRO | 0 | 1 | 0 | 0 | 0 | 2 | 3 | 0.4 |
| SXT, DXT, ATH, CRO | 2 | 4 | 0 | 2 | 2 | 0 | 10 | 0.5 |
| AUG, DXT, CRO | 0 | 0 | 0 | 1 | 0 | 4 | 5 | 0.4 |
| AUG, SXT, CIP, GM, CRO | 1 | 7 | 0 | 0 | 3 | 0 | 11 | 0.6 |
| AUG, MEM, SXT, DXT, GM, ATH, CRO | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.9 |
| AUG, CIP, GM, CRO | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| AUG, MEM, CRO | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.4 |
| SXT, DXT, CIP, ATH, CRO | 17 | 7 | 0 | 13 | 3 | 0 | 40 | 0.6 |
| DXT, CIP, ATH, CRO | 1 | 1 | 0 | 2 | 1 | 0 | 5 | 0.5 |
| DXT, CIP, CRO | 2 | 0 | 0 | 2 | 1 | 0 | 5 | 0.4 |
| DXT, GM, ATH, CRO | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0.5 |
| AUG, DXT, CIP, CRO | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 |
| AUG, DXT, CIP, ATH, CRO | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0.6 |
| Univariable Poisson Model | Multivariable Poisson Model | |||||
|---|---|---|---|---|---|---|
| Characteristic | IRR 1 | 95% CI 1 | p-Value | IRR 1 | 95% CI 1 | p-Value |
| Minimum Temperature | 1.02 | 1.00, 1.04 | 0.080 | 1.04 | 1.01, 1.08 | 0.019 |
| Maximum Temperature | 1.00 | 0.98, 1.02 | 0.821 | 1.00 | 0.97, 1.02 | 0.637 |
| Total rainfall in Chichiri | 1.00 | 1.00, 1.00 | 0.015 | 1.00 | 1.00, 1.00 | 0.561 |
| Total rainfall in Mpemba | 1.00 | 1.00, 1.00 | 0.133 | 1.00 | 1.00, 1.00 | 0.564 |
| Wastewater treatment plant | <0.001 | <0.001 | ||||
| Blantyre | — | — | — | — | ||
| Soche | 1.41 | 1.30, 1.53 | 1.33 | 1.22, 1.46 | ||
| Organism | <0.001 | <0.001 | ||||
| E. coli | — | — | — | — | ||
| Enterobacter cloacae | 0.27 | 0.23, 0.32 | 0.30 | 0.25, 0.35 | ||
| K. pneumoniae | 0.75 | 0.69, 0.82 | 0.71 | 0.65, 0.78 | ||
| Antibiotic | <0.001 | <0.001 | ||||
| Amoxicillin clavulanate (AUG) | — | — | — | — | ||
| Azithromycin (ATH) | 1.01 | 0.85, 1.20 | 0.95 | 0.79, 1.14 | ||
| Ceftriaxone (CRO) | 1.62 | 1.40, 1.88 | 1.74 | 1.48, 2.04 | ||
| Ciprofloxacin (CIP) | 1.33 | 1.14, 1.56 | 1.33 | 1.13, 1.58 | ||
| Cotrimoxazole (SXT) | 1.58 | 1.35, 1.84 | 1.52 | 1.29, 1.79 | ||
| Doxycycline (DXT) | 1.40 | 1.20, 1.63 | 1.47 | 1.25, 1.73 | ||
| Gentamicin (GM) | 0.86 | 0.71, 1.03 | 0.86 | 0.71, 1.04 | ||
| Meropenem (MEM) | 0.35 | 0.21, 0.53 | 0.31 | 0.19, 0.48 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, E.; Mkwanda, C.; Masoambeta, E.; Scudeller, L.; Kostyanev, T.; Twabi, H.H.; Diness, Y.K.; Chinkhumba, J.; Musaya, J.; Mkakosya, R.S.; et al. Prevalence of Extended-Spectrum β-Lactamase-Producing Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae in Wastewater Effluent in Blantyre, Malawi. Antibiotics 2025, 14, 562. https://doi.org/10.3390/antibiotics14060562
Ibrahim E, Mkwanda C, Masoambeta E, Scudeller L, Kostyanev T, Twabi HH, Diness YK, Chinkhumba J, Musaya J, Mkakosya RS, et al. Prevalence of Extended-Spectrum β-Lactamase-Producing Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae in Wastewater Effluent in Blantyre, Malawi. Antibiotics. 2025; 14(6):562. https://doi.org/10.3390/antibiotics14060562
Chicago/Turabian StyleIbrahim, Edna, Charity Mkwanda, Edward Masoambeta, Luigia Scudeller, Tomislav Kostyanev, Hussein H. Twabi, Yohane K. Diness, Jobiba Chinkhumba, Janelisa Musaya, Rajhab S. Mkakosya, and et al. 2025. "Prevalence of Extended-Spectrum β-Lactamase-Producing Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae in Wastewater Effluent in Blantyre, Malawi" Antibiotics 14, no. 6: 562. https://doi.org/10.3390/antibiotics14060562
APA StyleIbrahim, E., Mkwanda, C., Masoambeta, E., Scudeller, L., Kostyanev, T., Twabi, H. H., Diness, Y. K., Chinkhumba, J., Musaya, J., Mkakosya, R. S., Malhotra-Kumar, S., Morel, C. M., Kumwenda, S., & Msefula, C. L. (2025). Prevalence of Extended-Spectrum β-Lactamase-Producing Escherichia coli, Klebsiella pneumoniae and Enterobacter cloacae in Wastewater Effluent in Blantyre, Malawi. Antibiotics, 14(6), 562. https://doi.org/10.3390/antibiotics14060562







