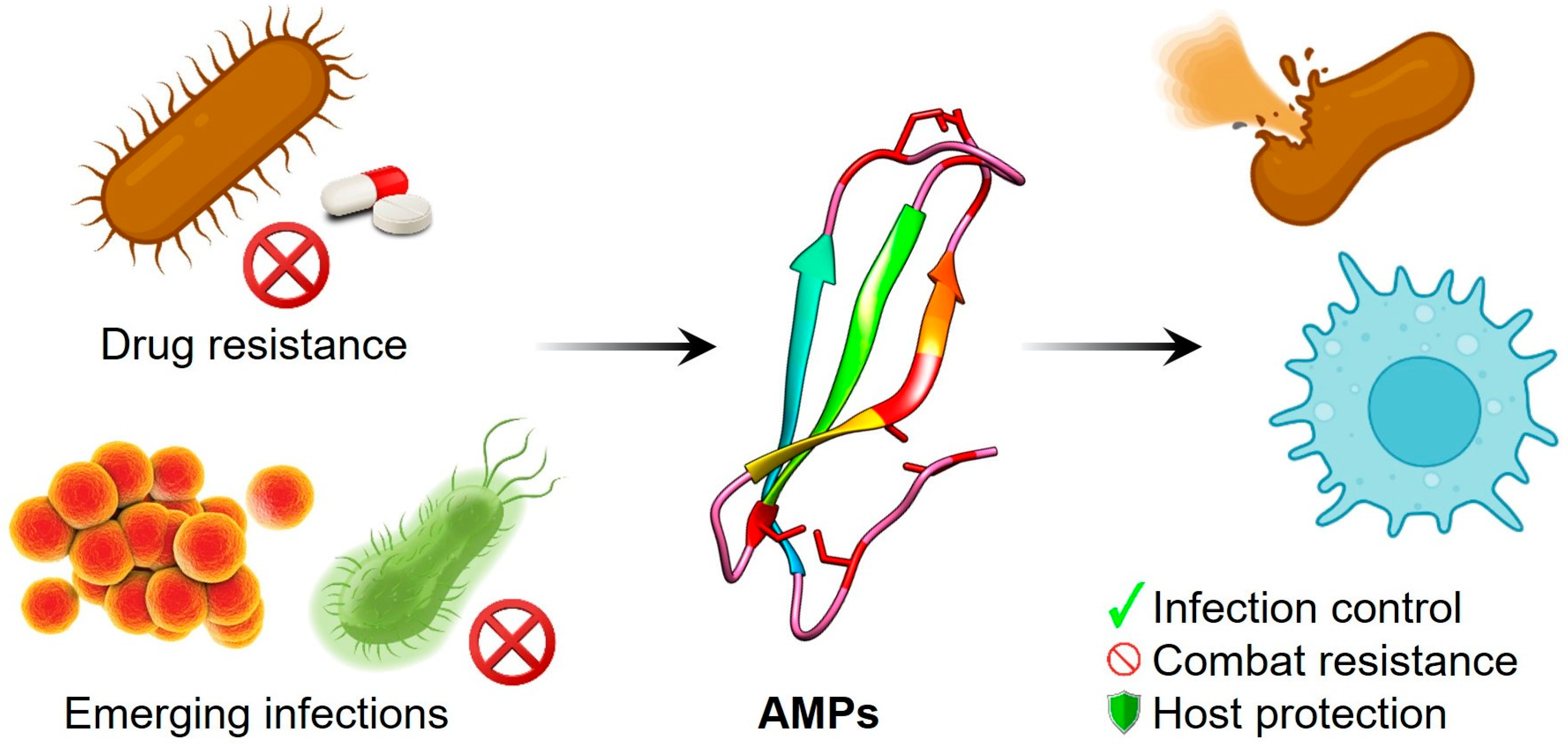
Antimicrobial Peptides: An Emerging Hope in the Era of New Infections and Resistance
- How do AMPs selectively target microbes over host cells? Can this selectivity be manipulated synthetically?
- How do the AMP producers maintain self-immunity?
- Is it possible to develop resistance against AMPs? If so, what are the AMP resistance genes and what is the global prevalence? Can it be transferred horizontally like conventional antibiotics?
- How do repeated AMP treatments influence microbial diversity?
- How does AMP treatment influence pro- and anti-inflammatory immune response?
- What signaling pathways are activated upon AMP–host cell interactions?
Acknowledgments
Conflicts of Interest
References
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, S.; Zhang, C. Antimicrobial peptides with antiviral and anticancer properties and their modification and nanodelivery systems. Curr. Res. Biotechnol. 2023, 5, 100121. [Google Scholar] [CrossRef]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial peptides’ immune modulation role in intracellular bacterial infection. Front. Immunol. 2023, 14, 1119574. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Z.; Xia, B.; Zhang, Y.; Liu, X.; Yu, Y.; Tang, N.; Tong, X.; Wang, M.; Ye, X.; et al. Identification of antimicrobial peptides from the human gut microbiome using deep learning. Nat. Biotechnol. 2022, 40, 921–931. [Google Scholar] [CrossRef]
- Ostaff, M.J.; Stange, E.F.; Wehkamp, J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol. Med. 2013, 5, 1465–1483. [Google Scholar] [CrossRef]
- Baindara, P.; Mandal, S.M. Gut-Antimicrobial Peptides: Synergistic Co-Evolution with Antibiotics to Combat Multi-Antibiotic Resistance. Antibiotics 2023, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Sharma, S.; Sinha, K.K. Evidence of Antibiotic Resistance and Virulence Factors in Environmental Isolates of Vibrio Species. Antibiotics 2023, 12, 1062. [Google Scholar] [CrossRef]
- Baindara, P.; Kumari, S.; Dinata, R.; Mandal, S.M. Antimicrobial peptides: Evolving soldiers in the battle against drug-resistant superbugs. Mol. Biol. Rep. 2025, 52, 432. [Google Scholar] [CrossRef]
- Sermkaew, N.; Atipairin, A.; Krobthong, S.; Aonbangkhen, C.; Yingchutrakul, Y.; Uchiyama, J.; Songnaka, N. Unveiling a New Antimicrobial Peptide with Efficacy against P. aeruginosa and K. pneumoniae from Mangrove-Derived Paenibacillus thiaminolyticus NNS5-6 and Genomic Analysis. Antibiotics 2024, 13, 846. [Google Scholar] [CrossRef]
- Yang, C.-L.; Wang, P.-P.; Zhou, Z.-Y.; Wu, X.-W.; Hua, Y.; Chen, J.-W.; Wang, H.; Wei, B. Discovery of naturally inspired antimicrobial peptides using deep learning. Bioorganic Chem. 2025, 160, 108444. [Google Scholar] [CrossRef]
- Klubthawee, N.; Adisakwattana, P.; Hanpithakpong, W.; Somsri, S.; Aunpad, R. A novel, rationally designed, hybrid antimicrobial peptide, inspired by cathelicidin and aurein, exhibits membrane-active mechanisms against Pseudomonas aeruginosa. Sci. Rep. 2020, 10, 9117. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Ridgway, Z.M.; Picciano, A.L.; Caputo, G.A. Impacts of Hydrophobic Mismatch on Antimicrobial Peptide Efficacy and Bilayer Permeabilization. Antibiotics 2023, 12, 1624. [Google Scholar] [CrossRef] [PubMed]
- Keeratikunakorn, K.; Chanapiwat, P.; Aunpad, R.; Ngamwongsatit, N.; Kaeoket, K. Effect of Antimicrobial Peptide BiF2_5K7K on Contaminated Bacteria Isolated from Boar Semen and Semen Qualities during Preservation and Subsequent Fertility Test on Pig Farm. Antibiotics 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Keeratikunakorn, K.; Chanapiwat, P.; Aunpad, R.; Ngamwongsatit, N.; Kaeoket, K. The Effects of Different Antimicrobial Peptides (A-11 and AP19) on Isolated Bacteria from Fresh Boar Semen and Semen Quality during Storage at 18 °C. Antibiotics 2024, 13, 489. [Google Scholar] [CrossRef]
- Finkina, E.I.; Bogdanov, I.V.; Shevchenko, O.V.; Fateeva, S.I.; Ignatova, A.A.; Balandin, S.V.; Ovchinnikova, T.V. Immunomodulatory Effects of the Tobacco Defensin NaD1. Antibiotics 2024, 13, 1101. [Google Scholar] [CrossRef]
- Roson-Calero, N.; Lucas, J.; Gomis-Font, M.A.; de Pedro-Jové, R.; Oliver, A.; Ballesté-Delpierre, C.; Vila, J. Cyclic Peptide MV6, an Aminoglycoside Efficacy Enhancer Against Acinetobacter baumannii. Antibiotics 2024, 13, 1147. [Google Scholar] [CrossRef]
- Yount, N.Y.; Yeaman, M.R. Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 7363–7368. [Google Scholar] [CrossRef]
- Baindara, P.; Singh, N.; Ranjan, M.; Nallabelli, N.; Chaudhry, V.; Pathania, G.L.; Sharma, N.; Kumar, A.; Patil, P.B.; Korpole, S. Laterosporulin10: A novel defensin like class iid bacteriocin from Brevibacillus sp. strain SKDU10 with inhibitory activity against microbial pathogens. Microbiology 2016, 162, 1286–1299. [Google Scholar] [CrossRef]
- Cardoso, M.H.; de Lima, L.R.; Pires, A.S.; Maximiano, M.R.; Harvey, P.J.; Freitas, C.G.; Costa, R.A.; Fensterseifer, I.C.M.; Rigueiras, P.O.; Migliolo, L.; et al. Discovery of Five Classes of Bacterial Defensins: Ancestral Precursors of Defensins from Eukarya? ACS Omega 2024, 9, 45297–45308. [Google Scholar] [CrossRef]
- Al Musaimi, O. FDA-Approved Antibacterials and Echinocandins. Antibiotics 2025, 14, 166. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins-a viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Telhig, S.; Ben Said, L.; Zirah, S.; Fliss, I.; Rebuffat, S. Bacteriocins to Thwart Bacterial Resistance in Gram Negative Bacteria. Front. Microbiol. 2020, 11, 586433. [Google Scholar] [CrossRef]
- Louca, S.; Mazel, F.; Doebeli, M.; Parfrey, L.W. A census-based estimate of earth’s bacterial and archaeal diversity. PLoS Biol. 2019, 17, e3000106. [Google Scholar] [CrossRef]
- Baindara, P.; Mandal, S.M. The interplay of gut-microbiome between infection and inflammation. Front. Cell. Infect. Microbiol. 2024, 14, 1413473. [Google Scholar] [CrossRef]
- Zeeuwen, P.L.J.M.; Grice, E.A. Skin microbiome and antimicrobial peptides. Exp. Dermatol. 2021, 30, 1362–1365. [Google Scholar] [CrossRef]
- Di, Y.P.; Kuhn, J.M.; Mangoni, M.L. Lung antimicrobial proteins and peptides: From host defense to therapeutic strategies. Physiol. Rev. 2024, 104, 1643–1677. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Torres, M.D.T.; Peng, J.; de la Fuente-Nunez, C. Deep-learning-enabled antibiotic discovery through molecular de-extinction. Nat. Biomed. Eng. 2024, 8, 854–871. [Google Scholar] [CrossRef]
- Santos-Júnior, C.D.; Torres, M.D.T.; Duan, Y.; Rodríguez del Río, Á.; Schmidt, T.S.B.; Chong, H.; Fullam, A.; Kuhn, M.; Zhu, C.; Houseman, A.; et al. Discovery of antimicrobial peptides in the global microbiome with machine learning. Cell 2024, 187, 3761–3778.e16. [Google Scholar] [CrossRef]
- Botelho Sampaio de Oliveira, K.; Lopes Leite, M.; Albuquerque Cunha, V.; Brito da Cunha, N.; Luiz Franco, O. Challenges and advances in antimicrobial peptide development. Drug Discov. Today 2023, 28, 103629. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baindara, P. Antimicrobial Peptides: An Emerging Hope in the Era of New Infections and Resistance. Antibiotics 2025, 14, 546. https://doi.org/10.3390/antibiotics14060546
Baindara P. Antimicrobial Peptides: An Emerging Hope in the Era of New Infections and Resistance. Antibiotics. 2025; 14(6):546. https://doi.org/10.3390/antibiotics14060546
Chicago/Turabian StyleBaindara, Piyush. 2025. "Antimicrobial Peptides: An Emerging Hope in the Era of New Infections and Resistance" Antibiotics 14, no. 6: 546. https://doi.org/10.3390/antibiotics14060546
APA StyleBaindara, P. (2025). Antimicrobial Peptides: An Emerging Hope in the Era of New Infections and Resistance. Antibiotics, 14(6), 546. https://doi.org/10.3390/antibiotics14060546




