Social Determinants of Health and Antibiotic Consumption
Abstract
1. Introduction
2. Results
2.1. The Observed and Projected Antibiotic Consumption Rates in Kazakhstan, Kyrgyzstan, Tajikistan, and Russia
2.2. Social Determinants of Health and Antibiotic Consumption in Kazakhstan, Kyrgyzstan, Tajikistan, and Russia
3. Discussion
3.1. Antibiotic Consumption Surveillance
3.2. Social Determinants of Health and Antibiotic Consumption
3.3. Implications for Public Health Policy
3.4. Study Limitations
4. Materials and Methods
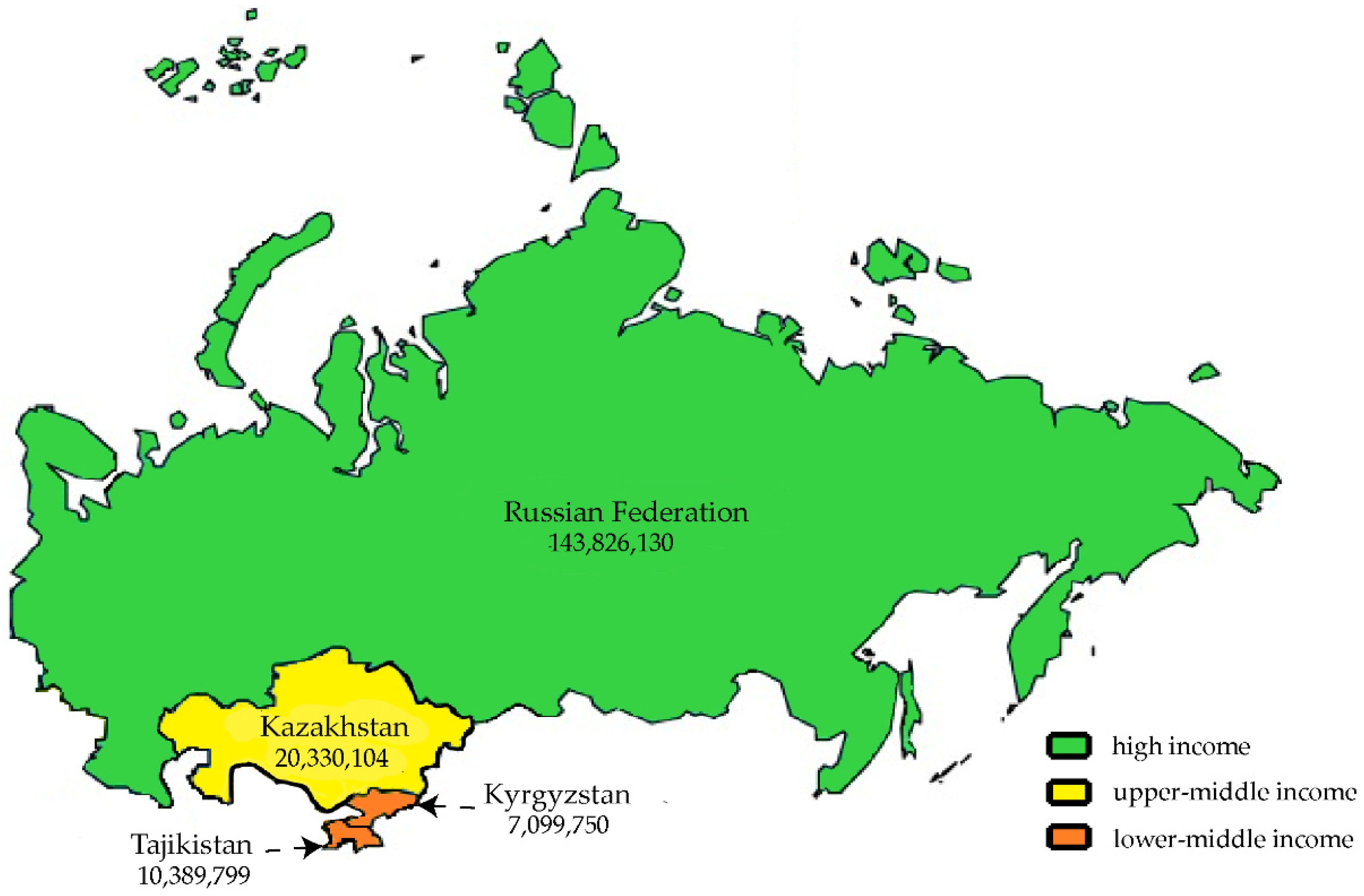
4.1. Countries Under Study
4.2. Data Sources
4.3. Study Variables
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAPS | Average Annual Percent Change |
| AMR | Antimicrobial Resistance |
| AMS | Antimicrobial Stewardship |
| DID | Defined Daily Doses per 1000 Inhabitants per Day |
| EU | European Union |
| GDP | Gross Domestic Product |
| HIV | Human Immunodeficiency Virus |
| LMICs | Low- and Middle-Income Countries |
| MoH | Ministry of Health |
| NAP | National Action Plan |
| OTC | Over-the-Counter |
| PM | Particulate Matter |
| SDH | Social Determinants of Health |
| SPSS | Statistical Package for the Social Sciences |
| TrACSS | Tracking AMR Country Self-Assessment Survey |
| WB | World Bank |
| WHO | World Health Organization |
| USA | United States of America |
| 95% CI | 95% Confidence Interval |
References
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chauhan, A.; Ranjan, A.; Mathkor, D.M.; Haque, S.; Ramniwas, S.; Tuli, H.S.; Jindal, T.; Yadav, V. Emerging challenges in antimicrobial resistance: Implications for pathogenic microorganisms, novel antibiotics, and their impact on sustainability. Front. Microbiol. 2024, 15, 1403168. [Google Scholar] [CrossRef]
- Otaigbe, I.I.; Elikwu, C.J. Drivers of inappropriate antibiotic use in low- and middle-income countries. JAC Antimicrob. Resist. 2023, 5, dlad062. [Google Scholar] [CrossRef]
- Wong, L.P.; Alias, H.; Husin, S.A.; Ali, Z.B.; Sim, B.; Ponnampalavanar, S.S.S. Factors influencing inappropriate use of antibiotics: Findings from a nationwide survey of the general public in Malaysia. PLoS ONE 2021, 16, e0258698. [Google Scholar] [CrossRef]
- World Health Organization. Social Determinants of Health. Available online: https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (accessed on 11 March 2025).
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2020, 44, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Amri, M.; Enright, T.; O’Campo, P.; Di Ruggiero, E.; Siddiqi, A.; Bump, J.B. Health promotion, the social determinants of health, and urban health: What does a critical discourse analysis of World Health Organization texts reveal about health equity? BMC Glob. Public Health 2023, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhou, Y.; Kivimäki, M.; Cai, Y.; Carrillo-Larco, R.M.; Xu, X.; Dai, X.; Xu, X. Socioeconomic inequalities in physical, psychological, and cognitive multimorbidity in middle-aged and older adults in 33 countries: A cross-sectional study. Lancet Healthy Longev. 2023, 4, e618–e628. [Google Scholar] [CrossRef]
- Semenova, Y.; Lim, L.; Salpynov, Z.; Gaipov, A.; Jakovljevic, M. Historical evolution of healthcare systems of post-soviet Russia, Belarus, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, Armenia, and Azerbaijan: A scoping review. Heliyon 2024, 10, e29550. [Google Scholar] [CrossRef]
- Zhazykhbayeva, D.; Bayesheva, D.; Kosherova, Z.; Semenova, Y. Antimicrobial Resistance Surveillance in Post-Soviet Countries: A Systematic Review. Antibiotics 2024, 13, 1129. [Google Scholar] [CrossRef]
- Semenova, Y.; Yergaliyeva, A.; Aimurziyeva, A.; Manatova, A.; Kuntuganova, A.; Makalkina, L.; Aldiyarova, N.; Semenov, D.; Lim, L. A Nationwide Evaluation of Antibiotic Consumption in Kazakhstan from 2019 to 2023. Antibiotics 2024, 13, 1123. [Google Scholar] [CrossRef]
- Robertson, J.; Vlahović-Palčevski, V.; Iwamoto, K.; Högberg, L.D.; Godman, B.; Monnet, D.L.; Garner, S.; Weist, K.; ESAC-Net Study Group; WHO Europe AMC Network Study Group. Variations in the Consumption of Antimicrobial Medicines in the European Region, 2014–2018: Findings and Implications from ESAC-Net and WHO Europe. Front. Pharmacol. 2021, 12, 639207. [Google Scholar] [CrossRef]
- Baktygul, K.; Marat, B.; Ashirali, Z.; Harun-Or-rashid, M.; Sakamoto, J. An assessment of antibiotics prescribed at the secondary health-care level in the Kyrgyz Republic. Nagoya J. Med. Sci. 2011, 73, 157–168. [Google Scholar] [PubMed]
- Zay Ya, K.; Patel, J.; Fink, G. Assessing the impact of antimicrobial resistance policies on antibiotic use and antimicrobial resistance-associated mortality in children and adults in low and middle-income countries: A global analysis. BMJ Public Health 2025, 3, e000511. [Google Scholar] [CrossRef] [PubMed]
- Fedotova, M.M.; Chigrina, V.P.; Shirinskaya, A.V.; Fedosenko, S.V.; Fedorova, O.S. Antibiotic prescribing practices and perceptions on antimicrobial resistance among healthcare practitioners in Russia. Public Health 2023, 225, 45–52. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. Antimicrobial Medicines Consumption (AMC) Network, AMC data 2023: Summary. Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2024-10997-50769-76929 (accessed on 14 March 2025).
- European Centre for Disease Prevention and Control. Latest Surveillance Data on Antimicrobial Consumption. Available online: https://qap.ecdc.europa.eu/public/extensions/AMC2_Dashboard/AMC2_Dashboard.html#eu-consumption-tab (accessed on 14 March 2025).
- World Bank Group. DataBank. Available online: https://databank.worldbank.org/home.aspx (accessed on 14 March 2025).
- Aslam, A.; Gajdács, M.; Zin, C.S.; Ab Rahman, N.S.; Ahmed, S.I.; Zafar, M.Z.; Jamshed, S. Evidence of the Practice of Self-Medication with Antibiotics among the Lay Public in Low- and Middle-Income Countries: A Scoping Review. Antibiotics 2020, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Ajulo, S.; Awosile, B. Global antimicrobial resistance and use surveillance system (GLASS 2022): Investigating the relationship between antimicrobial resistance and antimicrobial consumption data across the participating countries. PLoS ONE 2024, 19, e0297921. [Google Scholar] [CrossRef]
- World Health Organization. Review of Antibiotics in National Medicines Selection Lists in Eastern Europe and Central Asia. Available online: https://iris.who.int/bitstream/handle/10665/365989/9789289058582-eng.pdf (accessed on 14 March 2025).
- World Health Organization. Global Database for Tracking Antimicrobial Resistance Country Self-Assessment Survey. 2023. Available online: https://www.who.int/publications/m/item/tracking-amr-country-self-assessment-survey-tracss-(7.0)-2023 (accessed on 14 March 2025).
- Singh-Phulgenda, S.; Antoniou, P.; Wong, D.L.F.; Iwamoto, K.; Kandelaki, K. Knowledge, attitudes and behaviors on antimicrobial resistance among general public across 14 member states in the WHO European region: Results from a cross-sectional survey. Front. Public Health 2023, 11, 1274818. [Google Scholar] [CrossRef]
- Belkina, T.; Duvanova, N.; Karbovskaja, J.; Tebbens, J.D.; Vlcek, J. Antibiotic use practices of pharmacy staff: A cross-sectional study in Saint Petersburg, the Russian Federation. BMC Pharmacol. Toxicol. 2017, 18, 11. [Google Scholar] [CrossRef]
- Kassym, L.; Kussainova, A.; Semenov, D.; Aimurziyeva, A.; Uzbekova, S.; Semenova, Y. National trends in Azithromycin consumption during 2017–2023 in Kazakhstan: Impact of the COVID-19 pandemic and the imperative for enhanced clinical guidelines. Sci. Rep. 2025, 15, 6309. [Google Scholar] [CrossRef]
- Covino, M.; Buonsenso, D.; Gatto, A.; Morello, R.; Curatole, A.; Simeoni, B.; Franceschi, F.; Chiaretti, A. Determinants of antibiotic prescriptions in a large cohort of children discharged from a pediatric emergency department. Eur. J. Pediatr. 2022, 181, 2017–2030. [Google Scholar] [CrossRef]
- Fink, G.; D’Acremont, V.; Leslie, H.H.; Cohen, J. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: A cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect. Dis. 2020, 20, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tesema, G.A.; Biney, G.K.; Wang, V.Q.; Ameyaw, E.K.; Yaya, S. Antibiotic prescription sources and use among under-5 children with fever/cough in sub-Saharan Africa. Int. Health 2025, 17, 94–104. [Google Scholar] [CrossRef]
- Abenova, M.; Shaltynov, A.; Jamedinova, U.; Ospanov, E.; Semenova, Y. The Association between Parental Child Vaccination Refusal Rate and the Impact of Mass Vaccination against COVID-19 in Kazakhstan: An Interrupted Time Series Analysis with Predictive Modelling of Nationwide Data Sources from 2013 to 2022. Vaccines 2024, 12, 429. [Google Scholar] [CrossRef]
- Bruns, N.; Dohna-Schwake, C. Antibiotics in critically ill children-a narrative review on different aspects of a rational approach. Pediatr. Res. 2022, 91, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. News: Supporting Tajikistan in Tackling the Misuse of Antibiotics. Available online: https://www.who.int/europe/news/item/17-03-2020-supporting-tajikistan-in-tackling-the-misuse-of-antibiotics (accessed on 15 March 2025).
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Aslam, B.; Asghar, R.; Muzammil, S.; Shafique, M.; Siddique, A.B.; Khurshid, M.; Ijaz, M.; Rasool, M.H.; Chaudhry, T.H.; Aamir, A.; et al. AMR and Sustainable Development Goals: At a crossroads. Glob. Health 2024, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Shaltynov, A.; Semenova, Y.; Abenova, M.; Baibussinova, A.; Jamedinova, U.; Myssayev, A. An analysis of financial protection and financing incidence of out-of-pocket health expenditures in Kazakhstan from 2018 to 2021. Sci. Rep. 2024, 14, 8869. [Google Scholar] [CrossRef]
- Tichy, E.M.; Hoffman, J.M.; Tadrous, M.; Rim, M.H.; Cuellar, S.; Clark, J.S.; Newell, M.K.; Schumock, G.T. National trends in prescription drug expenditures and projections for 2024. Am. J. Health Syst. Pharm. 2024, 81, 583–598. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Lima, T.; Domingues, S.; Da Silva, G.J. Manure as a Potential Hotspot for Antibiotic Resistance Dissemination by Horizontal Gene Transfer Events. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- The Astana Times. Kazakhstan’s Grain Exports to Reach 12 Million Tons This Year. Available online: https://astanatimes.com/2024/12/kazakhstans-grain-exports-to-reach-12-million-tons-this-year/ (accessed on 15 March 2025).
- Cella, E.; Giovanetti, M.; Benedetti, F.; Scarpa, F.; Johnston, C.; Borsetti, A.; Ceccarelli, G.; Azarian, T.; Zella, D.; Ciccozzi, M. Joining Forces against Antibiotic Resistance: The One Health Solution. Pathogens 2023, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Abelenda-Alonso, G.; Satorra, P.; Marí-Dell’Olmo, M.; Tebé, C.; Padullés, A.; Vergara, A.; Gudiol, C.; Pujol, M.; Carratalà, J. Short-Term Exposure to Ambient Air Pollution and Antimicrobial Use for Acute Respiratory Symptoms. JAMA Netw. Open 2024, 7, e2432245. [Google Scholar] [CrossRef] [PubMed]
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ. Int. 2020, 142, 105876. [Google Scholar] [CrossRef] [PubMed]
- Beisenova, R.; Kuanyshevich, B.Z.; Turlybekova, G.; Yelikbayev, B.; Kakabayev, A.A.; Shamshedenova, S.; Nugmanov, A. Assessment of Atmospheric Air Quality in the Region of Central Kazakhstan and Astana. Atmosphere 2023, 14, 1601. [Google Scholar] [CrossRef]
- Papagiannis, S.; Abdullaev, S.F.; Vasilatou, V.; Manousakas, M.I.; Eleftheriadis, K.; Diapouli, E. Air quality challenges in Central Asian urban areas: A PM2.5 source apportionment analysis in Dushanbe, Tajikistan. Environ. Sci. Poll. Res. Int. 2024, 31, 39588–39601. [Google Scholar] [CrossRef]
- Gordeev, R.V.; Pyzhev, A.I.; Syrtsova, E.A. Effectiveness of the Federal ‘Clean Air’ Project to Improve Air Quality in the Most Polluted Russian Cities. Urban Sci. 2025, 9, 18. [Google Scholar] [CrossRef]
- van Houten, C.B.; Cohen, A.; Engelhard, D.; Hays, J.P.; Karlsson, R.; Moore, E.; Fernández, D.; Kreisberg, R.; Collins, L.V.; de Waal, W.; et al. Antibiotic misuse in respiratory tract infections in children and adults-a prospective, multicentre study (TAILORED Treatment). Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 505–514. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. Rapid Measles Outbreak Response Critical to Protect Millions of Vulnerable Children. Available online: https://www.who.int/europe/news/item/22-02-2024-rapid-measles-outbreak-response-critical-to-protect-millions-of-vulnerable-children (accessed on 15 March 2025).
- Sallam, M.; Snygg, J.; Allam, D.; Kassem, R. From Protection to Prevention: Redefining Vaccines in the Context of Antimicrobial Resistance. Cureus 2024, 16, e60551. [Google Scholar] [CrossRef]
- Shrivastava, B.; Bajracharya, O.; Shakya, R. Prioritizing intervention measures to prevent inappropriate self-medication practices using the Analytical Hierarchy Process. Explor. Res. Clin. Soc. Pharm. 2022, 5, 100117. [Google Scholar] [CrossRef] [PubMed]
- Mambula, G.; Nanjebe, D.; Munene, A.; Guindo, O.; Salifou, A.; Mamaty, A.A.; Rattigan, S.; Ellis, S.; Khavessian, N.; van der Pluijm, R.W.; et al. Practices and challenges related to antibiotic use in paediatric treatment in hospitals and health centres in Niger and Uganda: A mixed methods study. Antimicrob. Resist. Infect. Control. 2023, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Arghittu, A.; Deiana, G.; Dettori, M.; Castiglia, P. Vaccination, Public Health and Health Communication: A Network of Connections to Tackle Global Challenges. Vaccines 2025, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Kazakhstan. Population Health in the Republic of Kazakhstan and Activities of Healthcare Organizations. Available online: https://nrchd.kz/index.php/ru/?option=com_content&view=article&id=973 (accessed on 12 March 2025).
- National Statistical Committee of the Kyrgyz Republic. Publications Published by National Statistical Committee. Available online: http://www.stat.kg/en/ (accessed on 12 March 2025).
- Schmiege, D.; Evers, M.; Kistemann, T.; Falkenberg, T. What drives antibiotic use in the community? A systematic review of determinants in the human outpatient sector. Int. J. Hyg. Environ. Health 2020, 226, 113497. [Google Scholar] [CrossRef]
- Harvey, E.J.; De Brún, C.; Casale, E.; Finistrella, V.; Ashiru-Oredope, D. Influence of factors commonly known to be associated with health inequalities on antibiotic use in high-income countries: A systematic scoping review. J. Antimicrob. Chemother. 2020, 78, 861–870. [Google Scholar] [CrossRef]
- Harbarth, S.; Monnet, D.L. Cultural and Socioeconomic Determinants of Antibiotic Use. In Antibiotic Policies: Fighting Resistance; Gould, I.M., van der Meer, J.W., Eds.; Springer: Boston, MA, USA, 2008. [Google Scholar] [CrossRef]
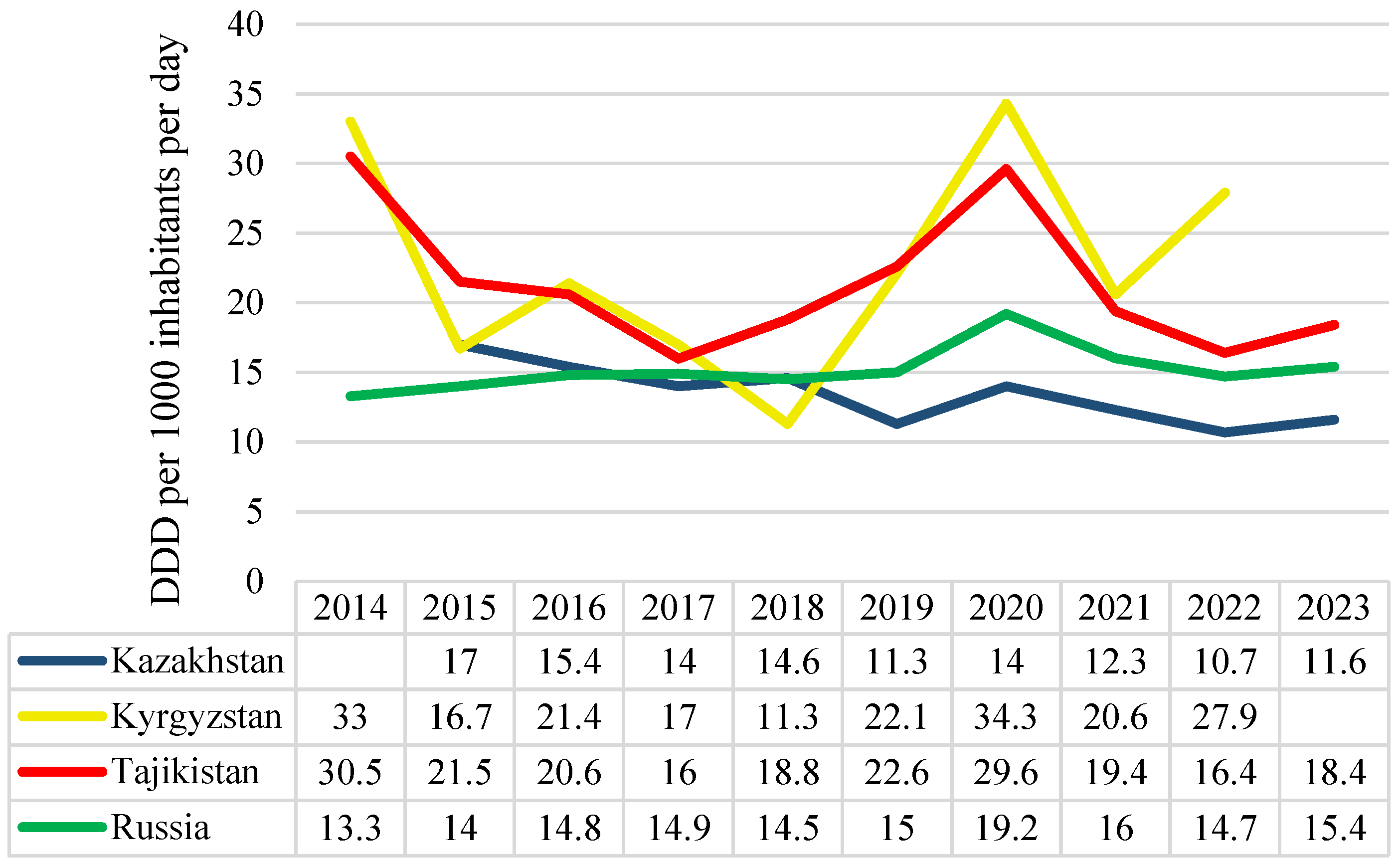

| Year | Country | |||
|---|---|---|---|---|
| Kazakhstan | Kyrgyzstan | Tajikistan | Russia | |
| 2024 | 10.2 | 22.7 | 21.4 | 15.2 |
| 2025 | 9.5 | 22.7 | 21.4 | 15.2 |
| 2026 | 8.9 | 22.7 | 21.4 | 15.2 |
| 2027 | 8.2 | 22.7 | 21.4 | 15.2 |
| 2028 | 7.6 | 22.7 | 21.4 | 15.2 |
| 2029 | 6.9 | 22.7 | 21.4 | 15.2 |
| 2030 | 6.2 | 22.7 | 21.4 | 15.2 |
| Model parameters | Holt, p = 0.509 | ARIMA (0.0.0) p < 0.001 + | ARIMA (0.0.0) p < 0.001 + | ARIMA (0.0.0) p < 0.001 + |
| Predictors | Model 1 (Enter Method) | Model 2 (Backward Method) | ||
|---|---|---|---|---|
| Standardized Coefficients (95% Confidence Interval) | p Value | Standardized Coefficients (95% Confidence Interval) | p Value | |
| Birth rate, crude | 0.509 (−0.888; 3.417) | 0.127 | 0.540 (0.221; 2.459) | 0.029 * |
| GDP per capita | −0.192 (−0.001; 0.001) | 0.351 | - | - |
| Inflation, consumer prices | −0.384 (−0.581; 0.184) | 0.155 | −0.435 (−0.416; −0.034) | 0.031 * |
| Access to clean fuels and technologies for cooking | −0.106 (−13.931; 10.265) | 0.581 | - | - |
| Cereal production | 0.636 (0.000; 0.000) | 0.095 | 0.705 (0.000; 0.000) | 0.015 * |
| Mortality rate, under 5 | 0.650 (0.188; 4.004) | 0.042 * | 0.685 (1.119; 3.294) | 0.005 ° |
| Model parameters | R2 = 0.971 | 0.085 | R2 = 0.949 | 0.008 ° |
| Predictors | Model 1 (Enter Method) | Model 2 (Backward Method) | ||
|---|---|---|---|---|
| Standardized Coefficients (95% Confidence Interval) | p Value | Standardized Coefficients (95% Confidence Interval) | p Value | |
| Immunization, measles | −1.055 (−9.595; 1.948) | 0.104 | −0.815 (−4.909; −1.000) | 0.010 * |
| Prevalence of undernourishment | 0.210 (−30.192; 36.313) | 0.730 | - | - |
| Access to clean fuels and technologies for cooking | −1.047 (−45.708; 8.281) | 0.096 | −0.788 (−23.722; −4.443) | 0.012 * |
| Inflation, consumer prices | −0.244 (−3.709; 2.923) | 0.661 | - | - |
| Gini index | 0.260 (−6.683; 10.009) | 0.482 | - | - |
| Cereal production | 0.344 (0.000; 0.000) | 0.327 | - | - |
| Model parameters | R2 = 0.897 | 0.279 | R2 = 0.758 | 0.014 * |
| Predictors | Model 1 (Enter Method) | Model 2 (Backward Method) | ||
|---|---|---|---|---|
| Standardized Coefficients (95% Confidence Interval) | p Value | Standardized Coefficients (95% Confidence Interval) | p Value | |
| Death rate, crude | 0.833 (0.394; 18.004) | 0.045 * | 0.703 (1.358; 14.162) | 0.023 * |
| Incidence of HIV | 0.863 (−558.161; 1617.036) | 0.219 | - | - |
| Incidence of tuberculosis | 1.556 (−0.500; 4.799) | 0.082 | - | - |
| Livestock production | 1.109 (−0.129; 0.406) | 0.198 | - | - |
| GDP per capita | −0.121 (−0.044; 0.042) | 0.954 | - | - |
| Population ages 0–14 | 1.577 (−17.142; 137.327) | 0.090 | - | - |
| Model parameters | R2 = 0.871 | 0.172 | R2 = 0.494 | 0.023 * |
| Predictors | Model 1 (Enter Method) | Model 2 (Backward Method) | ||
|---|---|---|---|---|
| Standardized Coefficients (95% Confidence Interval) | p Value | Standardized Coefficients (95% Confidence Interval) | p Value | |
| Immunization, measles | −1.043 (−6.745; 0.322) | 0.065 | −0.953 (−4.551; −1.319) | 0.004 ° |
| GDP per capita | −0.409 (−0.001; 0.000) | 0.302 | −0.682 (−0.001; 0.000) | 0.018 * |
| Unemployment, total | 0.429 (−1.327; 3.199) | 0.315 | - | - |
| Total fisheries production | 0.071 (0.000; 0.000) | 0.844 | - | - |
| Life expectancy at birth | 0.283 (−0.991; 1.910) | 0.429 | - | - |
| Model parameters | R2 = 0.827 | 0.109 | R2 = 0.738 | 0.009 ° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, Y.; Akhmetova, K.; Semenov, D.; Makalkina, L.; Surov, V.; Pivina, L.; Turgambayeva, A.; Belikhina, T.; Maukayeva, S.; Goremykina, M.; et al. Social Determinants of Health and Antibiotic Consumption. Antibiotics 2025, 14, 513. https://doi.org/10.3390/antibiotics14050513
Semenova Y, Akhmetova K, Semenov D, Makalkina L, Surov V, Pivina L, Turgambayeva A, Belikhina T, Maukayeva S, Goremykina M, et al. Social Determinants of Health and Antibiotic Consumption. Antibiotics. 2025; 14(5):513. https://doi.org/10.3390/antibiotics14050513
Chicago/Turabian StyleSemenova, Yuliya, Kamila Akhmetova, Daniil Semenov, Larissa Makalkina, Vladimir Surov, Lyudmila Pivina, Assiya Turgambayeva, Tatiana Belikhina, Saule Maukayeva, Maya Goremykina, and et al. 2025. "Social Determinants of Health and Antibiotic Consumption" Antibiotics 14, no. 5: 513. https://doi.org/10.3390/antibiotics14050513
APA StyleSemenova, Y., Akhmetova, K., Semenov, D., Makalkina, L., Surov, V., Pivina, L., Turgambayeva, A., Belikhina, T., Maukayeva, S., Goremykina, M., & Kumar, P. (2025). Social Determinants of Health and Antibiotic Consumption. Antibiotics, 14(5), 513. https://doi.org/10.3390/antibiotics14050513






