A Retrospective Study of the Impact of the COVID-19 Pandemic on the Utilization and Quality of Antibiotic Use in a Tertiary Care Teaching Hospital in Low-Resource Settings
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Data Collection
4.2. Antibiotic Utilization
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gagliotti, C.; Ricchizzi, E.; Buttazzi, R.; Tumietto, F.; Resi, D.; Moro, M.L. Hospital statistics for antibiotics: Defined versus prescribed daily dose. Infection 2014, 42, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Parra, J.; Muiño-Miguez, A.; Bendala-Estrada, A.D.; Ramos-Martínez, A.; Muñez-Rubio, E.; Carracedo, E.F.; Montes, J.T.; Rubio-Rivas, M.; Arnalich-Fernandez, F.; Pérez, J.L.B.; et al. Inappropriate antibiotic use in the COVID-19 era: Factors associated with inappropriate prescribing and secondary complications. Analysis of the registry SEMI-COVID. PLoS ONE 2021, 16, e0251340. [Google Scholar] [CrossRef] [PubMed]
- Sibani, M.; Canziani, L.M.; Tonolli, C.; Armellini, M.; Carrara, E.; Mazzaferri, F.; Conti, M.; SAVE Working Group; Mazzariol, A.; Micheletto, C.; et al. Antimicrobial Stewardship in COVID-19 Patients: Those Who Sow Will Reap Even through Hard Times. Antibiotics 2023, 12, 1009. [Google Scholar] [CrossRef]
- Robertson, J.; Vlahović-Palčevski, V.; Iwamoto, K.; Högberg, L.D.; Godman, B.; Monnet, D.L.; Garner, S.; Weist, K.; ESAC-Net Study Group; WHO Europe AMC Network Study Group. Variations in the Consumption of Antimicrobial Medicines in the European Region, 2014–2018: Findings and Implications from ESAC-Net and WHO Europe. Front. Pharmacol. 2021, 12, 639207. [Google Scholar]
- Vallès, J.; Fernández, S.; Cortés, E.; Morón, A.; Fondevilla, E.; Oliva, J.C.; Diaz, E. Comparison of the defined daily dose and days of treatment methods for evaluating the consumption of antibiotics and antifungals in the intensive care unit. Med. Intensiv. 2020, 44, 294–300. [Google Scholar] [CrossRef]
- Grau, S.; Bou, G.; Fondevilla, E.; Nicolás, J.; Rodríguez-Maresca, M.; Martínez-Martínez, L. How to measure and monitor antimicrobial consumption and resistance. Enferm. Infecc. Microbiol. Clin. 2013, 31 (Suppl. S4), 16–24. [Google Scholar] [CrossRef]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar]
- Nunes, P.H.C.; Moreira, J.P.d.L.; Thompson, A.d.F.; Machado, T.L.d.S.; Cerbino-Neto, J.; Bozza, F.A. Antibiotic Consumption and Deviation of Prescribed Daily Dose from the Defined Daily Dose in Critical Care Patients: A Point-Prevalence Study. Front. Pharmacol. 2022, 13, 913568. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Henig, O.; Kehat, O.; Meijer, S.E.; Chikly, A.; Weiss-Meilik, A.; Egoz, E.; Ben-Ami, R.; Paran, Y. Antibiotic Use during the COVID-19 Pandemic in a Tertiary Hospital with an Ongoing Antibiotic Stewardship Program. Antibiotics 2021, 10, 1056. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Rose, A.N.; Baggs, J.; Wolford, H.; Neuhauser, M.M.; Srinivasan, A.; Gundlapalli, A.V.; Reddy, S.; Kompaniyets, L.; Pennington, A.F.; Grigg, C.; et al. Trends in Antibiotic Use in United States Hospitals During the Coronavirus Disease 2019 Pandemic. Open Forum Infect. Dis. 2021, 8, ofab236. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N.; George, I.; Yaeger, L.H.; Chavez, M.A.; Tetteh, E.; Yarrabelli, S.; Pulcini, C.; et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1193–1202. [Google Scholar] [CrossRef]
- Kamel, A.M.; Monem, M.S.A.; Sharaf, N.A.; Magdy, N.; Farid, S.F. Efficacy and safety of azithromycin in COVID-19 patients: A systematic review and meta-analysis of randomized clinical trials. Rev. Med. Virol. 2022, 32, e2258. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Dufort, E.M.; Udo, T.; Wilberschied, L.A.; Kumar, J.; Tesoriero, J.; Weinberg, P.; Kirkwood, J.; Muse, A.; DeHovitz, J.; et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA 2020, 323, 2493–2502. [Google Scholar] [CrossRef]
- WHO News. WHO Reports Widespread Overuse of Antibiotics in Patients Hospitalized with COVID-19. 2025. Available online: https://www.who.int/news/item/26-04-2024-who-reports-widespread-overuse-of-antibiotics-in-patients--hospitalized-with-covid-19 (accessed on 20 March 2025).
- Walia, K.; Mendelson, M.; Kang, G.; Venkatasubramanian, R.; Sinha, R.; Vijay, S.; Veeraraghavan, B.; Basnyat, B.; Rodrigues, C.; Bansal, N.; et al. How can lessons from the COVID-19 pandemic enhance antimicrobial resistance surveillance and stewardship? Lancet Infect. Dis. 2023, 23, e301–e309. [Google Scholar] [CrossRef]
- Smith, L.; Karaba, S.M.; Amoah, J.; Jones, G.; Avery, R.K.; Dzintars, K.; Helsel, T.; Cosgrove, S.E.; Fabre, V. Hospital-acquired infections among adult patients admitted for coronavirus disease 2019 (COVID-19). Infect. Control Hosp. Epidemiol. 2022, 43, 1054–1057. [Google Scholar] [CrossRef]
- Bojanić, L.; Marković-Peković, V.; Škrbić, R.; Stojaković, N.; Ðermanović, M.; Bojanić, J.; Fürst, J.; Kurdi, A.B.; Godman, B. Recent Initiatives in the Republic of Srpska to Enhance Appropriate Use of Antibiotics in Ambulatory Care; Their Influence and Implications. Front. Pharmacol. 2018, 9, 442. [Google Scholar] [CrossRef]
- Sokolović, D.; Drakul, D.; Joksimović, B.; Lalović, N.; Avram, N.; Milić, M.; Nogo-Živanović, D.; Mijović, B. Consumption of Antibiotics in Primary Care Setting before and during COVID-19 Pandemic in Republic of Srpska, Bosnia and Herzegovina. Antibiotics 2022, 11, 1319. [Google Scholar] [CrossRef]
- Bednarčuk, N.; Jelić, A.G.; Šatara, S.S.; Stojaković, N.; Peković, V.M.; Stojiljković, M.P.; Popović, N.; Škrbić, R. Antibiotic Utilization during COVID-19: Are We Over-Prescribing? Antibiotics 2023, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Sokolović, D.; Drakul, D.; Vujić-Aleksić, V.; Joksimović, B.; Marić, S.; Nežić, L. Antibiotic consumption and antimicrobial resistance in the SARS-CoV-2 pandemic: A single-center experience. Front. Pharmacol. 2023, 14, 1067973. [Google Scholar] [CrossRef]
- Salehi, M.; Khalili, H.; Seifi, A.; Davoudi, H.; Darazam, I.A.; Jahangard-Rafsanjani, Z.; Mohammadnejad, E.; Heydari, B.; Siahkaly, S.J.M.; Tabarsi, P.; et al. Antibiotic use during the first 6 months of COVID-19 pandemic in Iran: A large-scale multi-centre study. J. Clin. Pharm. Ther. 2022, 47, 2140–2151. [Google Scholar] [CrossRef]
- Durà-Miralles, X.; Abelenda-Alonso, G.; Bergas, A.; Laporte-Amargós, J.; Sastre-Escolà, E.; Padullés, A.; Carratalà, J.; Gudiol, C. An Ocean between the Waves: Trends in Antimicrobial Consumption in Hospitalized Patients with COVID-19. Antibiotics 2024, 13, 55. [Google Scholar] [CrossRef]
- Abelenda-Alonso, G.; Padullés, A.; Rombauts, A.; Gudiol, C.; Pujol, M.; Alvarez-Pouso, C.; Jodar, R.; Carratalà, J. Antibiotic prescription during the COVID-19 pandemic: A biphasic pattern. Infect. Control Hosp. Epidemiol. 2020, 41, 1371–1372. [Google Scholar] [CrossRef]
- Meschiari, M.; Onorato, L.; Bacca, E.; Orlando, G.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Santoro, A.; Sarti, M.; et al. Long-Term Impact of the COVID-19 Pandemic on In-Hospital Antibiotic Consumption and Antibiotic Resistance: A Time Series Analysis (2015–2021). Antibiotics 2022, 11, 826. [Google Scholar] [CrossRef]
- Perrella, A.; Fortinguerra, F.; Pierantozzi, A.; Capoluongo, N.; Carannante, N.; Vecchio, A.L.; Bernardi, F.F.; Trotta, F.; Cangini, A. Hospital Antibiotic Use during COVID-19 Pandemic in Italy. Antibiotics 2023, 12, 168. [Google Scholar] [CrossRef]
- Bellanti, F.; Buglio, A.L.; Ricci, A.; Aquilino, A.; Labbate, A.; Vendemiale, G. In-hospital use of antibiotics in internal medicine: A cross-sectional study before, during and after the COVID-19 pandemic in a COVID-19-free ward. J. Infect. Public Health 2024, 17, 102490. [Google Scholar] [CrossRef]
- Bauer, K.A.; Puzniak, L.A.; Yu, K.C.; Klinker, K.P.; A Watts, J.; Moise, P.A.; Finelli, L.; Ai, C.; Gupta, V. A Multicenter Comparison of Prevalence and Predictors of Antimicrobial Resistance in Hospitalized Patients Before and During the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic. Open Forum Infect. Dis. 2022, 9, ofac537. [Google Scholar] [CrossRef]
- Benmerzouga, I.; Al-Zammay, S.A.; Al-Shammari, M.M.; A Alsaif, S.; Alhaidan, T.M.; Aljofan, M. Practices of patients consuming antibiotics and knowledge about antibiotic resistance in Hail region-Saudi Arabia. Future Sci. OA 2019, 5, FSO420. [Google Scholar] [CrossRef]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020, 98, 442–442A. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Godman, B.; Hassali, M.A.; Hashmi, F.K.; Azhar, F.; Rehman, I.U. Point prevalence surveys of health-care-associated infections: A systematic review. Pathog. Glob. Health 2019, 113, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, S.; Taylor, A.; Brown, A.; de Kraker, M.E.A.; El-Saed, A.; Alshamrani, M.; Hendriksen, R.S.; Jacob, M.; Löfmark, S.; Perovic, O.; et al. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: A global survey. J. Antimicrob. Chemother. 2021, 76, 3045–3058. [Google Scholar] [CrossRef]
- Kovacevic, P. Can the terms “low resource setting” and “low-income country” be used interchangeably in the context of intensive care medicine? Intensive Care Med. 2023, 49, 1274–1275. [Google Scholar] [CrossRef]
- Lucien, M.A.B.; Canarie, M.F.; Kilgore, P.E.; Jean-Denis, G.; Fénélon, N.; Pierre, M.; Cerpa, M.; Joseph, G.A.; Maki, G.; Zervos, M.J.; et al. Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings. Int. J. Infect. Dis. 2021, 104, 250–254. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Salman, M.; Aldeyab, M.; Kow, C.S.; Hasan, S.S. Antimicrobial consumption among hospitalized patients with COVID-19 in Pakistan. SN Compr. Clin. Med. 2021, 3, 1691–1695. [Google Scholar] [CrossRef]
- Grau, S.; Hernández, S.; Echeverría-Esnal, D.; Almendral, A.; Ferrer, R.; Limón, E.; Horcajada, J.P.; Catalan Infection Control and Antimicrobial Stewardship Program (VINCat-PROA). Antimicrobial Consumption among 66 Acute Care Hospitals in Catalonia: Impact of the COVID-19 Pandemic. Antibiotics 2021, 10, 943. [Google Scholar] [CrossRef]
- Tomic, T.; Henman, M.; Tadic, I.; Stankovic, J.A.; Milicevic, M.S.; Bukumiric, Z.; Lakic, D.; Odalovic, M. Immediate and long-term effects of COVID-19 on antibiotic dispensing: Increasing use of Watch antibiotics. J. Infect. Dev. Ctries. 2024, 18, 504–512. [Google Scholar] [CrossRef]
- Parra-Lara, L.G.; Martínez-Arboleda, J.J.; Rosso, F. Azithromycin and SARS-CoV-2 infection: Where we are now and where we are going. J. Glob. Antimicrob. Resist. 2020, 22, 680–684. [Google Scholar] [CrossRef]
- PRINCIPLE Trial Collaborative Group. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial. Lancet 2021, 397, 1063–1074. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 605–612. [Google Scholar]
- Čučković, M.; Drmić, Ž.; Pražetina, M.; Tipura, D.; Ćurčić, M.; Miko, I.; Mihelčić, A.; Romić, A.; Kukoč, A.; Blagaj, V.; et al. Epidemiological characteristics, baseline clinical features, and outcomes of critically ill patients treated in a coronavirus disease 2019 tertiary center in continental Croatia. Croat. Med. J. 2022, 63, 6–15. [Google Scholar] [CrossRef]
- Kovacevic, P.; Baric, G.; Dragic, S.; Momcicevic, D.; Zlojutro, B.; Jandric, M.; Kovacevic, T.; Lovric, D.; Palibrk, I.; Mallat, J. Intubation Versus Tracheotomy Outcomes in Critically Ill COVID-19 Patients in Low-Resource Settings: What Do We Know? J. Clin. Med. 2025, 14, 978. [Google Scholar] [CrossRef]
- Web Annex, C. WHO AWaRe (access, watch, reserve) classification of antibiotics for evaluation and monitoring of use, 2023. In The Selection and Use of Essential Medicines 2023: Executive Summary of the Report of the 24th WHO Expert Committee on the Selection and Use of Essential Medicines, 24–28 April 2023; World Health Organization: Geneva, Switzerland, 2023; (WHO/MHP/HPS/EML/2023.04); Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 25 March 2025).
- Al-Azzam, S.; Mhaidat, N.M.; Banat, H.A.; Alfaour, M.; Ahmad, D.S.; Muller, A.; Al-Nuseirat, A.; Lattyak, E.A.; Conway, B.R.; Aldeyab, M.A. An Assessment of the Impact of Coronavirus Disease (COVID-19) Pandemic on National Antimicrobial Consumption in Jordan. Antibiotics 2021, 10, 690. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net)-Annual Epidemiological Report 2023. Stockholm: ECDC. 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-consumption-eueea-esac-net-annual-epidemiological-report-2023 (accessed on 5 April 2025).
- World Health Organization Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index, 2025. Oslo, 2025. Available online: https://atcddd.fhi.no/atc_ddd_index/ (accessed on 25 March 2025).
- Suraj, B.; Somashekara, S.C.; Sandeep, B.; Desai, V.; Tanuja, V.H.; Srikanth. A prospective study on antibiotic usage and cost pattern in an intensive care unit of a tertiary care hospital. Natl. J. Physiol. Pharm. Pharmacol. 2021, 11, 238–241. [Google Scholar]
- Patel, M.K.; Barvaliya, M.J.; Patel, T.K.; Tripathi, C. Drug utilization pattern in critical care unit in a tertiary care teaching hospital in India. Int. J. Crit. Illn. Inj. Sci. 2013, 3, 250–255. [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology. DDD Indicators: Introduction to DDD Indicators, 2025. Oslo, 2025. Available online: https://www.who.int/tools/atc-ddd-toolkit/indicators (accessed on 25 March 2025).
- Aćimović, J.; Jandrić, L.; Đakovic-Dević, J.; Bojanić, J.; Subotić, B.; Radojčić, T.; Rodić-Vukmir, N.; Zeljković, B. Epidemiological Characteristics of COVID-19 Infection in the Republic of Srpska: A Hundred Days Survey. Scr. Med. 2020, 51, 74–80. [Google Scholar] [CrossRef]
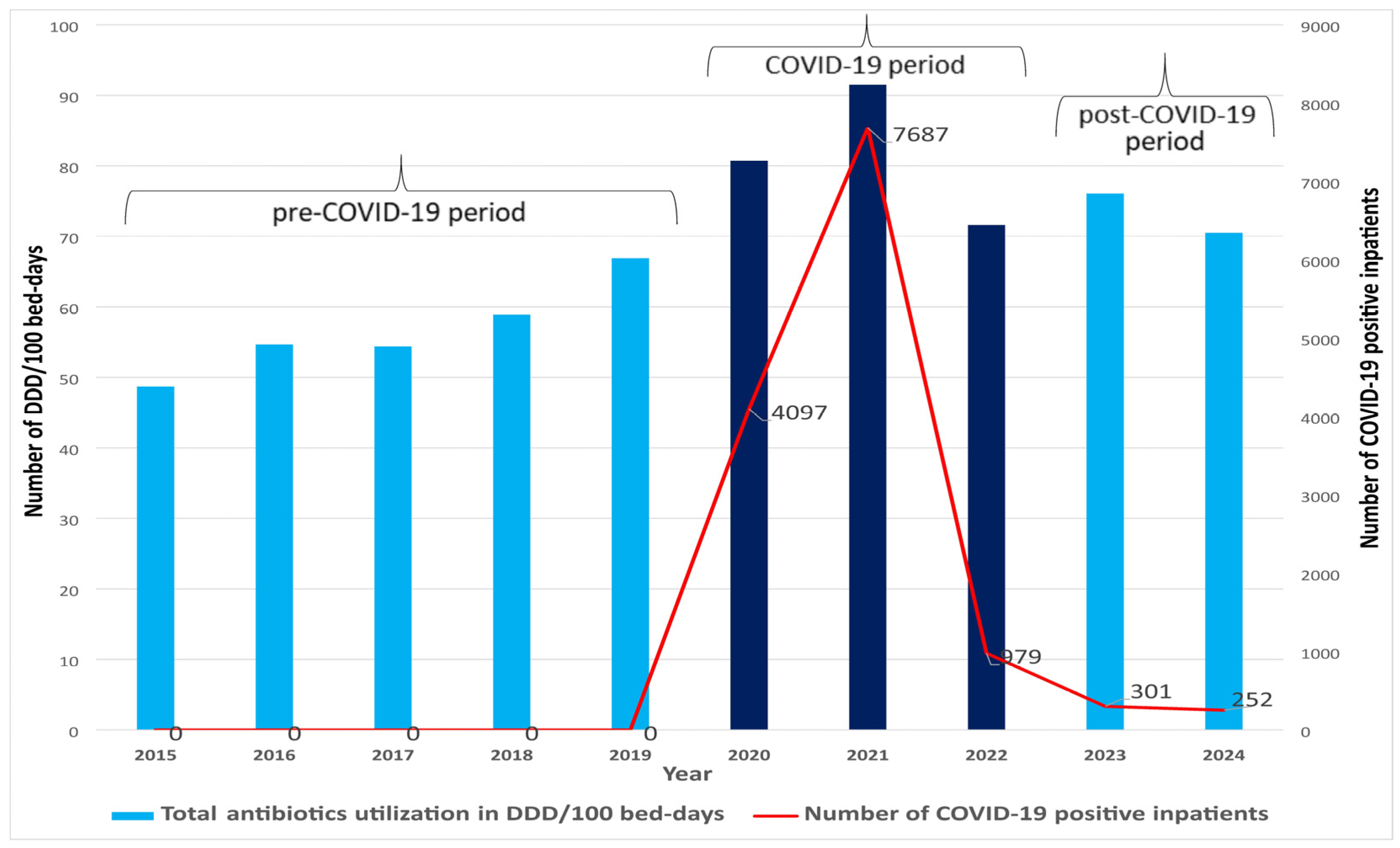
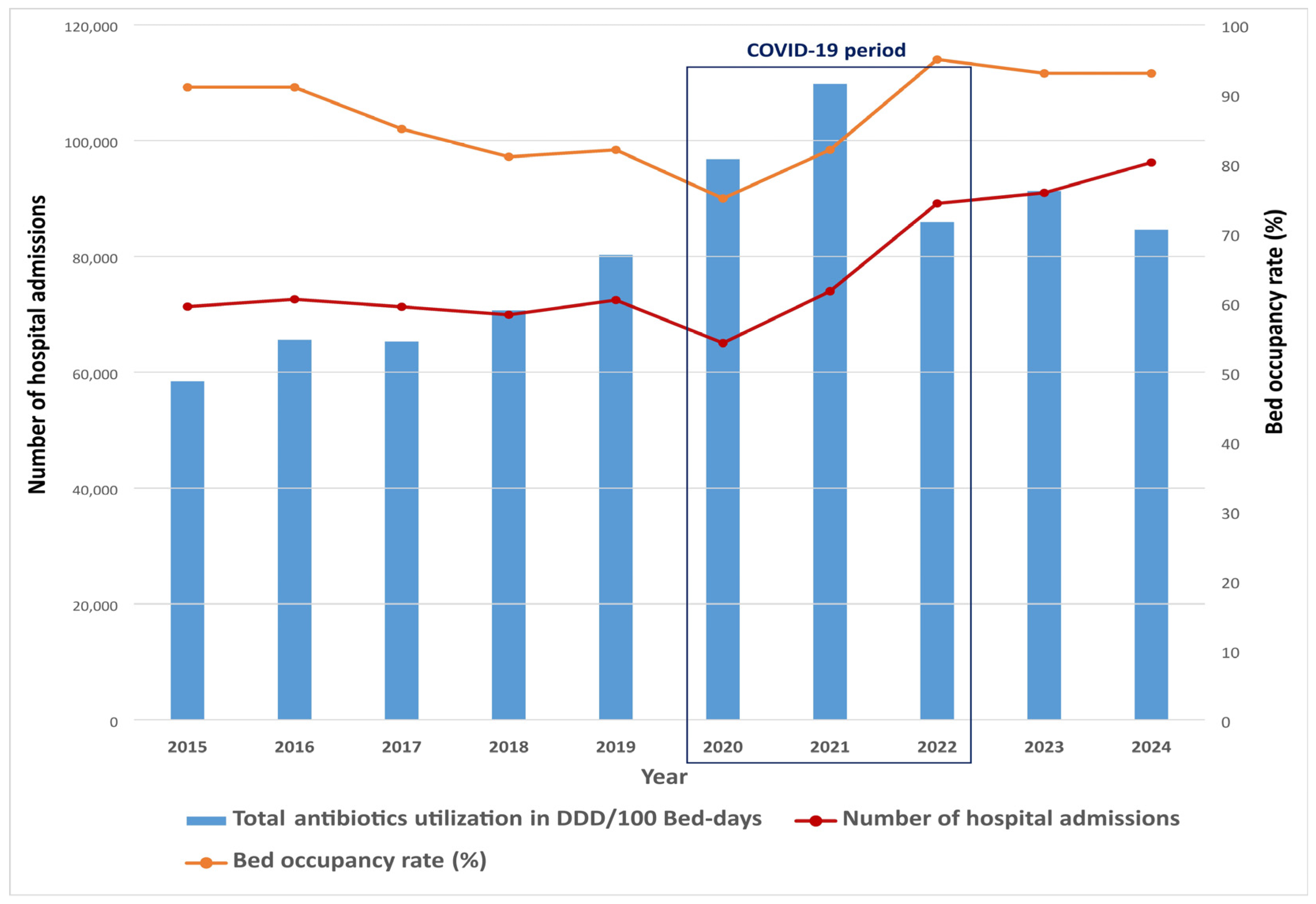
| DDD/100 Bed-Days | % Change 1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | COVID vs. Pre- 2 | Post- vs. Pre- 2 | Post- vs. COVID 2 | |
| Cephalosporins | 15.6 | 15.7 | 18.6 | 19.9 | 28.2 | 23.2 | 31.4 | 26.0 | 27.7 | 26.3 | 74.5 | 72.8 | −1.0 |
| Carbapenems | 1.2 | 1.7 | 1.8 | 2.5 | 2.8 | 5.6 | 8.4 | 5.0 | 5.7 | 6.1 | 390.5 | 313.9 | −15.6 |
| Penicillin | 8.3 | 9.9 | 7.4 | 9.7 | 5.3 | 9.9 | 8.6 | 6.3 | 7.0 | 6.0 | 1.6 | −28.5 | −29.7 |
| Tetracyclines | 3.0 | 3.5 | 3.2 | 2.5 | 2.3 | 4.4 | 5.7 | 1.6 | 1.7 | 1.5 | 55.7 | −50.4 | −68.1 |
| Quinolones | 6.5 | 6.7 | 6.1 | 5.8 | 5.6 | 6.7 | 8.9 | 6.0 | 6.7 | 6.0 | 18.3 | −3.3 | −18.2 |
| Aminoglycosides | 5.3 | 5.6 | 5.5 | 6.1 | 6.4 | 6.6 | 6.8 | 6.7 | 7.2 | 5.9 | 23.0 | 20.1 | −2.3 |
| Macrolides | 2.0 | 2.5 | 2.2 | 2.8 | 4.4 | 9.8 | 6.6 | 3.9 | 2.8 | 3.8 | 265.5 | 47.6 | −59.6 |
| Sulfonamides | 1.6 | 1.6 | 1.4 | 1.4 | 1.6 | 0.9 | 0.8 | 1.2 | 1.4 | 0.5 | −47.7 | −39.7 | 15.3 |
| Lincosamides | 0.5 | 0.4 | 0.8 | 0.9 | 0.4 | 0.6 | 1.3 | 1.1 | 1.0 | 0.9 | 110.5 | 108.5 | −1.0 |
| Nitroimidazole-metronidazole | 3.9 | 6.0 | 6.0 | 5.7 | 8.0 | 8.2 | 5.7 | 8.9 | 9.9 | 8.7 | 40.3 | 87.5 | 33.7 |
| Other antibiotics | 0.9 | 1.2 | 1.3 | 1.5 | 1.8 | 4.9 | 7.3 | 5.0 | 4.9 | 4.8 | 485.3 | 365.9 | −20.4 |
| Total | 48.7 | 54.7 | 54.4 | 58.9 | 66.9 | 80.7 | 91.5 | 71.6 | 76.1 | 70.5 | 66.6 | 41.8 | −14.9 |
| Pre-COVID-19 Period, Median (IQR) | COVID-19 Period, Median (IQR) | Post-COVID-19 Period, Median (IQR) | p-Value 1 | |
|---|---|---|---|---|
| Access | 38.5 (3.3) | 44.6 (0) | 36.1 (0) | 0.291 |
| Watch | 16.2 (8.2) | 32.9 (0) | 35.2 (0) | 0.032 |
| Reserve | 0.2 (0.3) | 2.2 (0) | 2 (0) | 0.032 |
| Total DDD/100 bed-days | 54.7 (11.4) | 80.7 (0) | 73.3 (0) | 0.028 |
| Cephalosporins | 18.6 (8.5) | 26 (0) | 27 (0) | 0.203 |
| Cefepime | 0.3 (0.2) | 1.0 (0) | 0.6 (0) | 0.057 |
| Ceftriaxone | 7.7 (6.9) | 18.4 (0) | 19.3 (0) | 0.051 |
| Carbapenems | 1.8 (1.2) | 5.6 (0) | 5.9 (0) | 0.032 |
| Meropenem | 1.3 (0.9) | 4.8 (0) | 4.8 (0) | 0.032 |
| Penicillin | 8.3 (3.4) | 8.6 (0) | 6.5 (0) | 0.422 |
| Amoxicillin | 2.5 (1.4) | 2.0 (0) | 1.9 (0) | 0.280 |
| Piperacillin/tazobactam | 0.2 (0.2) | 0.9 (0) | 0.8 (0) | 0.028 |
| Tetracyclines | 3.0 (0.9) | 4.4 (0) | 1.6 (0) | 0.170 |
| Quinolones | 6.1 (0.8) | 6.7 (0) | 6.4 (0) | 0.391 |
| Aminoglycosides | 5.6 (0.9) | 6.7 (0) | 6.5 (0) | 0.085 |
| Macrolides | 2.5 (1.5) | 6.6 (0) | 3.3 (0) | 0.072 |
| Azithromycin | 2.2 (1.5) | 6.5 (0) | 0.053 | |
| Sulfonamides | 1.6 (0.2) | 0.9 (0) | 0.9 (0) | 0.055 |
| Lincosamides | 0.5 (0.4) | 1.1 (0) | 0.9 (0) | 0.136 |
| Nitrozoimidazole-metronidazole | 6.0 (2.2) | 8.2 (0) | 9.3 (0) | 0.088 |
| Other | 1.3 (0.6) | 5.0 (0) | 4.8 (0) | 0.028 |
| Vancomycin | 1.1 (0.2) | 2.9 (0) | 2.7 (0) | 0.028 |
| Colistin | 0.2 (0.3) | 1.5 (0) | 1.1 (0) | 0.022 |
| N (%) | % Change 1 | |||||
|---|---|---|---|---|---|---|
| Pre-COVID-19 Period (2015–2019) | COVID-19 Period (2020–2022) | Post-COVID-19 Period (2023–2024) | COVID vs. Pre- 2 | Post- vs. Pre- 2 | Post- vs. COVID 2 | |
| Access | 190 (67) | 130.3 (53.5) | 72.1 (49.2) | 28.1 | −1.6 | −23.1 |
| Watch | 92.4 (32.6) | 106.3 (43.6) | 70.4 (48.0) | 145.2 | 135.0 | −4.2 |
| Reserve | 1.1 (0.4) | 7.1 (2.9) | 4.1 (2.8) | 3356.9 | 2781.5 | −16.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barišić, V.; Kovačević, T.; Travar, M.; Golić Jelić, A.; Kovačević, P.; Milaković, D.; Škrbić, R. A Retrospective Study of the Impact of the COVID-19 Pandemic on the Utilization and Quality of Antibiotic Use in a Tertiary Care Teaching Hospital in Low-Resource Settings. Antibiotics 2025, 14, 535. https://doi.org/10.3390/antibiotics14060535
Barišić V, Kovačević T, Travar M, Golić Jelić A, Kovačević P, Milaković D, Škrbić R. A Retrospective Study of the Impact of the COVID-19 Pandemic on the Utilization and Quality of Antibiotic Use in a Tertiary Care Teaching Hospital in Low-Resource Settings. Antibiotics. 2025; 14(6):535. https://doi.org/10.3390/antibiotics14060535
Chicago/Turabian StyleBarišić, Vedrana, Tijana Kovačević, Maja Travar, Ana Golić Jelić, Pedja Kovačević, Dragana Milaković, and Ranko Škrbić. 2025. "A Retrospective Study of the Impact of the COVID-19 Pandemic on the Utilization and Quality of Antibiotic Use in a Tertiary Care Teaching Hospital in Low-Resource Settings" Antibiotics 14, no. 6: 535. https://doi.org/10.3390/antibiotics14060535
APA StyleBarišić, V., Kovačević, T., Travar, M., Golić Jelić, A., Kovačević, P., Milaković, D., & Škrbić, R. (2025). A Retrospective Study of the Impact of the COVID-19 Pandemic on the Utilization and Quality of Antibiotic Use in a Tertiary Care Teaching Hospital in Low-Resource Settings. Antibiotics, 14(6), 535. https://doi.org/10.3390/antibiotics14060535







