Lack of Spontaneous and Adaptive Resistance Development in Staphylococcus aureus Against the Antimicrobial Peptide LTX-109
Abstract
1. Introduction
2. Results
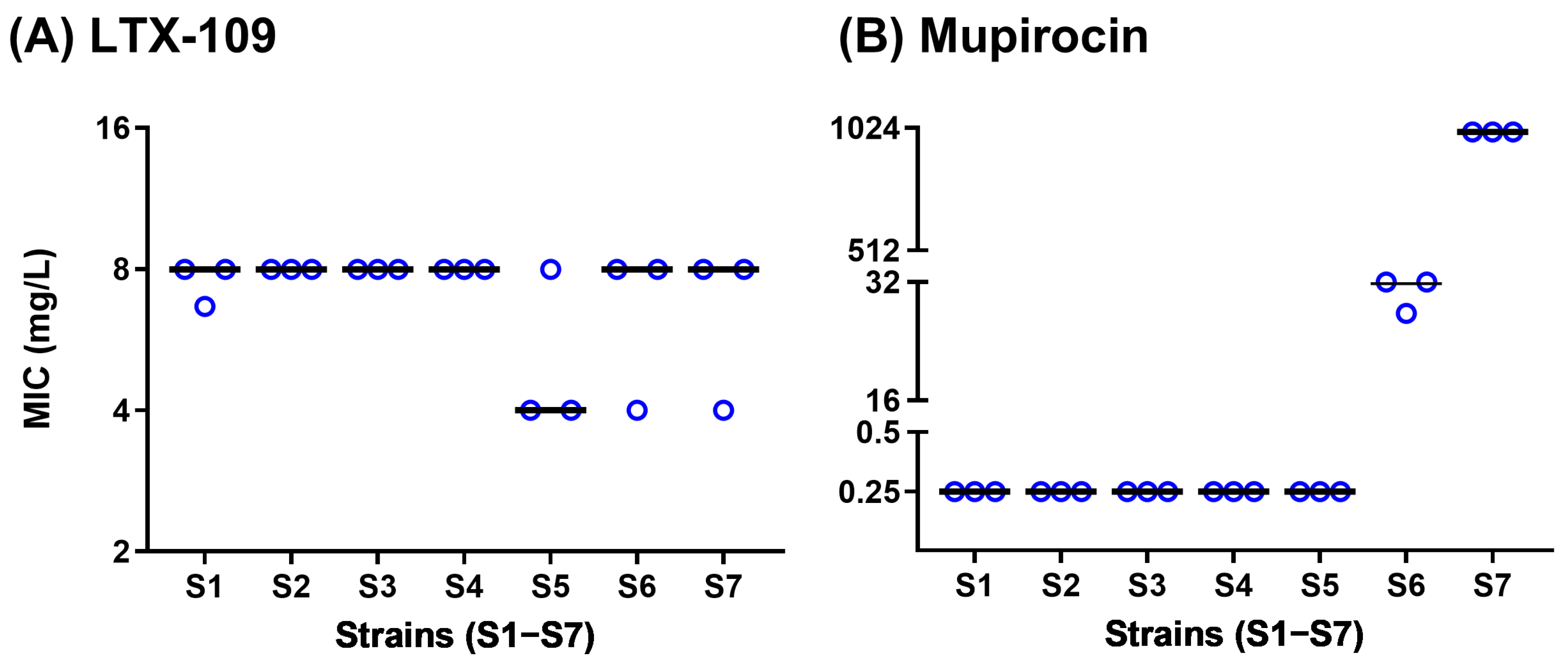
2.1. Broth Microdilution (BMD) Assay MIC Values Were in Agreement with Strain Characteristics
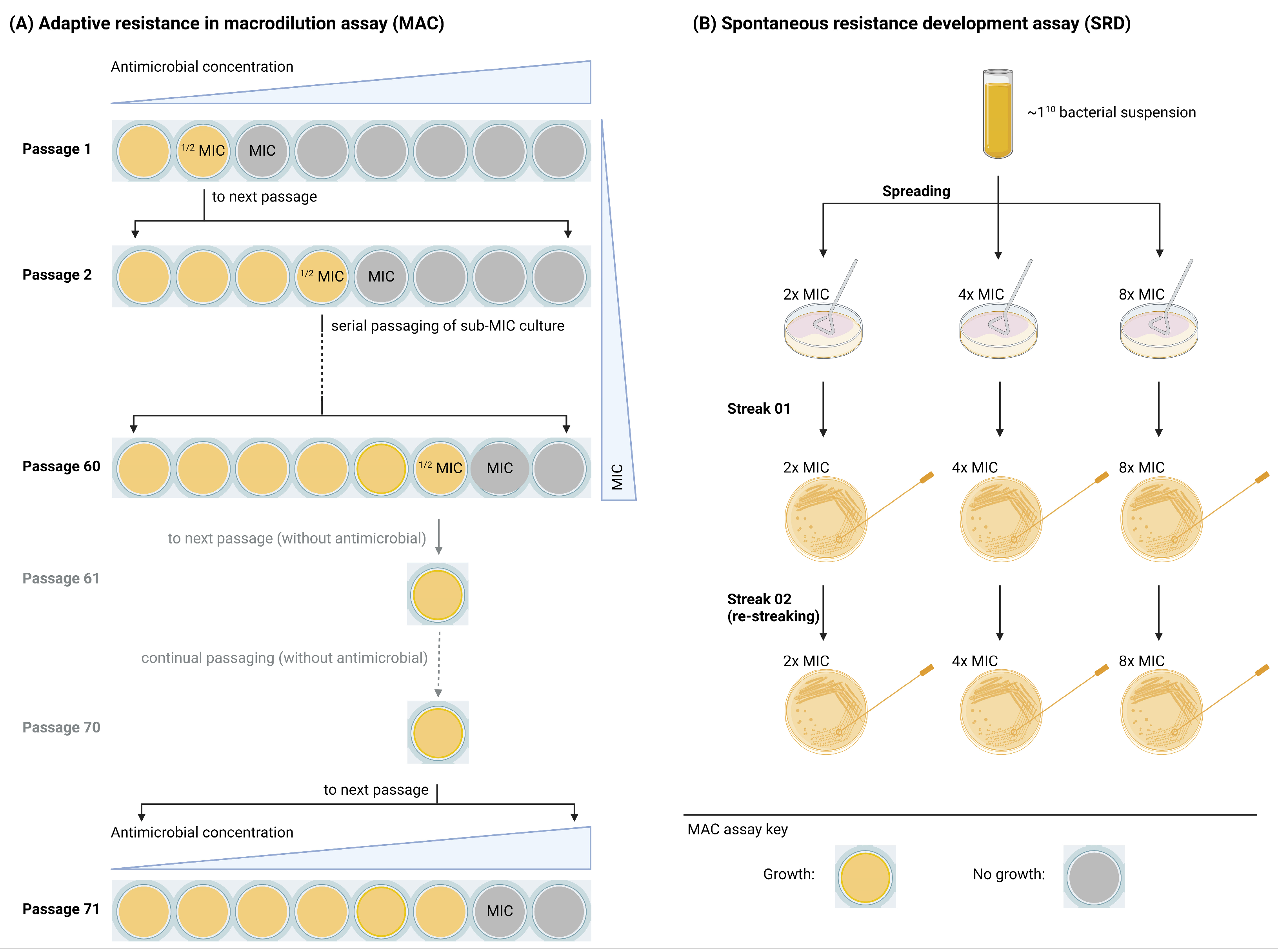
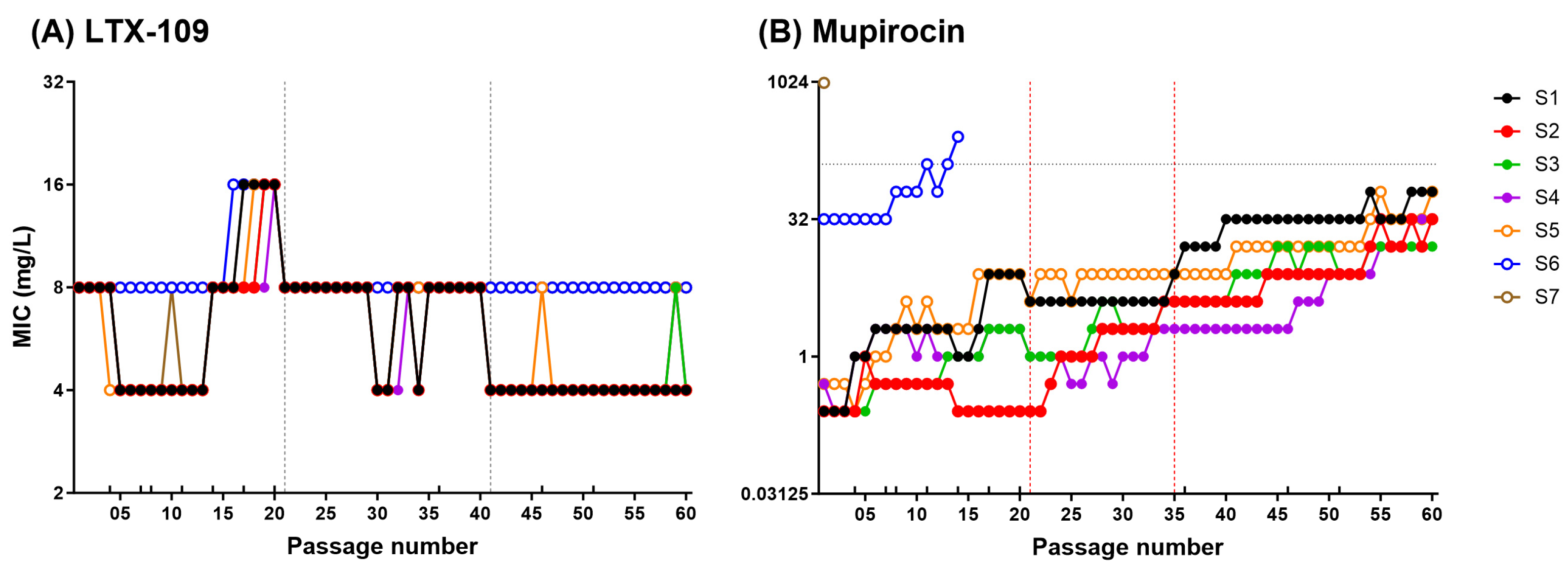
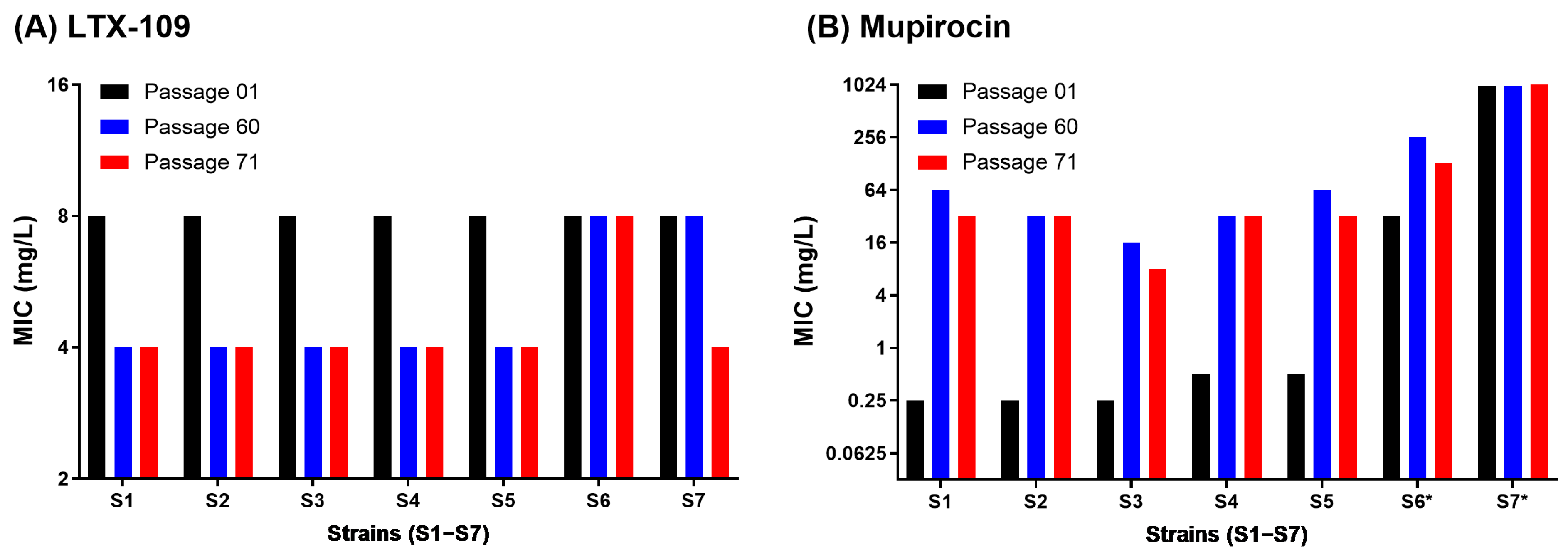
2.2. Adaptive and Mutational Resistance in Macrodilution (MAC) Assay Exhibited Steady LTX-109 MIC Values over 60 Passages
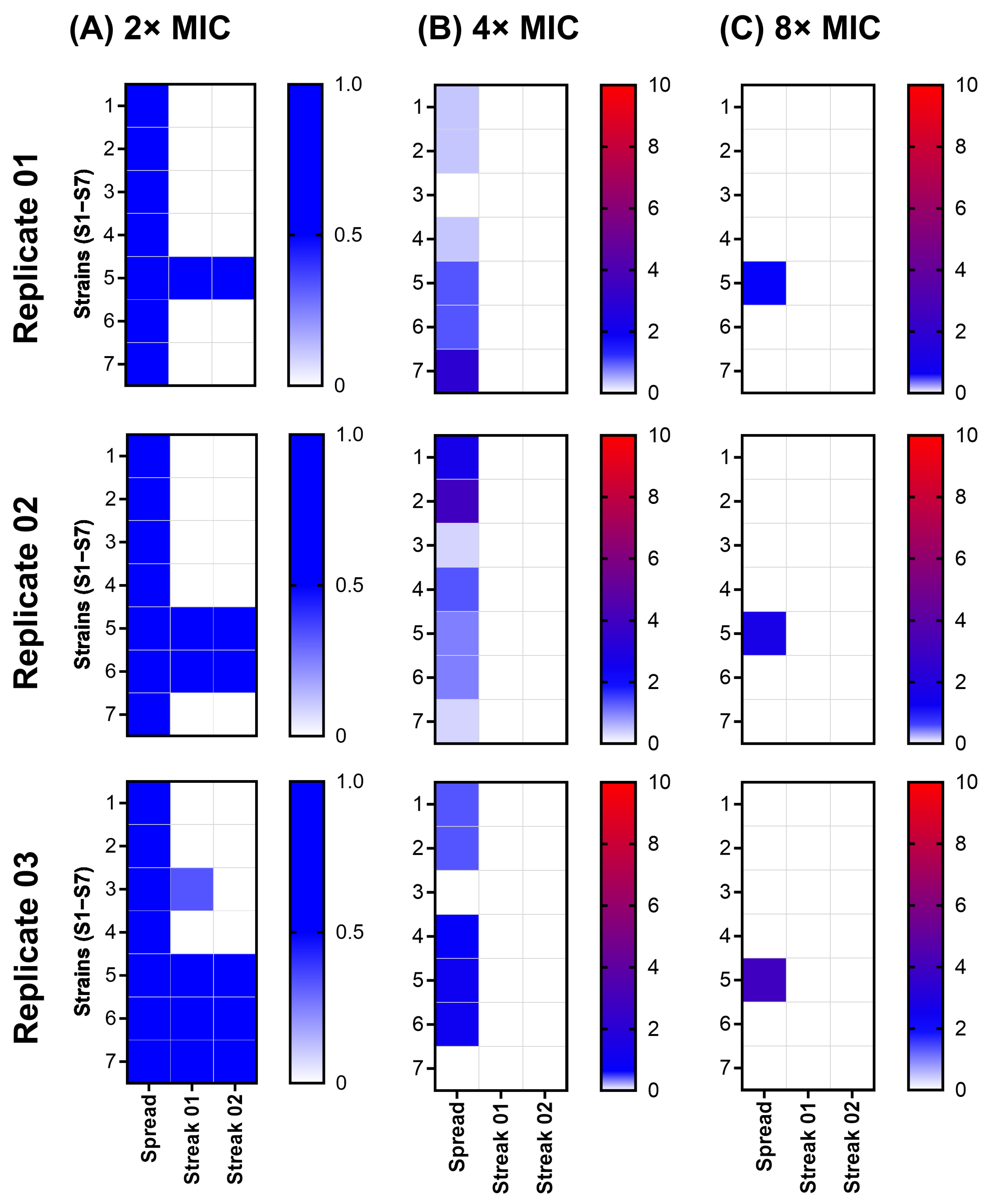
2.3. Spontaneous Resistance Development Assay (SRD) Indicated a Lack of Resistance Development
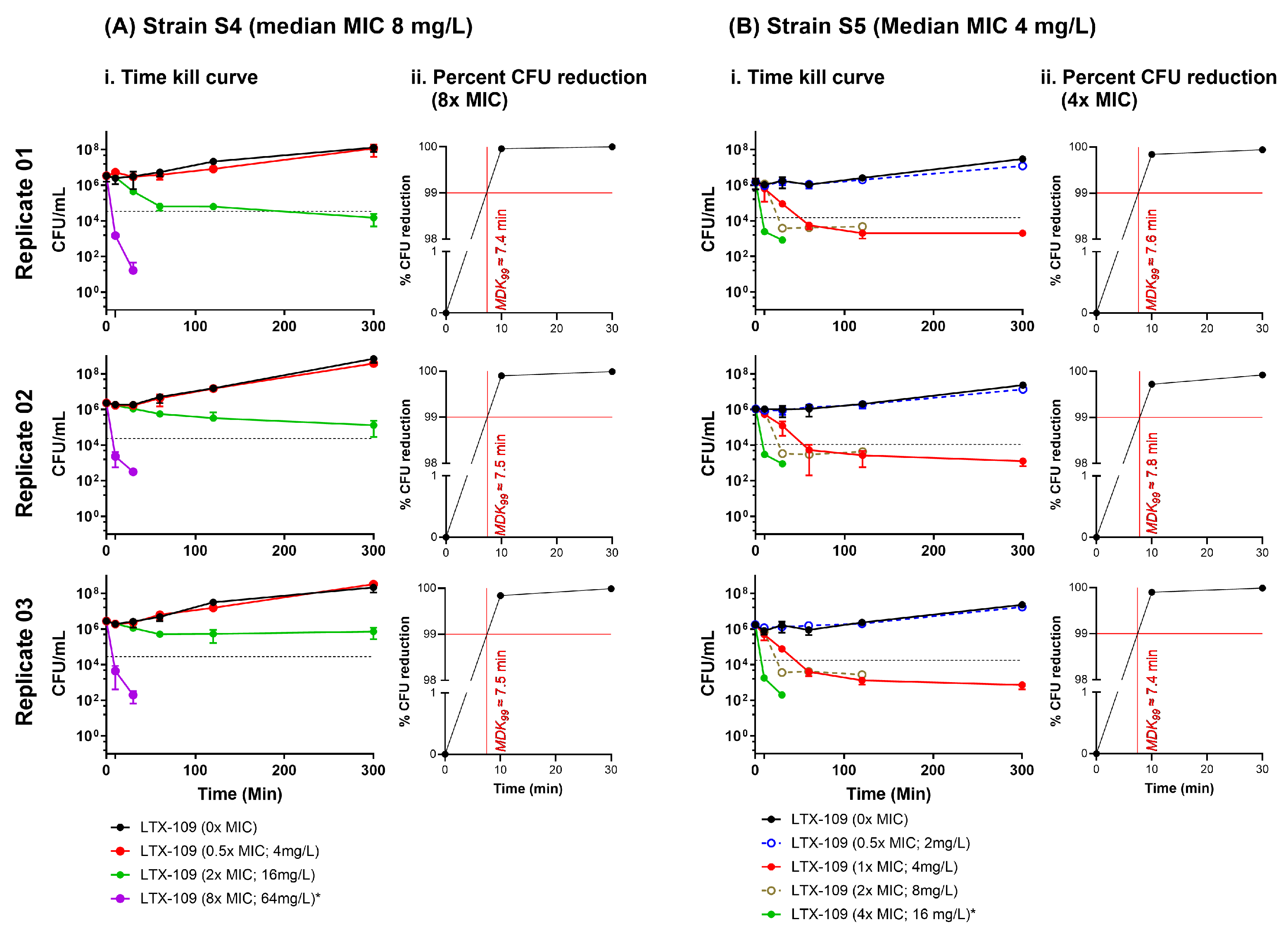
2.4. Time-Kill Curve Assay (TKC) Indicated That the Minimum Duration of Killing 99% Population (MKD99) Was <10 Min
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Growth Conditions
4.3. Antimicrobial Agents
4.4. Broth Microdilution (BMD) Assay
4.5. Adaptive and Mutational Resistance in Serial Macrodilution Assay (MAC)
4.6. Spontaneous Resistance Development Assay (SRD)
4.7. Time-Kill Curve Assay (TKC)
4.8. Statistical Methods Used
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S. aureus | Staphylococcus aureus |
| MRSA | Methicillin-resistant S. aureus |
| MSSA | Methicillin-susceptible S. aureus |
| SS | Surgical site |
| MuRSA | Mupirocin-resistant S. aureus |
| LLMuRSA | Low-level mupirocin resistance |
| HLMuRSA | High-level mupirocin resistance |
| MIC | Minimum inhibitory concentration |
| BMD | Broth microdilution assay |
| MAC | (Serial MH-broth) macrodilution assay |
| SRD | Spontaneous resistance development assay |
| TKC | Time-kill curve assay |
| MKD99 | Minimum duration for killing 99% of the population |
| ECOFF | Epidemiological cut-off |
| WT | Wild type |
| spa | S. aureus protein A |
| ST | Sequence type |
| SSI | Statens Serum Institute, Copenhagen |
| BA | Blood agar |
| DMSO | Dimethyl sulfoxide |
| MHB | Mueller Hinton Broth 2 |
| MH | Mueller Hinton |
| CFU | Colony-forming unit |
| PBS | Phosphate-buffered saline |
References
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Sollid, J.U.E.; Furberg, A.S.; Hanssen, A.M.; Johannessen, M. Staphylococcus aureus: Determinants of Human Carriage. Infect. Genet. Evol. 2014, 21, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The Role of Nasal Carriage in Staphylococcus aureus Infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Kluytmans, J.A.J.W.; Wertheim, H.F.L. Nasal Carriage of Staphylococcus aureus and Prevention of Nosocomial Infections. Infection 2005, 33, 3–8. [Google Scholar] [CrossRef]
- Perl, T.M.; Cullen, J.J.; Wenzel, R.P.; Zimmerman, M.B.; Pfaller, M.A.; Sheppard, D.; Twombley, J.; French, P.P.; Herwaldt, L.A. Intranasal Mupirocin to Prevent Postoperative Staphylococcus aureus Infections. N. Engl. J. Med. 2002, 346, 1871–1877. [Google Scholar] [CrossRef]
- Kluytmans, J.A.; Mouton, J.W.; VandenBergh, M.F.; Manders, M.J.; Maat, A.P.; Wagenvoort, J.H.; Michel, M.F.; Verbrugh, H.A. Reduction of Surgical-Site Infections in Cardiothoracic Surgery by Elimination of Nasal Carriage of Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 1996, 17, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Dadashi, M.; Hajikhani, B.; Darban-Sarokhalil, D.; van Belkum, A.; Goudarzi, M. Mupirocin Resistance in Staphylococcus aureus: A Systematic Review and Meta-Analysis. J. Glob. Antimicrob. Resist. 2020, 20, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, S.; Heidary, M.; Asadi, A.; Soleimani, S.; Motahar, M.; Savari, M.; Saki, M.; Abdi, M. A Review on Mechanism of Action, Resistance, Synergism, and Clinical Implications of Mupirocin against Staphylococcus aureus. Biomed. Pharmacother. 2019, 109, 1809–1818. [Google Scholar] [CrossRef]
- Hetem, D.J.; Bonten, M.J.M. Clinical Relevance of Mupirocin Resistance in Staphylococcus aureus. J. Hosp. Infect. 2013, 85, 249–256. [Google Scholar] [CrossRef]
- Gangwar, A.; Kumar, P.; Singh, R.; Kush, P. Recent Advances in Mupirocin Delivery Strategies for the Treatment of Bacterial Skin and Soft Tissue Infection. Future Pharmacol. 2021, 1, 80–103. [Google Scholar] [CrossRef]
- Isaksson, J.; Brandsdal, B.O.; Engqvist, M.; Flaten, G.E.; Svendsen, J.S.M.; Stensen, W. A Synthetic Antimicrobial Peptidomimetic (LTX 109): Stereochemical Impact on Membrane Disruption. J. Med. Chem. 2011, 54, 5786–5795. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Pawlak, J.; Johnson, L.; Bonilla, H.; Saravolatz, L.D.; Fakih, M.G.; Fugelli, A.; Olsen, W.M. In Vitro Activities of LTX-109, a Synthetic Antimicrobial Peptide, against Methicillin-Resistant, Vancomycin-Intermediate, Vancomycin-Resistant, Daptomycin-Nonsusceptible, and Linezolid-Nonsusceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 4478–4482. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Janson, H.; Wold, H.; Fugelli, A.; Andersson, K.; Håkangård, C.; Olsson, P.; Olsen, W.M. LTX-109 Is a Novel Agent for Nasal Decolonization of Methicillin-Resistant and -Sensitive Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 7. [Google Scholar] [CrossRef]
- Flemming, K.; Klingenberg, C.; Cavanagh, J.P.; Sletteng, M.; Stensen, W.; Svendsen, J.S.; Flaegstad, T. High in Vitro Antimicrobial Activity of Synthetic Antimicrobial Peptidomimetics against Staphylococcal Biofilms. J. Antimicrob. Chemother. 2008, 63, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, J.; Cavanagh, J.P.; Stensen, W.; Mortensen, B.; Svendsen, J.-S.; Svenson, J. In Vitro and in Vivo Antibacterial Properties of Peptide AMC-109 Impregnated Wound Dressings and Gels. J. Antibiot. 2021, 74, 337–345. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between Resistance, Tolerance and Persistence to Antibiotic Treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Eucast: MIC and Zone Distributions and ECOFFs. Available online: https://www.eucast.org/mic_and_zone_distributions_and_ecoffs (accessed on 25 April 2023).
- Samuelsen, Ø.; Haukland, H.H.; Jenssen, H.; Krämer, M.; Sandvik, K.; Ulvatne, H.; Vorland, L.H. Induced Resistance to the Antimicrobial Peptide Lactoferricin B in Staphylococcus aureus. FEBS Lett. 2005, 579, 3421–3426. [Google Scholar] [CrossRef]
- Farrell, D.J.; Robbins, M.; Rhys-Williams, W.; Love, W.G. Investigation of the Potential for Mutational Resistance to XF-73, Retapamulin, Mupirocin, Fusidic Acid, Daptomycin, and Vancomycin in Methicillin-Resistant Staphylococcus aureus Isolates during a 55-Passage Study. Antimicrob. Agents Chemother. 2011, 55, 1177–1181. [Google Scholar] [CrossRef]
- MacLean, R.C. Assessing the Potential for Staphylococcus aureus to Evolve Resistance to XF-73. Trends Microbiol. 2020, 28, 432–435. [Google Scholar] [CrossRef]
- Clark, C.; Kosowska-Shick, K.; McGhee, P.; Dewasse, B.; Beachel, L.; Appelbaum, P.C. Resistance Selection Studies Comparing the Activity of Razupenem (PTZ601) to Vancomycin and Linezolid against Eight Methicillin-Resistant and Two Methicillin-Susceptible Staphylococcus aureus Strains. Antimicrob. Agents Chemother. 2009, 53, 3118–3121. [Google Scholar] [CrossRef]
- Roland, K.L.; Martin, L.E.; Esther, C.R.; Spitznagel, J.K. Spontaneous pmrA Mutants of Salmonella Typhimurium LT2 Define a New Two-Component Regulatory System with a Possible Role in Virulence. J. Bacteriol. 1993, 175, 4154–4164. [Google Scholar] [CrossRef] [PubMed]
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Ravindran, A.; Selvin, J. An Antibiotic Agent Pyrrolo[1,2-a]Pyrazine-1,4-Dione,Hexahydro Isolated from a Marine Bacteria Bacillus tequilensis MSI45 Effectively Controls Multi-Drug Resistant Staphylococcus aureus. RSC Adv. 2018, 8, 17837–17846. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Lourenço, O.; Queiroz, J.A.; Domingues, F.C. Bacteriostatic versus Bactericidal Activity of Ciprofloxacin in Escherichia Coli Assessed by Flow Cytometry Using a Novel Far-Red Dye. J. Antibiot. 2011, 64, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.; Boon, R.J.; Griffin, K.E.; Masters, P.J.; Slocombe, B.; White, A.R. Antibacterial Activity of Mupirocin (Pseudomonic Acid), a New Antibiotic for Topical Use. Antimicrob. Agents Chemother. 1985, 27, 495–498. [Google Scholar] [CrossRef]
- MIC EUCAST. Available online: https://mic.eucast.org/search/?search%5Bmethod%5D=mic&search%5Bantibiotic%5D=-1&search%5Bspecies%5D=449&search%5Bdisk_content%5D=-1&search%5Blimit%5D=50 (accessed on 12 June 2022).
- Brauner, A.; Shoresh, N.; Fridman, O.; Balaban, N.Q. An Experimental Framework for Quantifying Bacterial Tolerance. Biophys. J. 2017, 112, 2664–2671. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and Guidelines for Research on Antibiotic Persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Antimicrobial Peptides Therapy: An Emerging Alternative for Treating Drug-Resistant Bacteria. Yale J. Biol. Med. 2022, 95, 445–463. [Google Scholar]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms and Consequences of Bacterial Resistance to Antimicrobial Peptides. Drug Resist. Updates 2016, 26, 43–57. [Google Scholar] [CrossRef]
- Malaviolle, X.; Nonhoff, C.; Denis, O.; Rottiers, S.; Struelens, M.J. Evaluation of Disc Diffusion Methods and Vitek 2 Automated System for Testing Susceptibility to Mupirocin in Staphylococcus aureus. J. Antimicrob. Chemother. 2008, 62, 1018–1023. [Google Scholar] [CrossRef]
- Mouton, J.W.; Meletiadis, J.; Voss, A.; Turnidge, J. Variation of MIC Measurements: The Contribution of Strain and Laboratory Variability to Measurement Precision. J. Antimicrob. Chemother. 2018, 73, 2374–2379. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-Based Dose Adjustment: Facts and Fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Inoculum Effect. Rev. Infect. Dis. 1989, 11, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Udekwu, K.I.; Parrish, N.; Ankomah, P.; Baquero, F.; Levin, B.R. Functional Relationship between Bacterial Cell Density and the Efficacy of Antibiotics. J. Antimicrob. Chemother. 2009, 63, 745–757. [Google Scholar] [CrossRef]
- LaPlante, K.L.; Rybak, M.J. Impact of High-Inoculum Staphylococcus aureus on the Activities of Nafcillin, Vancomycin, Linezolid, and Daptomycin, Alone and in Combination with Gentamicin, in an In Vitro Pharmacodynamic Model. Antimicrob. Agents Chemother. 2004, 48, 4665–4672. [Google Scholar] [CrossRef]
- Loffredo, M.R.; Savini, F.; Bobone, S.; Casciaro, B.; Franzyk, H.; Mangoni, M.L.; Stella, L. Inoculum Effect of Antimicrobial Peptides. Proc. Natl. Acad. Sci. USA 2021, 118, e2014364118. [Google Scholar] [CrossRef]
- Jacobsen, B.K.; Eggen, A.E.; Mathiesen, E.B.; Wilsgaard, T.; Njølstad, I. Cohort Profile: The Tromsø Study. Int. J. Epidemiol. 2012, 41, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Eggen, A.E.; Mathiesen, E.B.; Wilsgaard, T.; Jacobsen, B.K.; Njølstad, I. The Sixth Survey of the Tromso Study (Tromso 6) in 2007-08: Collaborative Research in the Interface between Clinical Medicine and Epidemiology: Study Objectives, Design, Data Collection Procedures, and Attendance in a Multipurpose Population-Based Health Survey. Scand. J. Public Health 2013, 41, 65–80. [Google Scholar] [CrossRef]
- Olsen, K.; Sangvik, M.; Simonsen, G.S.; Sollid, J.U.E.; Sundsfjord, A.; Thune, I.; Furberg, A.-S. Prevalence and Population Structure of Staphylococcus aureus Nasal Carriage in Healthcare Workers in a General Population. The Tromsø Staph and Skin Study. Epidemiol. Infect. 2013, 141, 143–152. [Google Scholar] [CrossRef]
- Treangen, T.J.; Maybank, R.A.; Enke, S.; Friss, M.B.; Diviak, L.F.; Karaolis, D.K.R.; Koren, S.; Ondov, B.; Phillippy, A.M.; Bergman, N.H.; et al. Complete Genome Sequence of the Quality Control Strain Staphylococcus aureus Subsp. Aureus ATCC 25923. Genome Announc. 2014, 2, e01110-14. [Google Scholar] [CrossRef]
- ISO 20776-1: 2006; ISO: Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. ISO: London, UK, 2006. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/04/70464.html (accessed on 12 June 2020).
- EUCAST: MIC Determination. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination/?no_cache=1 (accessed on 12 June 2020).
- Fernández, L.; Hancock, R.E.W. Adaptive and Mutational Resistance: Role of Porins and Efflux Pumps in Drug Resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef]
| Experimental ID | HMI ID 1 | Spa 2 | ST 3 | Sequencing | Origin | Characteristics |
|---|---|---|---|---|---|---|
| S1 | 68-01 | T012 | ST30 | Pac-bio 4 | Tromsø6 5 | MSSA |
| S2 | 68-02 | T065 | ST45 | Pac-bio | Tromsø6 | MSSA |
| S3 | 68-03 | T084 | ST15 | Pac-bio 6 | Tromsø6 | MSSA |
| S4 | 68-04 | T021 | ST243 | Yes 7 | ATCC 25923 | MSSA |
| S5 | 68-05 | T007 | ST39 | Yes 8 | ATCC 43300 | MRSA |
| S6 | 68-09 | T041 | N.P. 11 | Yes, CC5 9 | SSI 10 | MRSA, mupirocin MIC = 16 µg/mL, mupA negative strain |
| S7 | 68-11 | T067 | N.P. | Yes, CC5 | SSI | MRSA, mupirocin MIC > 512 µg/mL, mupA positive strain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, B.; Winkler, M.A.; Kabir, W.; Ericson, J.U.; Sundsfjord, A. Lack of Spontaneous and Adaptive Resistance Development in Staphylococcus aureus Against the Antimicrobial Peptide LTX-109. Antibiotics 2025, 14, 492. https://doi.org/10.3390/antibiotics14050492
Singh B, Winkler MA, Kabir W, Ericson JU, Sundsfjord A. Lack of Spontaneous and Adaptive Resistance Development in Staphylococcus aureus Against the Antimicrobial Peptide LTX-109. Antibiotics. 2025; 14(5):492. https://doi.org/10.3390/antibiotics14050492
Chicago/Turabian StyleSingh, Bhupender, Mia Angelique Winkler, Wasifa Kabir, Johanna U Ericson, and Arnfinn Sundsfjord. 2025. "Lack of Spontaneous and Adaptive Resistance Development in Staphylococcus aureus Against the Antimicrobial Peptide LTX-109" Antibiotics 14, no. 5: 492. https://doi.org/10.3390/antibiotics14050492
APA StyleSingh, B., Winkler, M. A., Kabir, W., Ericson, J. U., & Sundsfjord, A. (2025). Lack of Spontaneous and Adaptive Resistance Development in Staphylococcus aureus Against the Antimicrobial Peptide LTX-109. Antibiotics, 14(5), 492. https://doi.org/10.3390/antibiotics14050492







