Antimicrobial Susceptibility Profiles of Staphylococcus aureus Isolates from Domestic Pigeons in Hungary in 2022
Abstract
1. Introduction
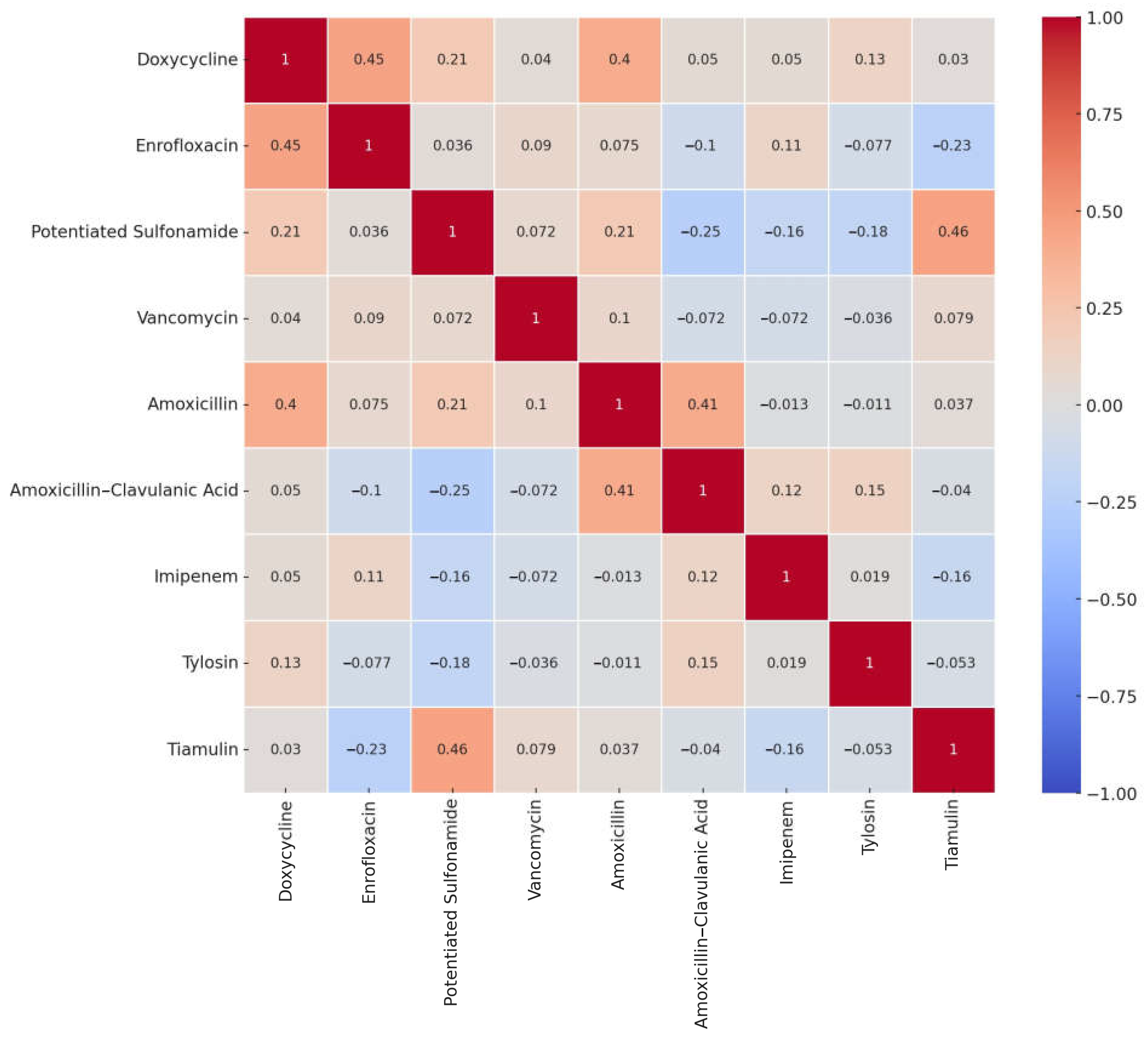
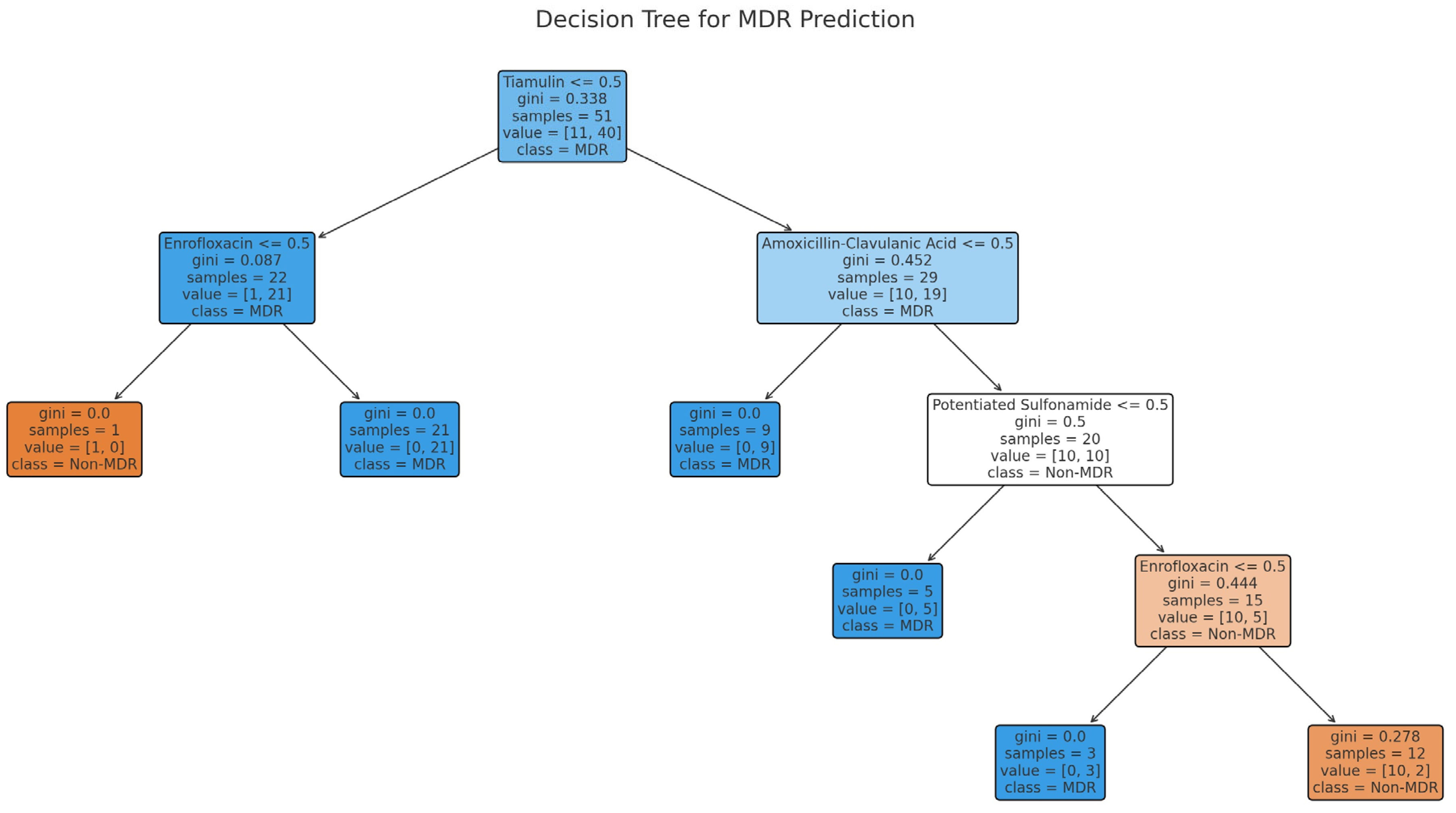
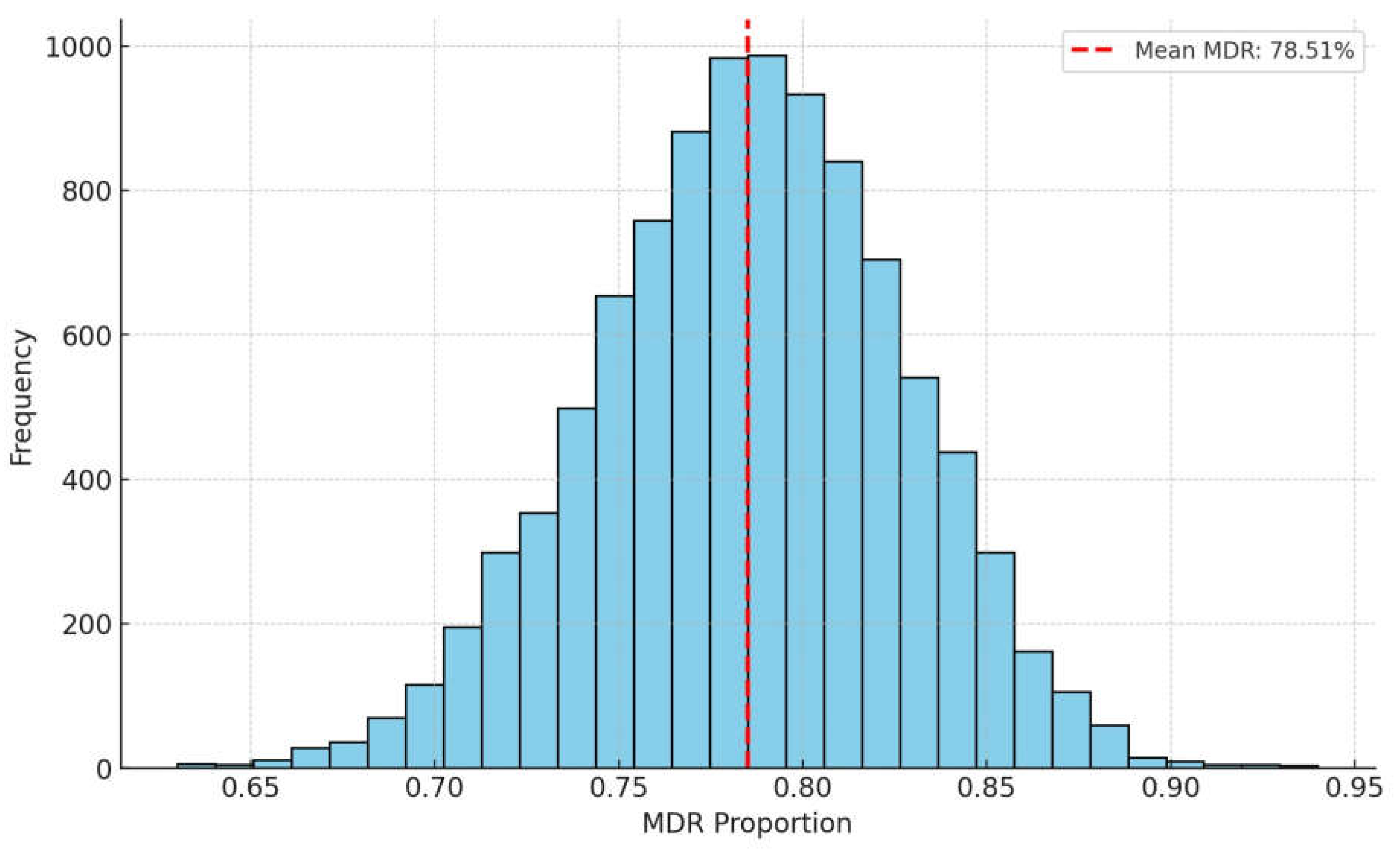
2. Results
3. Discussion
4. Materials and Methods
4.1. Origin of Strains and Human Data
4.2. Determination of Minimum Inhibitory Concentration (MIC)
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| CAMHB | Cation-adjusted Mueller Hinton Broth |
| CLSI | Clinical Laboratory Standards Institute |
| ECOFF | Epidemiological cutoff values |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| MDR | Multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| PDR | Pandrug-resistant |
| WHO | World Health Organization |
| XDR | Extensively drug-resistant |
References
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial Resistance, Mechanisms and Its Clinical Significance. Dis.-A-Mon. 2020, 66, 100971. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C. Multiple Drug Resistance Mechanisms in Cancer. Mol. Biotechnol. 2010, 46, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Littmann, J.; Viens, A.M. The Ethical Significance of Antimicrobial Resistance. Public Health Ethics 2015, 8, phv025. [Google Scholar] [CrossRef] [PubMed]
- Házigalambok|Brehm: Állatok Világa|Kézikönyvtár. Available online: https://www.arcanum.com/hu/online-kiadvanyok/Brehm-brehm-allatok-vilaga-8CCA/madarak-aves-3091/i-oregrend-tarajos-mellcsontuak-carinatae-323A/negyedik-rend-lileszeru-madarak-charadriiformes-40AD/elso-alrend-galambok-columbae-40B3/2-csalad-galamb-felek-columbidae-40CB/2-alcsalad-erdei-galamb-formak-columbinae-40E6/galamb-columba-linn-40E8/hazigalambok-4106/ (accessed on 9 July 2022).
- Johnston, R.F.; Janiga, M.; Janiga, R.O.M. Feral Pigeons; Oxford University Press: Oxford, UK, 1995; ISBN 978-0-19-508409-2. [Google Scholar]
- Audrey, S. Carrier Pigeons: From Past to Present. Mall 2018, 2, 23. [Google Scholar]
- Benmazouz, I.; Kövér, L.; Kardos, G. The Rise of Antimicrobial Resistance in Wild Birds: Potential AMR Sources and Wild Birds as AMR Reservoirs and Disseminators: Literature Review. Magy. Állatorvosok Lapja 2024, 146, 91–105. [Google Scholar] [CrossRef]
- Vasconcelos, R.H.; Teixeira, R.S.d.C.; Silva, I.N.G.d.; Lopes, E.d.S.; Maciel, W.C. Feral Pigeons (Columba livia) as Potential Reservoirs of Salmonella Sp. and Escherichia Coli. Arq. Inst. Biol. 2018, 85, e0412017. [Google Scholar] [CrossRef]
- Chrobak-Chmiel, D.; Kwiecień, E.; Golke, A.; Dolka, B.; Adamczyk, K.; Biegańska, M.J.; Spinu, M.; Binek, M.; Rzewuska, M. Pigeons as Carriers of Clinically Relevant Multidrug-Resistant Pathogens—A Clinical Case Report and Literature Review. Front. Vet. Sci. 2021, 8, 664226. [Google Scholar] [CrossRef]
- Madsen, A.M.; Zhang, F.; Zeng, Y.; Frederiksen, M.W. Airborne Methicillin-Resistant Staphylococcus aureus, Other Bacteria, Fungi, Endotoxin, and Dust in a Pigeon Exhibition. Environ. Res 2023, 216, 114642. [Google Scholar] [CrossRef]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Hetényi, N.; Bersényi, A.; Hullár, I. Physiological Effects of Medium-Chain Fatty Acids and Triglycerides, and Their Potential Use in Poultry and Swine Nutrition: A Literature Review. Magy. Állatorvosok Lapja 2024, 146, 651–659. [Google Scholar] [CrossRef]
- Jócsák, G.; Schilling-Tóth, B.; Bartha, T.; Tóth, I.; Ondrašovičová, S.; Kiss, D.S. Metal Nanoparticles—Immersion in the „tiny” World of Medicine. Magy. Állatorvosok Lapja 2025, 147, 115–127. [Google Scholar] [CrossRef]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antiprotozoal and Antifungal Efficiency of Propolis—Part 2. Magy. Állatorvosok Lapja 2022, 144, 691–704. [Google Scholar]
- Pomothy, J.M.; Barna, R.F.; Gere, E. The Effects of the Rosmarinic Acid in Livestock Animals: Literature Review. Magy. Állatorvosok Lapja 2020, 142, 567–576. [Google Scholar]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils—Animal Health Aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Kovács, L.; Hejel, P.; Farkas, M.; László, L. Könyves László Study Report on the Effect of a Litter Treatment Product Containing Bacillus licheniformis and Zeolite in Male Fattening Turkey Flock. Magy. Állatorvosok Lapja 2024, 146, 291–305. [Google Scholar] [CrossRef]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity Situation of Large-Scale Poultry Farms in Hungary According to the Databases of National Food Chain Safety Office Centre for Disease Control and Biosecurity Audit System of Poultry Product Board of Hungary in the Period of 2021–2022. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Mag, P.; Németh, K.; Somogyi, Z.; Jerzsele, Á. Antibacterial therapy based on pharmacokinetic/pharmacodynamic models in small animal medicine-1. Literature review. Magy. Állatorvosok Lapja 2023, 145, 419–438. [Google Scholar] [CrossRef]
- Foster, T. Staphylococcus. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Doneley, B. Pigeon Medicine and Surgery. In Proceedings of the North American Veterinary Conference, Orlando, FL, USA, 7–11 January 2006; Volume 6. [Google Scholar]
- Szafraniec, G.M.; Szeleszczuk, P.; Dolka, B. Review on Skeletal Disorders Caused by Staphylococcus spp. in Poultry. Vet. Q. 2022, 42, 21–40. [Google Scholar] [CrossRef]
- Heidemann Olsen, R.; Christensen, H.; Kabell, S.; Bisgaard, M. Characterization of Prevalent Bacterial Pathogens Associated with Pododermatitis in Table Egg Layers. Avian Pathol. 2018, 47, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Harlin, R.W. Pigeon Therapeutics. Vet. Clin. N. Am. Exot. Anim. Pr. 2000, 3, 19–34. [Google Scholar] [CrossRef]
- Bengtsson, B.; Greko, C. Antibiotic Resistance—Consequences for Animal Health, Welfare, and Food Production. Upsala J. Med. Sci. 2014, 119, 96–102. [Google Scholar] [CrossRef]
- Waters, A.E.; Contente-Cuomo, T.; Buchhagen, J.; Liu, C.M.; Watson, L.; Pearce, K.; Foster, J.T.; Bowers, J.; Driebe, E.M.; Engelthaler, D.M.; et al. Multidrug-Resistant Staphylococcus aureus in US Meat and Poultry. Clin. Infect. Dis. 2011, 52, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Laniesse, D.; Beaufrère, H.; Mackenzie, S.; Singh, A.; Samman, A.; Susta, L. Perforating Foreign Body in the Ventriculus of a Pet Pigeon (Columba livia domestica). J. Am. Vet. Med. Assoc. 2018, 253, 1610–1616. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.-P.; Mouton, J.W. Oral Amoxicillin and Amoxicillin–Clavulanic Acid: Properties, Indications and Usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Bodey, G.P.; Nance, J. Amoxicillin: In Vitro and Pharmacological Studies. Antimicrob. Agents Chemother. 1972, 1, 358–362. [Google Scholar] [CrossRef]
- Jerzsele, A.; Nagy, G.; Lehel, J.; Semjen, G. Oral Bioavailability and Pharmacokinetic Profile of the Amoxicillin-Clavulanic Acid Combination after Intravenous and Oral Gavage Administration in Broiler Chickens. J. Vet. Pharmacol. Ther. 2009, 32, 506–509. [Google Scholar] [CrossRef]
- Jerzsele, Á. Comparative Pharmacokinetics of the Amoxicillin- Clavulanic Acid Combination in Broiler Chickens and Turkeys, Susceptibility and Stability Tests of the Combination. Ph.D. Thesis, Szent István University, Gödöllő, Hungary, 2009; p. 10. [Google Scholar]
- Canada, H. Maximum Residue Limits (MRLs). Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/maximum-residue-limits-mrls.html (accessed on 8 August 2022).
- EMA Maximum Residue Limits (MRL). Available online: https://www.ema.europa.eu/en/veterinary-regulatory/research-development/maximum-residue-limits-mrl (accessed on 8 August 2022).
- Esposito, J.F. Respiratory Medicine in Pigeons. Vet. Clin. N. Am. Exot. Anim. Pr. 2000, 3, 395–402. [Google Scholar] [CrossRef]
- Vinothini, P.; Ramesh, S.; Sooraj Nair, V.; Preetha, S.P.; Sriram, P. Pharmacokinetics and Relative Bioavailability of Tiamulin in Broiler Chicken as Influenced by Different Routes of Administration. J. Vet. Pharmacol. Ther. 2019, 42, 447–451. [Google Scholar] [CrossRef]
- Ziv, G. Preliminary Clinical Pharmacological Investigations of Tylosin and Tiamulin in Chickens. Vet. Q. 1980, 2, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.; Apley, M.; Dau, D.; Bustamante, A. In-Vitro Antimicrobial Activity of Tiamulin and Chlortetracycline against Field Swine Pathogens; Scientific Publications: Dubai, United Arab Emirates, 2008. [Google Scholar]
- Baughn, C.O.; Alpaugh, W.C.; Linkenheimer, W.H.; Maplesden, D.C. Effect of Tiamulin in Chickens and Turkeys Infected Experimentally with Avian Mycoplasma. Avian Dis. 1978, 22, 620. [Google Scholar] [CrossRef] [PubMed]
- Drews, J.; Georgopoulos, A.; Laber, G.; Schütze, E.; Unger, J. Antimicrobial Activities of 81.723 Hfu, a New Pleuromutilin Derivative. Antimicrob Agents Chemother 1975, 7, 507–516. [Google Scholar] [CrossRef]
- European Medicines Agency. Tiamulin—Summary Report; European Medicines Agency (EMA); Committee for Veterinary Medicinal Products: Amsterdam, The Netherlands, 1999; Available online: https://www.ema.europa.eu/documents/mrl-report/tiamulin-summary-report-1-committee-veterinary-medicinal-products_en.pdf (accessed on 15 May 2025).
- Losito, P.; Vergara, A.; Muscariello, T.; Ianieri, A. Antimicrobial Susceptibility of Environmental Staphylococcus aureus Strains Isolated from a Pigeon Slaughterhouse in Italy. Poult. Sci. 2005, 84, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, T.; Bancerz-Kisiel, A.; Tykalowski, B.; Smialek, M.; Pestka, D.; Koncicki, A. Antimicrobial Resistance in Bacteria Isolated from Pigeons in Poland. Pol. J. Vet. Sci. 2014, 17, 169–171. [Google Scholar] [CrossRef]
- Nemati, M.; Hermans, K.; Lipinska, U.; Denis, O.; Deplano, A.; Struelens, M.; Devriese, L.A.; Pasmans, F.; Haesebrouck, F. Antimicrobial Resistance of Old and Recent Staphylococcus aureus Isolates from Poultry: First Detection of Livestock-Associated Methicillin-Resistant Strain ST398. Antimicrob. Agents Chemother. 2008, 52, 3817–3819. [Google Scholar] [CrossRef]
- Wilson, T.K.; Zishiri, O.T.; El Zowalaty, M.E. Molecular Detection of Multidrug and Methicillin Resistance in Staphylococcus aureus Isolated from Wild Pigeons (Columba livia) in South Africa. One Health 2024, 18, 100671. [Google Scholar] [CrossRef]
- Ferdous, T.; Ferdouse, S.; Hossain, M.S.; Sohidullah, M.; Marma, Y.N.F.; Nath, S.K.; Biswas, P.K. Prevalence of Escherichia coli Isolated from Oropharynx and Trachea of Clinically Sick Poultry and Antimicrobial Resistance Pattern of the Strains Isolated. Biochem. Biophys. Rep. 2023, 36, 101555. [Google Scholar] [CrossRef]
- CLSI Standards VET06; Methods For Antimicrobial Susceptibility Testing Of Infrequently Isolated Or Fastidious Bacteria Isolated From Animals; 1st ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- EUCAST: MIC and Zone Distributions and ECOFFs. Available online: https://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 1 May 2022).
- Moawad, A.A.; Hotzel, H.; Awad, O.; Roesler, U.; Hafez, H.M.; Tomaso, H.; Neubauer, H.; El-Adawy, H. Evolution of Antibiotic Resistance of Coagulase-Negative Staphylococci Isolated from Healthy Turkeys in Egypt: First Report of Linezolid Resistance. Microorganisms 2019, 7, 476. [Google Scholar] [CrossRef]
- Lin, J.; Yeh, K.-S.; Liu, H.-T.; Lin, J.-H. Staphylococcus Aureus Isolated from Pork and Chicken Carcasses in Taiwan: Prevalence and Antimicrobial Susceptibility. J. Food Prot. 2009, 72, 608–611. [Google Scholar] [CrossRef]
- Nemeghaire, S.; Argudín, M.A.; Haesebrouck, F.; Butaye, P. Molecular Epidemiology of Methicillin-Resistant Staphylococcus sciuri in Healthy Chickens. Vet. Microbiol. 2014, 171, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Brian, V.L. VET01SEd5|Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 5th ed. Available online: https://clsi.org/standards/products/veterinary-medicine/documents/vet01s/ (accessed on 8 May 2022).
- CLSI Standards M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-Test? On Assumptions for Hypothesis Tests and Multiple Interpretations of Decision Rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]



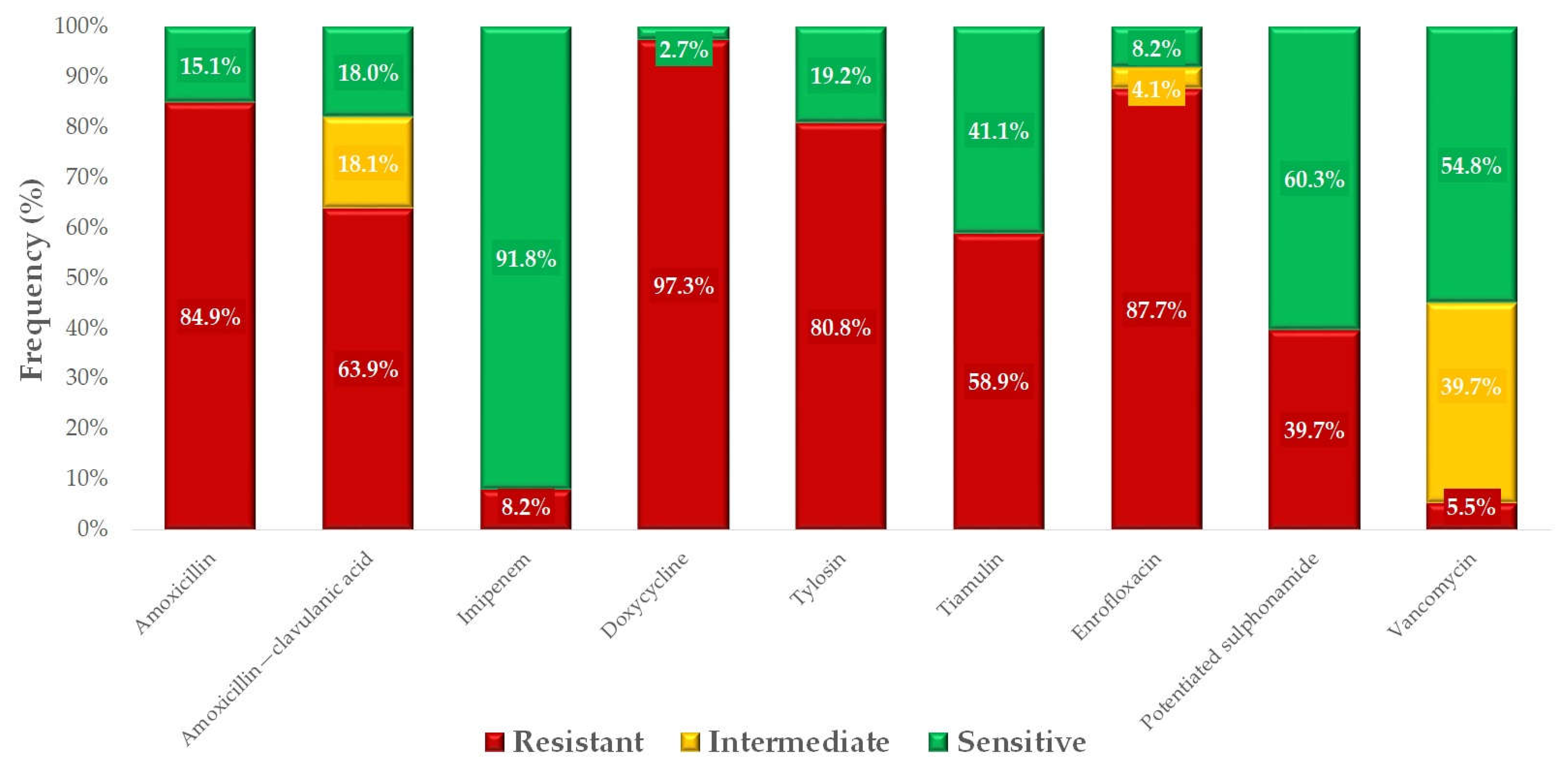

| Antibiotics | Breakpoint | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.031 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | MIC50 | MIC90 | 3 ECOFF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/mL | |||||||||||||||||||||||||
| Amoxicillin | 0.5 | 6 | 5 | 7 | 8 | 6 | 11 | 9 | 6 | 1 | 14 | 4 | 64 | 0.5 | |||||||||||
| 8.2% | 6.8% | 9.6% | 11.0% | 8.2% | 15.1% | 12.3% | 8.2% | 1.4% | 19.2% | ||||||||||||||||
| 1 Amoxicillin-clavulanic acid | 1 | 1 | 3 | 2 | 7 | 13 | 13 | 14 | 9 | 6 | 4 | 1 | 8 | 0.5 | |||||||||||
| 1.4% | 4.1% | 2.7% | 9.6% | 17.8% | 17.8% | 19.2% | 12.3% | 8.2% | 5.5% | ||||||||||||||||
| Doxycycline | 0.5 | 2 | 0 | 0 | 0 | 6 | 2 | 6 | 10 | 23 | 14 | 6 | 4 | 16 | 64 | 0.5 | |||||||||
| 2.7% | 0.0% | 0.0% | 0.0% | 8.2% | 2.7% | 8.2% | 13.7% | 31.5% | 19.2% | 8.2% | 5.5% | ||||||||||||||
| Enrofloxacin | 4 | 4 | 1 | 0 | 1 | 2 | 1 | 4 | 10 | 8 | 42 | 32 | 32 | 0.5 | |||||||||||
| 5.5% | 1.4% | 0.0% | 1.4% | 2.7% | 1.4% | 5.5% | 13.7% | 11.0% | 57.5% | ||||||||||||||||
| Imipenem | 8 | 10 | 11 | 13 | 7 | 4 | 2 | 1 | 19 | 2 | 1 | 3 | 0.25 | 4 | 0.125 | ||||||||||
| 13.7% | 15.1% | 17.8% | 9.6% | 5.5% | 2.7% | 1.4% | 26.0% | 2.7% | 1.4% | 4.1% | |||||||||||||||
| 2 Potentiated sulphonamide | 4 | 2 | 1 | 14 | 12 | 5 | 22 | 10 | 2 | 2 | 2 | 1 | 8 | 16 | 0.25 | ||||||||||
| 2.7% | 1.4% | 19.2% | 16.4% | 6.8% | 30.1% | 13.7% | 2.7% | 2.7% | 2.7% | 1.4% | |||||||||||||||
| Tiamulin | 4 | 8 | 7 | 2 | 2 | 7 | 4 | 3 | 2 | 8 | 6 | 5 | 19 | 16 | 128 | 2 | |||||||||
| 11.0% | 9.6% | 2.7% | 2.7% | 9.6% | 5.5% | 4.1% | 2.7% | 11.0% | 8.2% | 6.8% | 26.0% | ||||||||||||||
| Tylosin | 64 | 1 | 1 | 1 | 3 | 1 | 4 | 1 | 2 | 2 | 57 | 128 | 128 | 2 | |||||||||||
| 1.4% | 1.4% | 1.4% | 4.1% | 1.4% | 5.5% | 1.4% | 2.7% | 2.7% | 78.1% | ||||||||||||||||
| Vancomycin | 32 | 1 | 0 | 5 | 10 | 10 | 14 | 13 | 16 | 4 | 4 | 16 | 2 | ||||||||||||
| 1.4% | 0.0% | 6.8% | 13.7% | 13.7% | 19.2% | 17.8% | 21.9% | 5.5% | |||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerek, Á.; Szabó, Á.; Jerzsele, Á. Antimicrobial Susceptibility Profiles of Staphylococcus aureus Isolates from Domestic Pigeons in Hungary in 2022. Antibiotics 2025, 14, 525. https://doi.org/10.3390/antibiotics14050525
Kerek Á, Szabó Á, Jerzsele Á. Antimicrobial Susceptibility Profiles of Staphylococcus aureus Isolates from Domestic Pigeons in Hungary in 2022. Antibiotics. 2025; 14(5):525. https://doi.org/10.3390/antibiotics14050525
Chicago/Turabian StyleKerek, Ádám, Ábel Szabó, and Ákos Jerzsele. 2025. "Antimicrobial Susceptibility Profiles of Staphylococcus aureus Isolates from Domestic Pigeons in Hungary in 2022" Antibiotics 14, no. 5: 525. https://doi.org/10.3390/antibiotics14050525
APA StyleKerek, Á., Szabó, Á., & Jerzsele, Á. (2025). Antimicrobial Susceptibility Profiles of Staphylococcus aureus Isolates from Domestic Pigeons in Hungary in 2022. Antibiotics, 14(5), 525. https://doi.org/10.3390/antibiotics14050525







