Drug Susceptibility, Siderophore Production, and Genome Analysis of Staphylococcus aureus Clinical Isolates from a University Hospital in Chiang Mai, Thailand
Abstract
1. Introduction
2. Results
2.1. Specimen Collection and Biochemical Tests
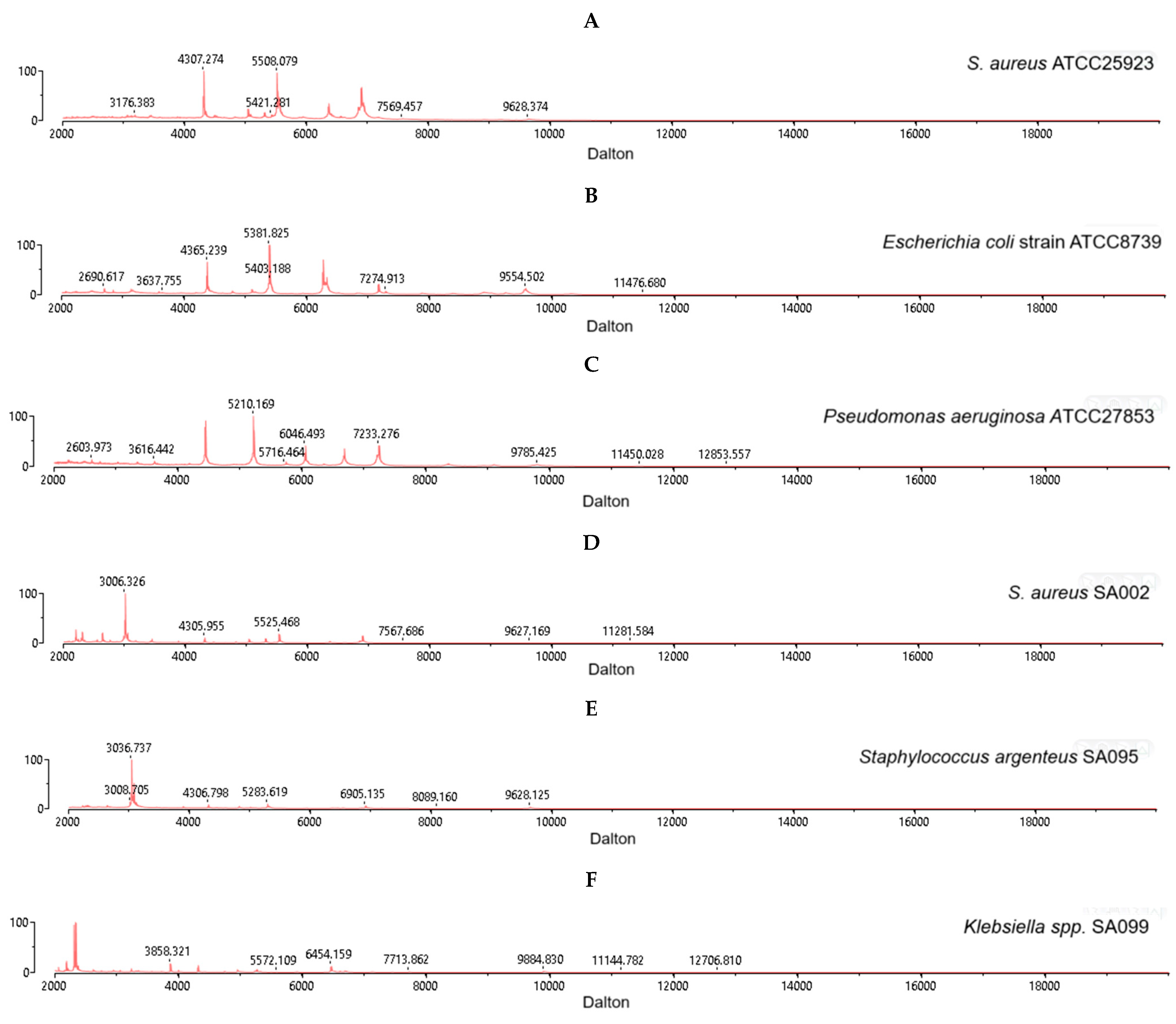
2.2. MALDI–TOF MS Identification of S. aureus
2.3. Drug Susceptibility Test for S. aureus Isolates
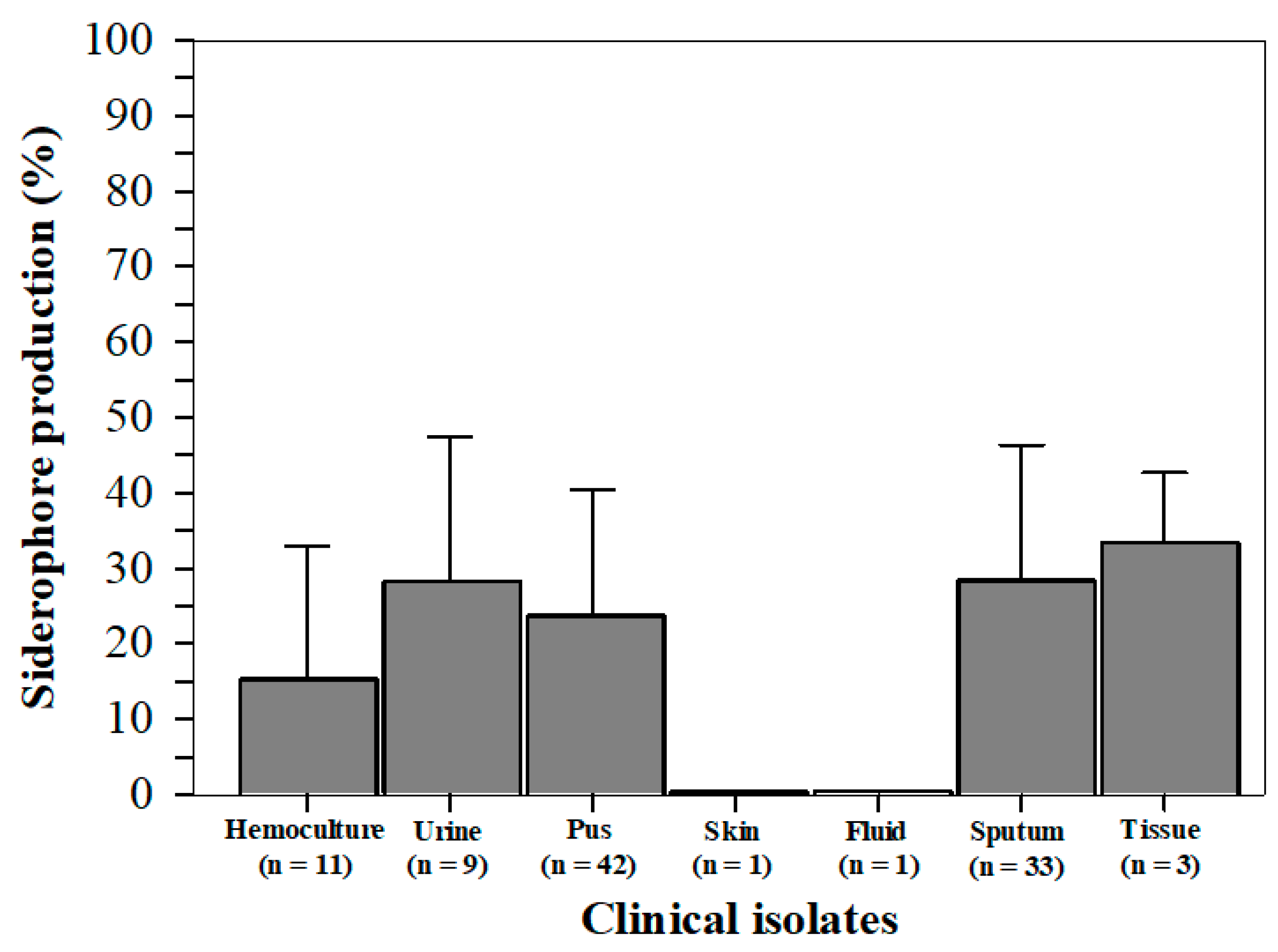
2.4. Siderophore Production from S. aureus Isolates
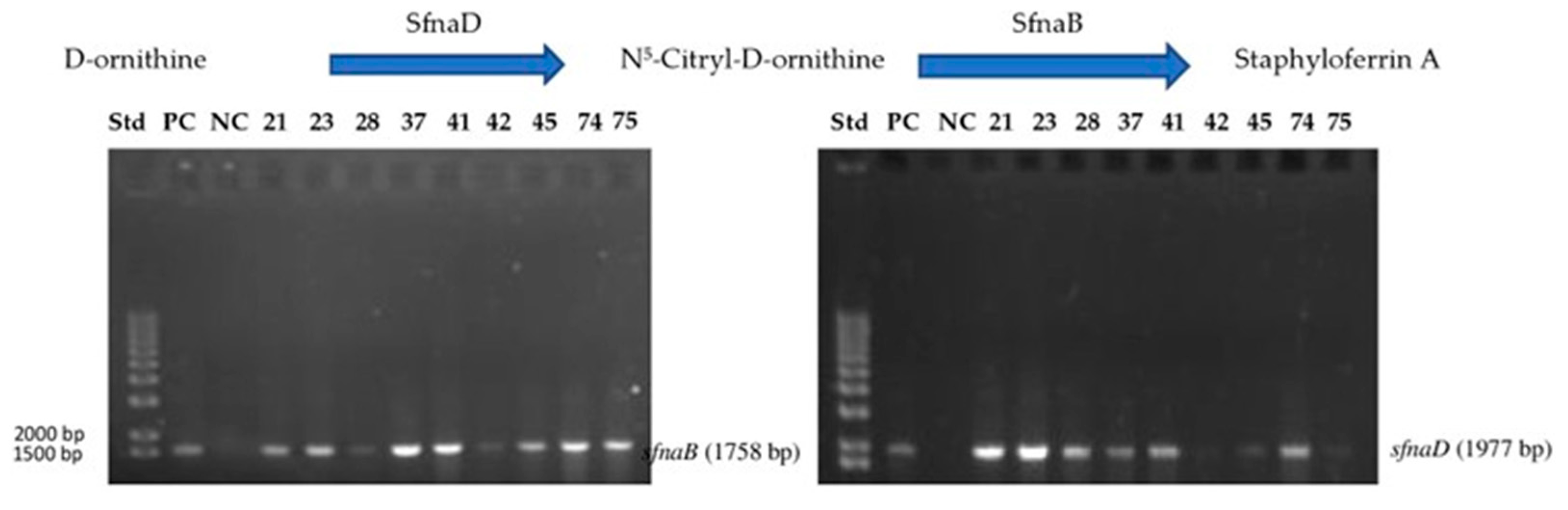
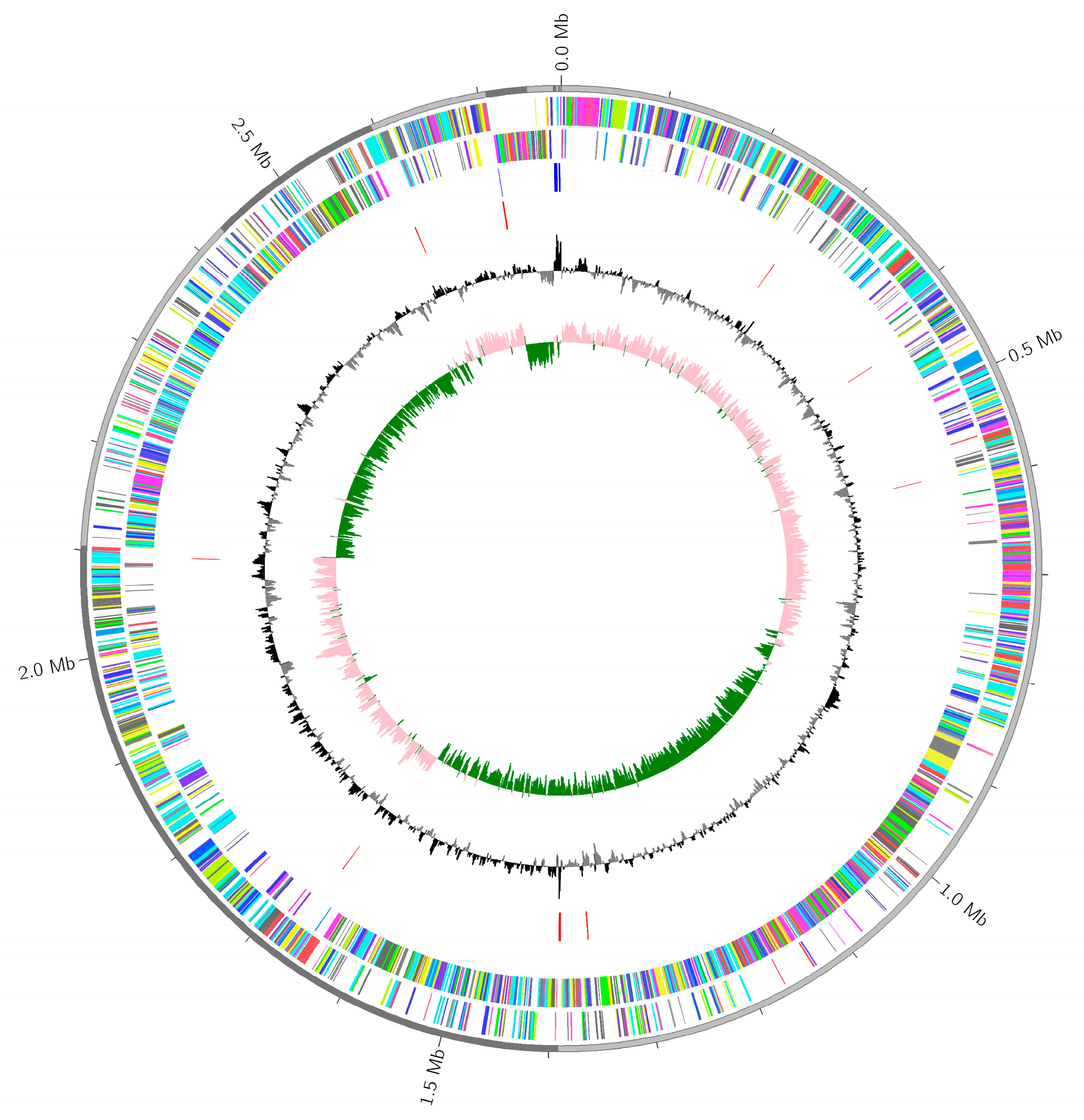
2.5. Genomic DNA and Staphyloferrin A Biosynthesis Gene
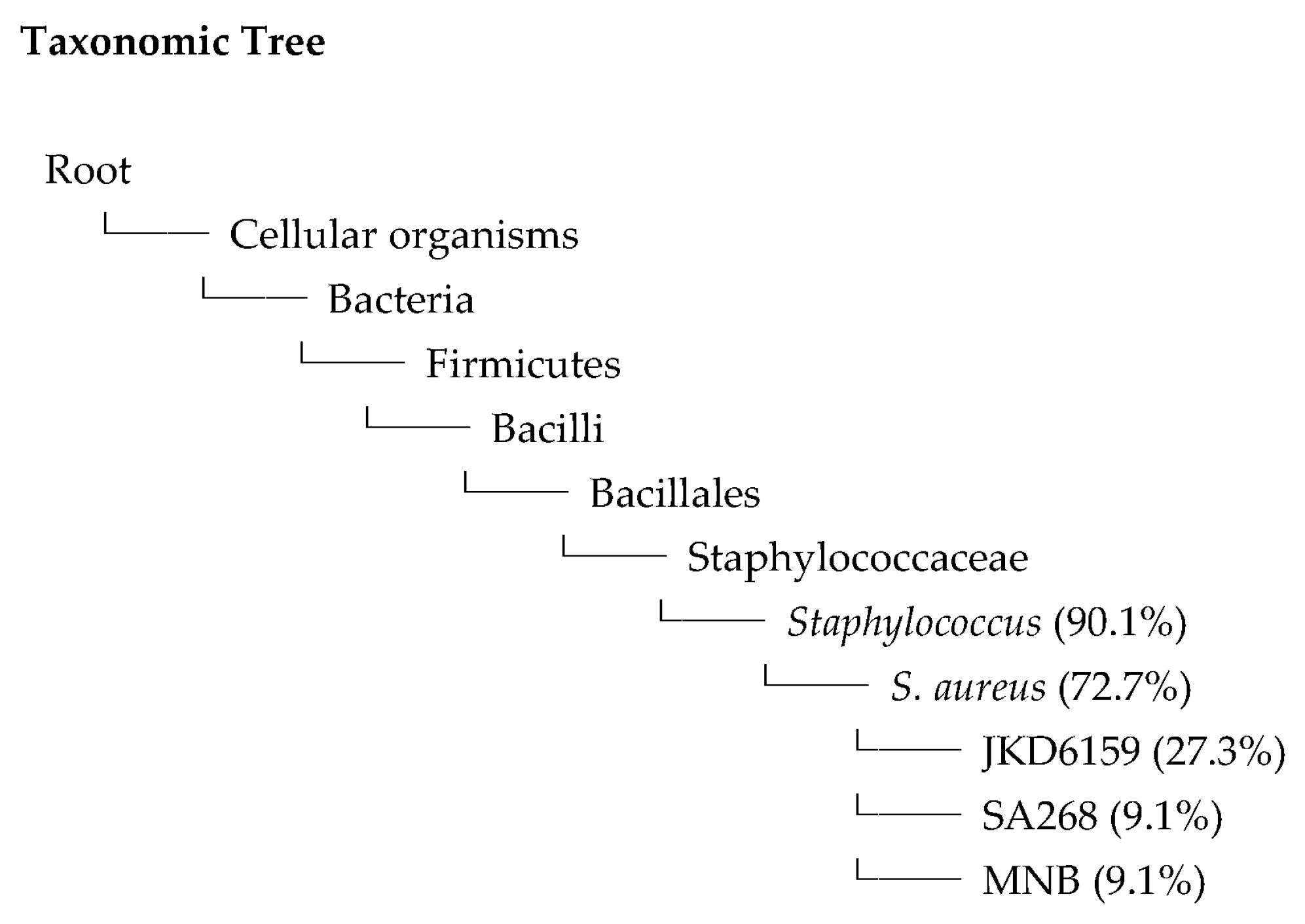
2.6. Whole Genome Sequences
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Ethics
4.3. Institutional Review Board Statement
4.4. Specimen Collection and Staphylococcus Culturing
4.5. Biochemical Identification of S. aureus
4.6. MALDI-TOF/MS Analysis of Microbial–Specific Protein
4.7. Drug Susceptibility Testing
4.8. Detection of Siderophore Production by Staphylococcus Isolates
4.8.1. Preparation of Siderophore
4.8.2. Colorimetric CAS Assay
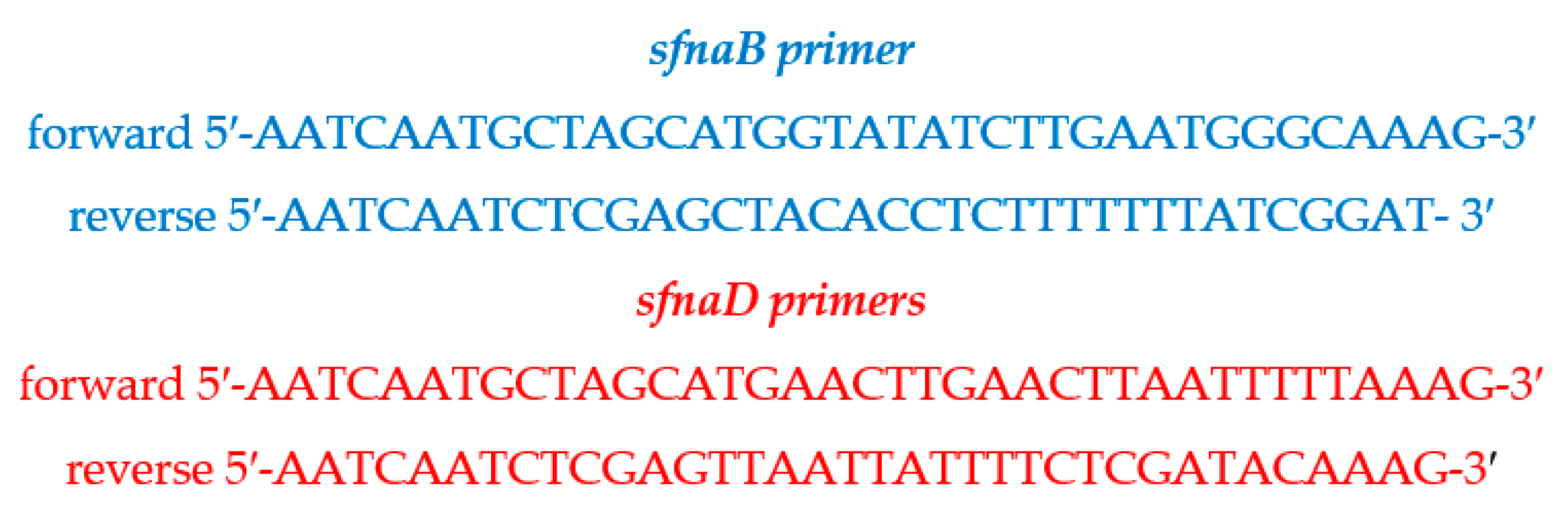
4.9. Identification of sfnaB and sfnaD Genes
4.9.1. Genomic DNA Extraction
4.9.2. Polymerase Chain Reaction Amplification
4.9.3. Nucleotide Sequencing and Data Analysis
4.10. Whole Genome Sequencing and Phylogenetic Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP synthase-binding cassette |
| C | Cytosine |
| CAMH | Cation-adjusted Mueller–Hinton (broth) |
| CAMRSA | Community-acquired methicillin-resistant S. aureus |
| CAS | Chrome azurol S |
| CAT | Catalase |
| CDS | Coding sequencing |
| CLSI | Clinical and Laboratory Standards Institute |
| COG | Cluster of orthologous groups |
| DFO | Desferrioxamine |
| DI | Deionized water |
| DNA | Deoxyribonucleic acid |
| dNTPs | Deoxyribonucleoside triphosphates |
| EDDHA | Ethylenediamine–di(o–hydroxyphenylacetic acid) |
| EDTA | Ethylenediamine tetraacetic acid |
| Fe3+ | Ferric ion |
| Fe2+ | Ferrous ion |
| G | Guanine |
| HDTMA | Hexadecyltrimethylammonium bromide |
| IBC | Institutional Biosafety Committee |
| IUS | Iron utilization system |
| LB | Luria–Bertani (broth) |
| MALDI–TOF/MS | Matrix-assisted laser desorption ionization–time of flight mass spectrometry |
| MIC | Minimal inhibitory concentration |
| MRS | Man, Rogosa and Sharpe |
| MRSA | Methicillin-resistant S. aureus |
| m/z | Mass-to-charge ratio |
| NC | Negative control |
| NIS | Non-ribosomal peptide synthetase |
| OD | Optical density |
| PC | Positive control |
| PCR | Polymerase chain reaction |
| PIPES | Piperazine–N, N′–bis(2–ethanesulfonic acid) |
| rRNA | Ribosomal ribonucleic acid |
| S. aureus | Staphylococcus aureus |
| SD | Standard deviation |
| sfna | Staphyloferrin A |
| sfnb | Staphyloferrin B |
| S. epidermidis | Staphylococcus epidermidis |
| S. hominis | Staphylococcus hominis |
| S. hyicus | Staphylococcus hyicus |
| S. lugdunensis | Staphylococcus lugdunensis |
| SPU | Siderophore production unit |
| TAE | Tris–acetate ethylenediamine tetraacetic acid |
| TMS | Tris–minimal succinate |
| T/S | Trimethoprim/sulfamethoxazole |
| VRSA | Vancomycin-resistant S. aureus |
| v/v | Volume by volume |
| w/v | Weight by volume |
References
- Jones, T.F.; Kellum, M.E.; Porter, S.S.; Bell, M.; Schaffner, W. An outbreak of community-acquired foodborne illness caused by methicillin-resistant Staphylococcus aureus. Emerg. Infect. Dis. 2002, 8, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Holban, A.M.; Giurcaneanu, C.; Popa, L.G.; Buzea, M.; Filipov, M.; Lazar, V.; Chifiriuc, M.C.; Popa, M.I. Identification and phenotypic characterization of the most frequent bacterial etiologies in chronic skin ulcers. Rom. J. Morphol. Embryol. 2014, 55, 1401–1408. [Google Scholar]
- Adhikari, R.P.; Karauzum, H.; Sarwar, J.; Abaandou, L.; Mahmoudieh, M.; Boroun, A.R.; Vu, H.; Nguyen, T.; Devi, V.S.; Shulenin, S.; et al. Novel structurally designed vaccine for S. aureus alpha-hemolysin: Protection against bacteremia and pneumonia. PLoS ONE 2012, 7, e38567. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Rasooly, A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000, 61, 1–10. [Google Scholar] [CrossRef]
- McDanel, J.S.; Perencevich, E.N.; Diekema, D.J.; Herwaldt, L.A.; Smith, T.C.; Chrischilles, E.A.; Dawson, J.D.; Jiang, L.; Goto, M.; Schweizer, M.L. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin. Infect. Dis. 2015, 61, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Davidov, Y.; Tejman-Yarden, N.; Robinson, A.; Rahav, G.; Nissan, I. Enterobactin and salmochelin S4 inhibit the growth of Staphylococcus aureus. Front. Cell Infect. Microbiol. 2025, 15, 1456046. [Google Scholar] [CrossRef]
- Ippolito, G.; Leone, S.; Lauria, F.N.; Nicastri, E.; Wenzel, R.P. Methicillin-resistant Staphylococcus aureus: The superbug. Int. J. Infect. Dis. 2010, 14 (Suppl. S4), S7–S11. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Demling, R.H.; Waterhouse, B. The increasing problem of wound bacterial burden and infection in acute and chronic soft-tissue wounds caused by methicillin-resistant Staphylococcus aureus. J. Burns Wounds 2007, 7, e8. [Google Scholar]
- Huang, H.; Flynn, N.M.; King, J.H.; Monchaud, C.; Morita, M.; Cohen, S.H. Comparisons of community-associated methicillin-resistant Staphylococcus aureus (MRSA) and hospital-associated MSRA infections in Sacramento, California. J. Clin. Microbiol. 2006, 44, 2423–2427. [Google Scholar] [CrossRef]
- Hartzen, S.H.; Frimodt-Moller, N.; Frolund Thomsen, V. The antibacterial activity of a siderophore. 1. In vitro activity of deferoxamine alone and in combination with ascorbic acid on Staphylococcus aureus. APMIS 1989, 97, 419–424. [Google Scholar] [CrossRef]
- Al-Azemi, A.; Fielder, M.D.; Abuknesha, R.A.; Price, R.G. Effects of chelating agent and environmental stresses on microbial biofilms: Relevance to clinical microbiology. J. Appl. Microbiol. 2011, 110, 1307–1313. [Google Scholar] [CrossRef]
- Bernheimer, A.W.; Avigad, L.S.; Grushoff, P. Lytic effects of staphylococcal alpha-toxin and delta-hemolysin. J. Bacteriol. 1968, 96, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791. [Google Scholar] [CrossRef]
- Biswas, S.; Rolain, J.M. Use of MALDI-TOF mass spectrometry for identification of bacteria that are difficult to culture. J. Microbiol. Methods 2013, 92, 14–24. [Google Scholar] [CrossRef]
- Moon, H.W.; Lee, S.H.; Chung, H.S.; Lee, M.; Lee, K. Performance of the Vitek MS matrix-assisted laser desorption ionization time-of-flight mass spectrometry system for identification of Gram-positive cocci routinely isolated in clinical microbiology laboratories. J. Med. Microbiol. 2013, 62, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Ayeni, F.A.; Andersen, C.; Norskov-Lauritsen, N. Comparison of growth on mannitol salt agar, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, VITEK((R)) 2 with partial sequencing of 16S rRNA gene for identification of coagulase-negative Staphylococci. Microb. Pathog. 2017, 105, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.F. The regulation of iron absorption and homeostasis. Clin. Biochem. Rev. 2016, 37, 51–62. [Google Scholar]
- Hammer, N.D.; Skaar, E.P. Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu. Rev. Microbiol. 2011, 65, 129–147. [Google Scholar] [CrossRef]
- Sheldon, J.R.; Heinrichs, D.E. Recent developments in understanding the iron acquisition strategies of gram positive pathogens. FEMS Microbiol. Rev. 2015, 39, 592–630. [Google Scholar] [CrossRef]
- Challis, G.L. A widely distributed bacterial pathway for siderophore biosynthesis independent of nonribosomal peptide synthetases. Chembiochem 2005, 6, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J.C.; Cooper, J.D.; Cheung, J.; Heinrichs, D.E.; Murphy, M.E. The Staphylococcus aureus siderophore receptor HtsA undergoes localized conformational changes to enclose staphyloferrin A in an arginine-rich binding pocket. J. Biol. Chem. 2010, 285, 11162–11171. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and characterization of antibacterial siderophores secreted by endophytic fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef]
- Perry, W.J.; Spraggins, J.M.; Sheldon, J.R.; Grunenwald, C.M.; Heinrichs, D.E.; Cassat, J.E.; Skaar, E.P.; Caprioli, R.M. Staphylococcus aureus exhibits heterogeneous siderophore production within the vertebrate host. Proc. Natl. Acad. Sci. USA 2019, 116, 21980–21982. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, H.; Freund, S.; Nicholson, G.; Haag, H.; Jung, O.; Zahner, H.; Jung, G. Purification and chemical characterization of staphyloferrin B, a hydrophilic siderophore from Staphylococci. Biometals 1993, 6, 185–192. [Google Scholar] [CrossRef]
- Beasley, F.C.; Cheung, J.; Heinrichs, D.E. Mutation of L-2,3-diaminopropionic acid synthase genes blocks staphyloferrin B synthesis in Staphylococcus aureus. BMC Microbiol. 2011, 11, 199. [Google Scholar] [CrossRef]
- Beasley, F.C.; Vines, E.D.; Grigg, J.C.; Zheng, Q.; Liu, S.; Lajoie, G.A.; Murphy, M.E.; Heinrichs, D.E. Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus. Mol. Microbiol. 2009, 72, 947–963. [Google Scholar] [CrossRef]
- Meiwes, J.; Fiedler, H.P.; Haag, H.; Zahner, H.; Konetschny-Rapp, S.; Jung, G. Isolation and characterization of staphyloferrin A, a compound with siderophore activity from Staphylococcus hyicus DSM 20459. FEMS Microbiol. Lett. 1990, 55, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Flannagan, R.S.; Brozyna, J.R.; Kumar, B.; Adolf, L.A.; Power, J.J.; Heilbronner, S.; Heinrichs, D.E. In vivo growth of Staphylococcus lugdunensis is facilitated by the concerted function of heme and non-heme iron acquisition mechanisms. J. Biol. Chem. 2022, 298, 101823. [Google Scholar] [CrossRef]
- Hannauer, M.; Sheldon, J.R.; Heinrichs, D.E. Involvement of major facilitator superfamily proteins SfaA and SbnD in staphyloferrin secretion in Staphylococcus aureus. FEBS Lett. 2015, 589, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Cotton, J.L.; Tao, J.; Balibar, C.J. Identification and characterization of the Staphylococcus aureus gene cluster coding for staphyloferrin A. Biochemistry 2009, 48, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.M.; Banerjee, P.; Hsieh, Y.H.; Miranda, N.; Simpson, S.; Kerdahi, K. Rapid detection of Staphylococcus aureus and related species isolated from food, environment, cosmetics, a medical device, and clinical samples using the VITEK MS microbial identification system. J. AOAC Int. 2018, 101, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Uchida-Fujii, E.; Niwa, H.; Kinoshita, Y.; Nukada, T. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) for identification of bacterial isolates from horses. J. Equine Vet. Sci. 2020, 95, 103276. [Google Scholar] [CrossRef]
- Pizauro, L.J.L.; de Almeida, C.C.; Silva, S.R.; MacInnes, J.I.; Kropinski, A.M.; Zafalon, L.F.; de Avila, F.A.; de Mello Varani, A. Genomic comparisons and phylogenetic analysis of mastitis-related Staphylococci with a focus on adhesion, biofilm, and related regulatory genes. Sci. Rep. 2021, 11, 17392. [Google Scholar] [CrossRef]
- Meyer, S.; Hernandez-Padilla, A.C.; Fedou, A.L.; Daix, T.; Chainier, D.; Ploy, M.C.; Vignon, P.; Francois, B.; Barraud, O. Longitudinal two-year comparative genomic analysis of respiratory Staphylococcus aureus isolates from intensive care unit mechanically ventilated patients. J. Hosp. Infect. 2024, 154, 37–44. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Paiva, D.D.Q.; Amando, B.R.; Melgarejo, C.M.A.; Freitas, A.S.; Gomes, F.I.F.; Ocadaque, C.J.; Costa, C.L.; Guedes, G.M.M.; Lima-Neto, R.G.; et al. Antimicrobial susceptibility and production of virulence factors by bacteria recovered from bitches with pyometra. Reprod. Domest. Anim. 2022, 57, 1063–1073. [Google Scholar] [CrossRef]
- Lindsay, J.A.; Aravena-Roman, M.A.; Riley, T.V. Identification of Staphylococcus epidermidis and Staphylococcus hominis from blood cultures by testing susceptibility to desferrioxamine. Eur. J. Clin. Microbiol. Infect. Dis. 1993, 12, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.A.; Riley, T.V. Susceptibility to desferrioxamine: A new test for the identification of Staphylococcus epidermidis. J. Med. Microbiol. 1991, 35, 45–48. [Google Scholar] [CrossRef]
- Antunes, A.L.; Secchi, C.; Reiter, K.C.; Perez, L.R.; de Freitas, A.L.; D’Azevedo, P.A. Feasible identification of Staphylococcus epidermidis using desferrioxamine and fosfomycin disks. APMIS 2008, 116, 16–20. [Google Scholar] [CrossRef]
- Temel, A.; Aksoyalp, Z.S. A preliminary study on the effect of deferoxamine on the disruption of bacterial biofilms and antimicrobial resistance. Turk. J. Pharm. Sci. 2024, 21, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.A.; Riley, T.V.; Mee, B.J. Production of siderophore by coagulase-negative staphylococci and its relation to virulence. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 1063–1066. [Google Scholar] [CrossRef]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M. Macrocyclic polyketides with siderophore mode of action from marine heterotrophic Shewanella algae: Prospective anti-infective leads attenuate drug-resistant pathogens. J. Appl. Microbiol. 2021, 130, 1552–1570. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Varghese, C.; Asharaf, S.; Chakraborty, R.D. Antibiotic-active heterotrophic Firmicutes sheltered in seaweeds: Can they add new dimensions to future antimicrobial agents? Arch. Microbiol. 2022, 204, 183. [Google Scholar] [CrossRef]
- Amiri-Eliasi, B.; Fenselau, C. Characterization of protein biomarkers desorbed by MALDI from whole fungal cells. Anal. Chem. 2001, 73, 5228–5231. [Google Scholar] [CrossRef] [PubMed]
- Pineda, F.J.; Lin, J.S.; Fenselau, C.; Demirev, P.A. Testing the significance of microorganism identification by mass spectrometry and proteome database search. Anal. Chem. 2000, 72, 3739–3744. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Miranda, N.; Hook, W.; Mendoza, J.; Kumfert, Q.; Barnes, T.; Sung, K.; Khan, S.; Nawaz, M.; Banerjee, P.; et al. A single-laboratory performance evaluation of MALDI-TOF MS in rapid identification of Staphylococcus aureus, Cronobacter sakazakii, Vibrio parahaemolyticus, and some closely related bacterial species of public health importance. J. AOAC Int. 2023, 106, 1574–1588. [Google Scholar] [CrossRef]
- Chung, J.H.; Park, M.H.; Kim, J.H.; Lim, Y.; Shin, S.H. Growth and siderophore production of Staphylococci in human peritoneal dialysate. J. Korean Med. Sci. 2003, 18, 158–162. [Google Scholar] [CrossRef]
- Le Masters, T.; Johnson, S.; Jeraldo, P.R.; Greenwood-Quaintance, K.E.; Cunningham, S.A.; Abdel, M.P.; Chia, N.; Patel, R. Comparative transcriptomic analysis of Staphylococcus aureus associated with periprosthetic joint infection under in vivo and in vitro conditions. J. Mol. Diagn. 2021, 23, 986–999. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. Pseudomonas aeruginosa alters Staphylococcus aureus sensitivity to vancomycin in a biofilm model of cystic fibrosis infection. mBio 2017, 8, e00873-17. [Google Scholar] [CrossRef]
- Vollenweider, V.; Rehm, K.; Chepkirui, C.; Perez-Berlanga, M.; Polymenidou, M.; Piel, J.; Bigler, L.; Kummerli, R. Antimicrobial activity of iron-depriving pyoverdines against human opportunistic pathogens. eLife 2024, 13, RP92493. [Google Scholar] [CrossRef]
- Coker, M.S.; Forbes, L.V.; Plowman-Holmes, M.; Murdoch, D.R.; Winterbourn, C.C.; Kettle, A.J. Interactions of staphyloxanthin and enterobactin with myeloperoxidase and reactive chlorine species. Arch. Biochem. Biophys. 2018, 646, 80–89. [Google Scholar] [CrossRef]
- Mishra, R.P.; Mariotti, P.; Fiaschi, L.; Nosari, S.; Maccari, S.; Liberatori, S.; Fontana, M.R.; Pezzicoli, A.; De Falco, M.G.; Falugi, F.; et al. Staphylococcus aureus FhuD2 is involved in the early phase of staphylococcal dissemination and generates protective immunity in mice. J. Infect. Dis. 2012, 206, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Millar, B.C.; Moore, J.E.; Murphy, K.; Webb, H.; Fox, A.J.; Cafferkey, M.; Crowe, M.J. Employment of broad-range 16S rRNA PCR to detect aetiological agents of infection from clinical specimens in patients with acute meningitis--rapid separation of 16S rRNA PCR amplicons without the need for cloning. J. Appl. Microbiol. 2003, 94, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiong, Y.; Bao, C.; Wei, Y.; Wen, Z.; Cao, X.; Yu, Z.; Deng, X.; Li, G.; Deng, Q. Antibacterial and anti-biofilm activity of radezolid against Staphylococcus aureus clinical isolates from China. Front. Microbiol. 2023, 14, 1131178. [Google Scholar] [CrossRef] [PubMed]
- Ronco, T.; Klaas, I.C.; Stegger, M.; Svennesen, L.; Astrup, L.B.; Farre, M.; Pedersen, K. Genomic investigation of Staphylococcus aureus isolates from bulk tank milk and dairy cows with clinical mastitis. Vet. Microbiol. 2018, 215, 35–42. [Google Scholar] [CrossRef]
- Watanabe, S.; Aiba, Y.; Tan, X.E.; Li, F.Y.; Boonsiri, T.; Thitiananpakorn, K.; Cui, B.; Sato’o, Y.; Kiga, K.; Sasahara, T.; et al. Complete genome sequencing of three human clinical isolates of Staphylococcus caprae reveals virulence factors similar to those of S. epidermidis and S. capitis. BMC Genom. 2018, 19, 810. [Google Scholar] [CrossRef]
- Lindsay, J.A.; Riley, T.V.; Mee, B.J. Staphylococcus aureus but not Staphylococcus epidermidis can acquire iron from transferrin. Microbiology 1995, 141 Pt 1, 197–203. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Anwar, H.; Brown, M.R.; Shand, G.H.; Ward, K.H. Media for study of growth kinetics and envelope properties of iron-deprived bacteria. J. Clin. Microbiol. 1987, 25, 849–855. [Google Scholar] [CrossRef]
- Liu, X.; Su, T.; Hsu, Y.S.; Yu, H.; Yang, H.S.; Jiang, L.; Zhao, Z. Rapid identification and discrimination of methicillin-resistant Staphylococcus aureus strains via matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2021, 35, e8972. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. M100 Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, MI, USA, 2021. [Google Scholar]
- Santos, S.; Neto, I.F.; Machado, M.D.; Soares, H.M.; Soares, E.V. Siderophore production by Bacillus megaterium: Effect of growth phase and cultural conditions. Appl. Biochem. Biotechnol. 2014, 172, 549–560. [Google Scholar] [CrossRef]
- Queipo-Ortuno, M.I.; De Dios Colmenero, J.; Macias, M.; Bravo, M.J.; Morata, P. Preparation of bacterial DNA template by boiling and effect of immunoglobulin G as an inhibitor in real-time PCR for serum samples from patients with brucellosis. Clin. Vaccine Immunol. 2008, 15, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
| Specimen | Total (Female/Male) | Age (Years) | CAT | Coagulase |
|---|---|---|---|---|
| Pus | 42 (19/23) | 40.1 ± 21.1 | + (42) | + (42) |
| Sputum | 33 (8/25) | 62.5 ± 21.8 | + (33) | + (33) |
| Hemoculture | 11 (2/9) | 58.5 ± 17.7 | + (11) | + (11) |
| Urine | 9 (2/7) | 63.2 ± 10.3 | + (9) | + (9) |
| Tissue | 3 (0/3) | 47.3 ± 9.2 | + (3) | + (3) |
| Fluid | 1 (0/1) | 33 | + (1) | + (1) |
| Skin scrap | 1 (0/1) | 19 | + (1) | + (1) |
| Isolates | Antimicrobial Drugs | |||||||
|---|---|---|---|---|---|---|---|---|
| Clindamycin | Erythromycin | Gentamycin | Linezolid | Moxifloxacin | Oxacillin | Trimethoprim/Sulfamethoxazole | Vancomycin | |
| Pus | 35S, 7R | 34S, 1I, 7R | 42S | 42S | 38S, 4R | 37S, 5R | 41S, 1R | 42S |
| Sputum | 28S, 5R | 28S, 5R | 32S, 1R | 33S | 32S, 1R | 29S, 4R | 33S | 33S |
| Hemoculture | 8S,3R | 8S,3R | 10S,1R | 11S | 11S | 10S,1R | 11S | 11S |
| Urine | 7S, 2R | 7S, 2R | 8S, 1R | 9S | 8S, 1R | 7S, 2R | 9S | 9S |
| Tissue | 2S, 1R | 2S, 1R | 3S | 3S | 3S | 3S | 3S | 3S |
| Fluid | 1S | 1S | 1S | 1S | 1S | 1S | 1S | 1S |
| Skin scarp | 1S | 1S | 1S | 1S | 1S | 1S | 1S | 1S |
| Frequency | Identification | |||
|---|---|---|---|---|
| Relative | Absolute | Role | Taxon ID | Taxon, Species and Strain |
| 100% | 11 | 0 R | 1 | Root |
| 100% | 11 | 0 R1 | 131567 | Cellular organism |
| 100% | 11 | 1 D | 2 | Bacteria |
| 90.1% | 10 | 0 D1 | 1783272 | Terrabacteria group |
| 90.1% | 10 | 0 P | 1239 | Firmicutes |
| 90.1% | 10 | 0 C | 91061 | Bacilli |
| 90.1% | 10 | 0 O | 1385 | Bacillales |
| 90.1% | 10 | 0 F | 90964 | Staphylococcaceae |
| 90.1% | 10 | 2 G | 1279 | Staphylococcus |
| 72.7% | 8 | 3 S | 1280 | Staphylococcus aureus |
| 27.3% | 3 | 3 S1 | 869816 | Staphylococcus aureus JKD6159 |
| 9.1% | 1 | 1 S1 | 1368166 | Staphylococcus aureus SA268 |
| 9.1% | 1 | 1 S1 | 548470 | Staphylococcus aureus MNB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prommachote, W.; Deeudom, M.; Koonyosying, P.; Srichomphoo, P.; Somnabut, R.; Khamnoi, P.; Cilibrizzi, A.; Ravikumar, Y.; Srichairatanakool, S. Drug Susceptibility, Siderophore Production, and Genome Analysis of Staphylococcus aureus Clinical Isolates from a University Hospital in Chiang Mai, Thailand. Antibiotics 2025, 14, 521. https://doi.org/10.3390/antibiotics14050521
Prommachote W, Deeudom M, Koonyosying P, Srichomphoo P, Somnabut R, Khamnoi P, Cilibrizzi A, Ravikumar Y, Srichairatanakool S. Drug Susceptibility, Siderophore Production, and Genome Analysis of Staphylococcus aureus Clinical Isolates from a University Hospital in Chiang Mai, Thailand. Antibiotics. 2025; 14(5):521. https://doi.org/10.3390/antibiotics14050521
Chicago/Turabian StylePrommachote, Warinda, Manu Deeudom, Pimpisid Koonyosying, Phronpawee Srichomphoo, Ratchanee Somnabut, Phadungkiat Khamnoi, Agostino Cilibrizzi, Yuvaraj Ravikumar, and Somdet Srichairatanakool. 2025. "Drug Susceptibility, Siderophore Production, and Genome Analysis of Staphylococcus aureus Clinical Isolates from a University Hospital in Chiang Mai, Thailand" Antibiotics 14, no. 5: 521. https://doi.org/10.3390/antibiotics14050521
APA StylePrommachote, W., Deeudom, M., Koonyosying, P., Srichomphoo, P., Somnabut, R., Khamnoi, P., Cilibrizzi, A., Ravikumar, Y., & Srichairatanakool, S. (2025). Drug Susceptibility, Siderophore Production, and Genome Analysis of Staphylococcus aureus Clinical Isolates from a University Hospital in Chiang Mai, Thailand. Antibiotics, 14(5), 521. https://doi.org/10.3390/antibiotics14050521






