A Pilot Study in Humans on the Urinary Tract Excretion of the FimH Inhibitor 1-Deoxymannose
Abstract
1. Introduction
2. Results
2.1. Preparation of Test Material and Purities
2.2. Safety Analysis of DM
2.3. Human Trial
2.4. Utilization of Carbohydrates by E. coli
3. Discussion
4. Materials and Methods
4.1. Test Materials
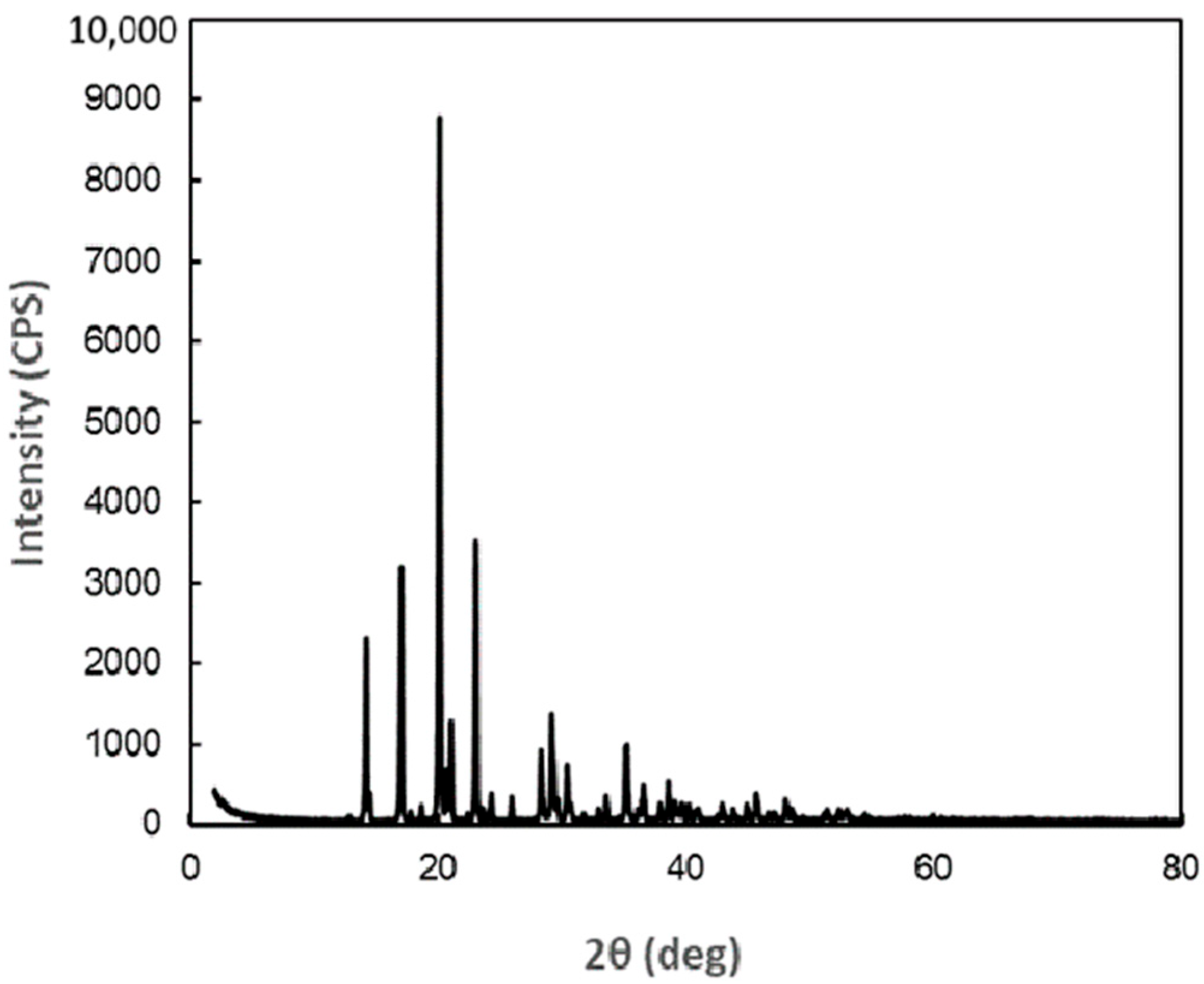
4.2. X-Ray Crystal Structure Analysis of DM
4.3. Urinary DM Concentrations
4.4. Urinary Man Concentrations
4.5. Acute Oral Toxicity Test of DM Using Female Mice
4.6. Bacterial Reverse Mutation Test of DM
4.7. Humane Trial
4.8. Carbohydrate Utilization Test
4.9. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | 1-Deoxymannose |
| Man | Ⅾ-Mannose |
| UPEC | Uropathogenic Escherichia coli |
| UTI | Urinary tract infection |
Appendix A
| Peak No. | Diffraction Angle (deg) | Surface Spacing (nm) | Intensity (cps) | Integrated Intensity (a.u) |
|---|---|---|---|---|
| 1 | 12.89 | 0.6862 | 51 | 5 |
| 2 | 14.26 | 0.6206 | 2,253 | 374 |
| 3 | 14.54 | 0.6087 | 256 | 24 |
| 4 | 17.08 | 0.5187 | 3,129 | 493 |
| 5 | 17.81 | 0.4976 | 103 | 15 |
| 6 | 18.71 | 0.4739 | 182 | 30 |
| 7 | 20.16 | 0.4401 | 8,698 | 1438 |
| 8 | 20.75 | 0.4277 | 488 | 60 |
| 9 | 21.08 | 0.4211 | 1,113 | 141 |
| 10 | 22.43 | 0.3961 | 78 | 11 |
| 11 | 23.1 | 0.3847 | 3,435 | 530 |
| 12 | 23.64 | 0.3761 | 125 | 17 |
| 13 | 24.35 | 0.3652 | 329 | 61 |
| 14 | 25.97 | 0.3428 | 296 | 43 |
| 15 | 28.35 | 0.3146 | 867 | 175 |
| 16 | 29.21 | 0.3055 | 1,268 | 307 |
| 17 | 29.67 | 0.3009 | 167 | 18 |
| 18 | 30.44 | 0.2934 | 667 | 138 |
| 19 | 30.74 | 0.2906 | 188 | 46 |
| 20 | 31.79 | 0.2813 | 83 | 14 |
| 21 | 32.97 | 0.2715 | 131 | 18 |
| 22 | 33.61 | 0.2664 | 292 | 51 |
| 23 | 35.2 | 0.2548 | 535 | 42 |
| 24 | 36.16 | 0.2482 | 83 | 17 |
| 25 | 36.61 | 0.2453 | 410 | 108 |
| 26 | 37.96 | 0.2368 | 192 | 34 |
| 27 | 38.61 | 0.233 | 454 | 69 |
| 28 | 39.13 | 0.23 | 173 | 29 |
| 29 | 39.79 | 0.2264 | 143 | 30 |
| 30 | 40.26 | 0.2238 | 157 | 33 |
| 31 | 41.01 | 0.2199 | 134 | 33 |
| 32 | 42.98 | 0.2103 | 171 | 53 |
| 33 | 43.87 | 0.2062 | 131 | 28 |
| 34 | 44.96 | 0.2015 | 183 | 38 |
| 35 | 45.72 | 0.1983 | 322 | 81 |
| 36 | 46.71 | 0.1943 | 73 | 14 |
| 37 | 47.14 | 0.1926 | 74 | 13 |
| 38 | 48.08 | 0.1891 | 252 | 60 |
| 39 | 48.54 | 0.1874 | 99 | 13 |
References
- Sihra, N.; Goodman, A.; Zakri, R.; Sahai, A.; Malde, S. Nonantibiotic prevention and management of recurrent urinary tract infection. Nat. Rev. Urol. 2018, 15, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, K.; Hsieh, A.F.; Lin, Z.; Bueno, H.; Ross, J.S.; Horwitz, L.I.; Barreto-Filho, J.A.; Kim, N.; Bernheim, S.M.; Suter, L.G.; et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013, 309, 355–363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Nunzio, C.; Bartoletti, R.; Tubaro, A.; Simonato, A.; Ficarra, V. Role of D-mannose in the prevention of recurrent uncomplicated cystitis: State of the art and future perspectives. Antibiotics 2021, 10, 373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ala-Jaakkola, R.; Laitila, A.; Ouwehand, A.C.; Lehtoranta, L. Role of D-mannose in urinary tract infections—A narrative review. Nutr. J. 2022, 21, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayward, G.; Mort, S.; Hay, A.D.; Moore, M.; Thomas, N.P.B.; Cook, J.; Robinson, J.; Williams, N.; Maeder, N.; Edeson, R.; et al. d-Mannose for Prevention of Recurrent urinary tract infection among Women: A Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 619–628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, E.; Dashti, M.; Fuentes, J.; Reitzer, L.; Christie, A.L.; Zimmern, P.E. d-mannosuria levels measured 1 h after d-mannose intake can select out favorable responders: A pilot study. Neurourol. Urodyn. 2023, 42, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.N.; Goguen, J.D.; Sun, D.; Klemm, P.; Beachey, E.H. Identification of two ancillary subunits of Escherichia coli type 1 fimbriae by using antibodies against synthetic oligopeptides of fim gene products. J. Bacteriol. 1987, 169, 5530–5536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, B.; Zhou, G.; Chan, S.Y.; Shapiro, E.; Kong, X.P.; Wu, X.R.; Sun, T.T.; Costello, C.E. Distinct glycan structures of uroplakins Ia and Ib: Structural basis for the selective binding of FimH adhesin to uroplakin Ia. J. Biol. Chem. 2006, 281, 14644–14653. [Google Scholar] [CrossRef] [PubMed]
- Krammer, E.M.; de Ruyck, J.; Roos, G.; Bouckaert, J.; Lensink, M.F. Targeting dynamical binding processes in the design of non-antibiotic anti-adhesives by molecular simulation-the example of FimH. Molecules 2018, 23, 1641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kühn, A.; Yu, S.; Giffhorn, F. Catabolism of 1,5-anhydro-D-fructose in Sinorhizobium morelense S-30.7.5: Discovery, characterization, and overexpression of a new 1,5-anhydro-D-fructose reductase and its application in sugar analysis and rare sugar synthesis. Appl. Environ. Microbiol. 2006, 72, 1248–1257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izumi, S.; Hirota, T.; Yoshinaga, K.; Abe, J. Bioconversion of 1,5-anhydro-D-fructose to 1,5-anhydro-D-glucitol and 1,5-anhydro-D-mannitol using Saccharomyces cerevisiae. J. Appl. Glycosci. 2012, 59, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.M.; Lundt, I.; Marcussen, J.; Yu, S. 1,5-Anhydro-D-fructose; a versatile chiral building block: Biochemistry and chemistry. Carbohydr. Res. 2002, 337, 873–890. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Krammer, E.M.; Roos, G.; Zalewski, A.; Preston, R.; Eid, S.; Zihlmann, P.; Prévost, M.; Lensink, M.F.; Thompson, A.; et al. Mutation of Tyr137 of the universal Escherichia coli fimbrial adhesin FimH relaxes the tyrosine gate prior to mannose binding. IUCrJ 2017, 4, 7–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kisiela, D.I.; Kramer, J.J.; Tchesnokova, V.; Aprikian, P.; Yarov-Yarovoy, V.; Clegg, S.; Sokurenko, E.V. Allosteric catch bond properties of the FimH adhesin from Salmonella enterica serovar Typhimurium. J. Biol. Chem. 2011, 286, 38136–38147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Old, D.C. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. J. Gen. Microbiol. 1972, 71, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Anan, G.; Kikuchi, D.; Omae, K.; Hirose, T.; Okada, K.; Mori, T. Sodium-glucose cotransporter-2 inhibitors increase urinary tract infections?-a cross sectional analysis of a nationwide Japanese claims database. Endocr. J. 2023, 70, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Confederat, L.G.; Condurache, M.I.; Alexa, R.E.; Dragostin, O.M. Particularities of urinary tract infections in diabetic patients: A concise review. Medicina 2023, 59, 1747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spaulding, C.N.; Klein, R.D.; Ruer, S.; Kau, A.L.; Schreiber, H.L.; Cusumano, Z.T.; Dodson, K.W.; Pinkner, J.S.; Fremont, D.H.; Janetka, J.W.; et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 2017, 546, 528–532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujisue, M.; Yoshinaga, K.; Muroya, K.; Abel, J.; Hizukuri, S. Preparation and antioxidative activity of 1,5-anhydrofructose. J. Appl. Glycosci. 1999, 46, 439–444. [Google Scholar] [CrossRef][Green Version]




| 1-Deoxymannose | D-Mannose | |
|---|---|---|
| KD value (µM: µg/mL) | 1.125:0.185 * | 1.672:0.301 ** |
| Peak urine concentration after oral intake (µg/mL) | 665–5780 | 2.15–22.9 |
| Peak urine concentration/KD | 3600–31,200 | 66.3–707 |
| AUC (µg·h/mL) | 437–2240 | 1.43–19.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, H.; Miyazaki, N.; Kawakami, T.; Izumi, S.; Yoshinaga, K. A Pilot Study in Humans on the Urinary Tract Excretion of the FimH Inhibitor 1-Deoxymannose. Antibiotics 2025, 14, 498. https://doi.org/10.3390/antibiotics14050498
Hayashi H, Miyazaki N, Kawakami T, Izumi S, Yoshinaga K. A Pilot Study in Humans on the Urinary Tract Excretion of the FimH Inhibitor 1-Deoxymannose. Antibiotics. 2025; 14(5):498. https://doi.org/10.3390/antibiotics14050498
Chicago/Turabian StyleHayashi, Hiromi, Naoto Miyazaki, Takuya Kawakami, Shusaku Izumi, and Kazuhiro Yoshinaga. 2025. "A Pilot Study in Humans on the Urinary Tract Excretion of the FimH Inhibitor 1-Deoxymannose" Antibiotics 14, no. 5: 498. https://doi.org/10.3390/antibiotics14050498
APA StyleHayashi, H., Miyazaki, N., Kawakami, T., Izumi, S., & Yoshinaga, K. (2025). A Pilot Study in Humans on the Urinary Tract Excretion of the FimH Inhibitor 1-Deoxymannose. Antibiotics, 14(5), 498. https://doi.org/10.3390/antibiotics14050498





