Virological and Pharmaceutical Properties of Clinically Relevant Phages
Abstract
1. Introduction
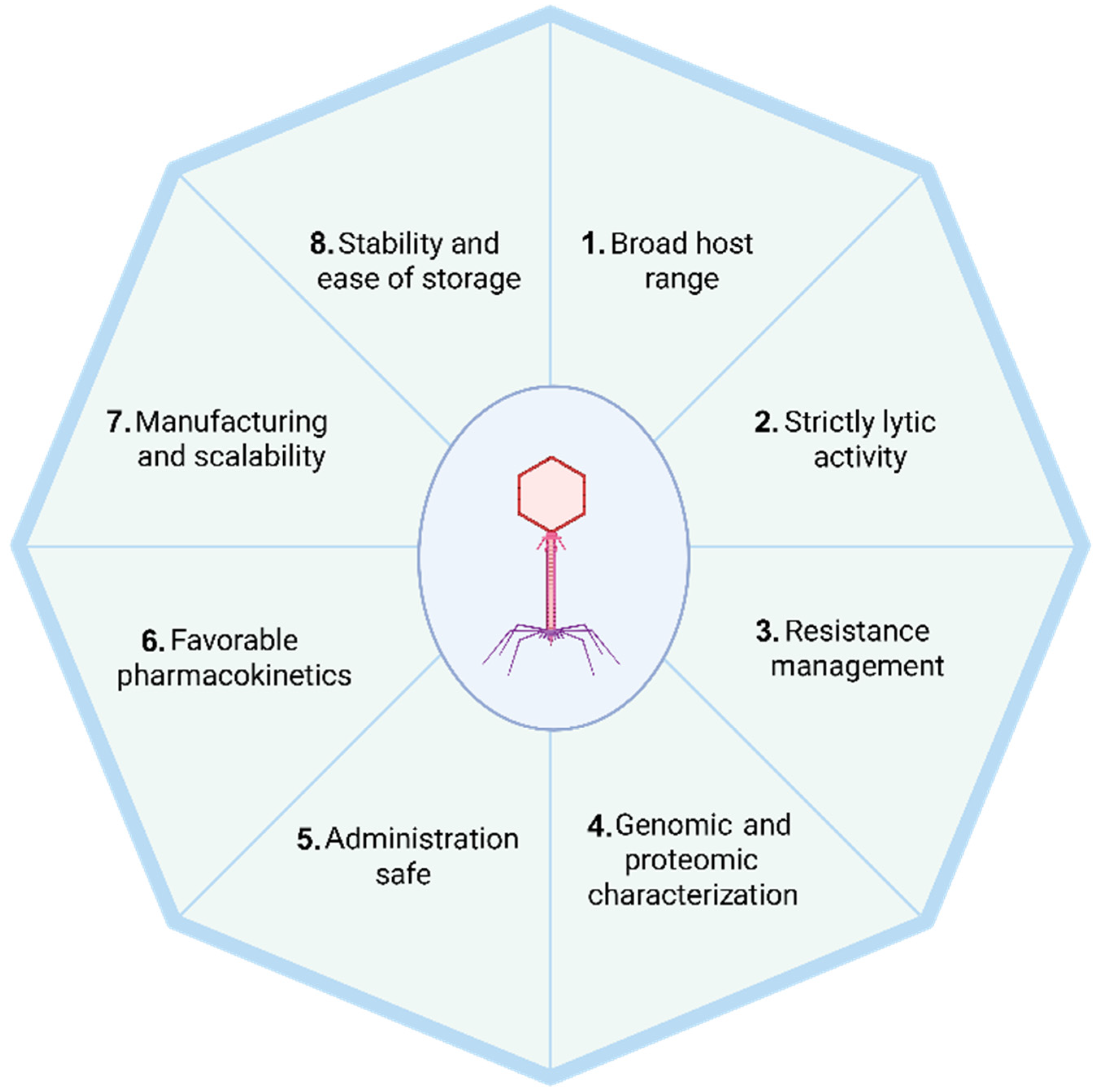
2. The Eight Essential Characteristics of Therapeutic Phages
2.1. Broad Host Range
2.2. Strictly Lytic Life Cycle-Driven Phages
2.3. Resistance Management
2.4. Genomic and Proteomic Characterization
2.5. Safe for Administration
2.6. Favorable Pharmacokinetics
2.7. Manufacturing and Scalability
2.8. Stability and Ease of Storage
3. Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jdeed, G.; Kravchuk, B.; Tikunova, N.V. Factors Affecting Phage-Bacteria Coevolution Dynamics. Viruses 2025, 17, 235. [Google Scholar] [CrossRef] [PubMed]
- Rohde, C.; Wittmann, J. Phage Diversity for Research and Application. Antibiotics 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Olszak, T.; Latka, A.; Roszniowski, B.; Valvano, M.A.; Drulis-Kawa, Z. Phage Life Cycles Behind Bacterial Biodiversity. Curr. Med. Chem. 2017, 24, 3987–4001. [Google Scholar] [CrossRef]
- Salmond, G.P.C.; Fineran, P.C. A Century of the Phage: Past, Present and Future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Summers, W.C. Bacteriophage Therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Ferous, S.; Petsimeri, A.; Gioula, G.; Tsakris, A. Phage-Based Therapy in Combination with Antibiotics: A Promising Alternative against Multidrug-Resistant Gram-Negative Pathogens. Pathogens 2024, 13, 896. [Google Scholar] [CrossRef]
- Straka, M.; Dubinová, M.; Liptáková, A. Phascinating Phages. Microorganisms 2022, 10, 1365. [Google Scholar] [CrossRef]
- Faltus, T. The Medicinal Phage—Regulatory Roadmap for Phage Therapy under EU Pharmaceutical Legislation. Viruses 2024, 16, 443. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T.; Vermeulen, W. Bacteriophage–Host Interactions and the Therapeutic Potential of Bacteriophages. Viruses 2024, 16, 478. [Google Scholar] [CrossRef]
- Boeckaerts, D.; Stock, M.; Ferriol-González, C.; Oteo-Iglesias, J.; Sanjuán, R.; Domingo-Calap, P.; De Baets, B.; Briers, Y. Prediction of Klebsiella Phage-Host Specificity at the Strain Level. Nat. Commun. 2024, 15, 4355. [Google Scholar] [CrossRef]
- Lewis, J.M.; Sagona, A.P. Armed Phages Are Heading for Clinical Trials. Nat. Microbiol. 2023, 8, 1191–1192. [Google Scholar] [CrossRef]
- Oromí-Bosch, A.; Antani, J.D.; Turner, P.E. Developing Phage Therapy That Overcomes the Evolution of Bacterial Resistance. Annu. Rev. Virol. 2023, 10, 503–524. [Google Scholar] [CrossRef]
- Grigson, S.R.; Giles, S.K.; Edwards, R.A.; Papudeshi, B. Knowing and Naming: Phage Annotation and Nomenclature for Phage Therapy. Clin. Infect. Dis. 2023, 77, S352–S359. [Google Scholar] [CrossRef] [PubMed]
- Hibstu, Z.; Belew, H.; Akelew, Y.; Mengist, H.M. Phage Therapy: A Different Approach to Fight Bacterial Infections. Biologics 2022, 16, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.J.; Kwon, J.; Kim, S.G.; Lee, S.-J. The Biotechnological Application of Bacteriophages: What to Do and Where to Go in the Middle of the Post-Antibiotic Era. Microorganisms 2023, 11, 2311. [Google Scholar] [CrossRef]
- Dunne, M.; Rupf, B.; Tala, M.; Qabrati, X.; Ernst, P.; Shen, Y.; Sumrall, E.; Heeb, L.; Plückthun, A.; Loessner, M.J.; et al. Reprogramming Bacteriophage Host Range through Structure-Guided Design of Chimeric Receptor Binding Proteins. Cell Rep. 2019, 29, 1336–1350.e4. [Google Scholar] [CrossRef] [PubMed]
- Alseth, E.O.; Roush, C.; Irby, I.; Kopylov, M.; Bobe, D.; Diggs, M.W.; Nguyen, K.; Xu, H.; Schmidt-Krey, I.; Bryksin, A.V.; et al. Mystique, a Broad Host Range Acinetobacter Phage, Reveals the Impact of Culturing Conditions on Phage Isolation and Infectivity. PLoS Pathog. 2025, 21, e1012986. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.R.; Sillankorva, S.; Azeredo, J. Bacteriophage-Encoded Depolymerases: Their Diversity and Biotechnological Applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef]
- Sun, C.; Chen, J.; Jin, M.; Zhao, X.; Li, Y.; Dong, Y.; Gao, N.; Liu, Z.; Bork, P.; Zhao, X.-M.; et al. Long-Read Sequencing Reveals Extensive DNA Methylations in Human Gut Phagenome Contributed by Prevalently Phage-Encoded Methyltransferases. Adv. Sci. 2023, 10, 2302159. [Google Scholar] [CrossRef]
- Murtazalieva, K.; Mu, A.; Petrovskaya, A.; Finn, R.D. The Growing Repertoire of Phage Anti-Defence Systems. Trends Microbiol. 2024, 32, 1212–1228. [Google Scholar] [CrossRef]
- Philipson, C.W.; Voegtly, L.J.; Lueder, M.R.; Long, K.A.; Rice, G.K.; Frey, K.G.; Biswas, B.; Cer, R.Z.; Hamilton, T.; Bishop-Lilly, K.A. Characterizing Phage Genomes for Therapeutic Applications. Viruses 2018, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Niaz, H.; Skurnik, M.; Adnan, F. Genomic and Proteomic Characterization of Four Novel Schitoviridae Family Phages Targeting Uropathogenic Escherichia Coli Strain. Virol. J. 2025, 22, 83. [Google Scholar] [CrossRef]
- Nour El-Din, H.T.; Kettal, M.; Granados Maciel, J.C.; Beaudoin, G.; Oktay, U.; Hrapovic, S.; Sad, S.; Dennis, J.J.; Peters, D.L.; Chen, W. Isolation, Characterization, and Genomic Analysis of Bacteriophages Against Pseudomonas Aeruginosa Clinical Isolates from Early and Chronic Cystic Fibrosis Patients for Potential Phage Therapy. Microorganisms 2025, 13, 511. [Google Scholar] [CrossRef]
- Qin, J.; Wei, L.; Feng, Y.; Zong, Z. Genome Sequence of the Broad-Host-Range Phage Phi1_092033 against Acinetobacter Baumannii. Microbiol. Resour. Announc. 2025, 14, e0006225. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Y.; Yu, D.; Fan, M.-M.; Zhang, X.; Jin, Z.-Y.; Tang, C.; Liu, X.-F. Antimicrobial Resistance Crisis: Could Artificial Intelligence Be the Solution? Mil. Med. Res. 2024, 11, 7. [Google Scholar] [CrossRef]
- Culot, A.; Abriat, G.; Furlong, K.P. High-Performance Genome Annotation for a Safer and Faster-Developing Phage Therapy. Viruses 2025, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, Z.; Liu, Y. PHPGAT: Predicting Phage Hosts Based on Multimodal Heterogeneous Knowledge Graph with Graph Attention Network. Brief. Bioinform. 2025, 26, bbaf017. [Google Scholar] [CrossRef]
- Reuter, M.; Kruger, D.H. Approaches to Optimize Therapeutic Bacteriophage and Bacteriophage-Derived Products to Combat Bacterial Infections. Virus Genes 2020, 56, 136–149. [Google Scholar] [CrossRef]
- Yukgehnaish, K.; Rajandas, H.; Parimannan, S.; Manickam, R.; Marimuthu, K.; Petersen, B.; Clokie, M.R.J.; Millard, A.; Sicheritz-Pontén, T. PhageLeads: Rapid Assessment of Phage Therapeutic Suitability Using an Ensemble Machine Learning Approach. Viruses 2022, 14, 342. [Google Scholar] [CrossRef]
- Palma, M.; Qi, B. Advancing Phage Therapy: A Comprehensive Review of the Safety, Efficacy, and Future Prospects for the Targeted Treatment of Bacterial Infections. Infect. Dis. Rep. 2024, 16, 1127–1181. [Google Scholar] [CrossRef]
- Santacroce, L.; Di Domenico, M.; Montagnani, M.; Jirillo, E. Antibiotic Resistance and Microbiota Response. Curr. Pharm. Des. 2023, 29, 356–364. [Google Scholar] [CrossRef]
- Manoussopoulos, Y.N.; Anastassopoulou, C.G. Virome: The Prodigious Little Cousin of the Family. In Microbiomics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 53–73. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 2019, 25, 803–814.e5. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Lu, X.-J.; Zhang, Y.; Cheung, C.P.; Lam, S.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Zhao, R.; Chan, P.K.S.; et al. Gut Mucosal Virome Alterations in Ulcerative Colitis. Gut 2019, 68, 1169–1179. [Google Scholar] [CrossRef]
- Qu, J.; Zou, J.; Zhang, J.; Qu, J.; Lu, H. Phage Therapy for Extensively Drug Resistant Acinetobacter Baumannii Infection: Case Report and in Vivo Evaluation of the Distribution of Phage and the Impact on Gut Microbiome. Front. Med. 2024, 11, 1432703. [Google Scholar] [CrossRef]
- Nang, S.C.; Lin, Y.-W.; Petrovic Fabijan, A.; Chang, R.Y.K.; Rao, G.G.; Iredell, J.; Chan, H.-K.; Li, J. Pharmacokinetics/Pharmacodynamics of Phage Therapy: A Major Hurdle to Clinical Translation. Clin. Microbiol. Infect. 2023, 29, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Kopač, T.; Lisac, A.; Mravljak, R.; Ručigaj, A.; Krajnc, M.; Podgornik, A. Bacteriophage Delivery Systems Based on Composite PolyHIPE/Nanocellulose Hydrogel Particles. Polymers 2021, 13, 2648. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Harjai, K.; Katare, O.P.; Chhibber, S. Encapsulation of Bacteriophage in Liposome Accentuates Its Entry in to Macrophage and Shields It from Neutralizing Antibodies. PLoS ONE 2016, 11, e0153777. [Google Scholar] [CrossRef]
- Pathak, V.; Chan, H.-K.; Zhou, Q.T. Formulation of Bacteriophage for Inhalation to Treat Multidrug-Resistant Pulmonary Infections. Kona 2025, 42, 200–212. [Google Scholar] [CrossRef]
- Jończyk-Matysiak, E.; Łodej, N.; Kula, D.; Owczarek, B.; Orwat, F.; Międzybrodzki, R.; Neuberg, J.; Bagińska, N.; Weber-Dąbrowska, B.; Górski, A. Factors Determining Phage Stability/Activity: Challenges in Practical Phage Application. Expert Rev. Anti-Infect. Ther. 2019, 17, 583–606. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Animal and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Malik, D.J.; Resch, G. Editorial: Manufacturing, Formulation and Delivery Issues for Phage Therapy to Become A Reality. Front. Microbiol. 2020, 11, 584137. [Google Scholar] [CrossRef]
- Bretaudeau, L.; Tremblais, K.; Aubrit, F.; Meichenin, M.; Arnaud, I. Good Manufacturing Practice (GMP) Compliance for Phage Therapy Medicinal Products. Front. Microbiol. 2020, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J. Approaches for Manufacture, Formulation, Targeted Delivery and Controlled Release of Phage-Based Therapeutics. Curr. Opin. Biotechnol. 2021, 68, 262–271. [Google Scholar] [CrossRef]
- Mutti, M.; Corsini, L. Robust Approaches for the Production of Active Ingredient and Drug Product for Human Phage Therapy. Front. Microbiol. 2019, 10, 2289. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.; Salabarria, A.-C.; Edwards, R.A.; Roach, D.R. Standardized Bacteriophage Purification for Personalized Phage Therapy. Nat. Protoc. 2020, 15, 2867–2890. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, Stabilisation and Encapsulation of Bacteriophage for Phage Therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [PubMed]
- Flint, R.; Laucirica, D.R.; Chan, H.-K.; Chang, B.J.; Stick, S.M.; Kicic, A. Stability Considerations for Bacteriophages in Liquid Formulations Designed for Nebulization. Cells 2023, 12, 2057. [Google Scholar] [CrossRef]
- Lin, Y.; Yoon Kyung Chang, R.; Britton, W.J.; Morales, S.; Kutter, E.; Li, J.; Chan, H.-K. Storage Stability of Phage-Ciprofloxacin Combination Powders against Pseudomonas Aeruginosa Respiratory Infections. Int. J. Pharm. 2020, 591, 119952. [Google Scholar] [CrossRef]
- Uyttebroek, S.; Bessems, L.; Metsemakers, W.-J.; Debaveye, Y.; Van Gerven, L.; Dupont, L.; Depypere, M.; Wagemans, J.; Lavigne, R.; Merabishvili, M.; et al. Stability of Magistral Phage Preparations before Therapeutic Application in Patients with Chronic Rhinosinusitis, Sepsis, Pulmonary, and Musculoskeletal Infections. Microbiol. Spectr. 2023, 11, e0290723. [Google Scholar] [CrossRef]
- Karn, S.L.; Gangwar, M.; Kumar, R.; Bhartiya, S.K.; Nath, G. Phage Therapy: A Revolutionary Shift in the Management of Bacterial Infections, Pioneering New Horizons in Clinical Practice, and Reimagining the Arsenal against Microbial Pathogens. Front. Med. 2023, 10, 1209782. [Google Scholar] [CrossRef]
- Sertkaya, A.; Wong, H.-H.; Jessup, A.; Beleche, T. Key Cost Drivers of Pharmaceutical Clinical Trials in the United States. Clin. Trials 2016, 13, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Alseth, E.O.; Custodio, R.; Sundius, S.A.; Kuske, R.A.; Brown, S.P.; Westra, E.R. The Impact of Phage and Phage Resistance on Microbial Community Dynamics. PLoS Biol. 2024, 22, e3002346. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.; Malik, D.J. Microencapsulation of Bacteriophages Using Membrane Emulsification in Different pH-Triggered Controlled Release Formulations for Oral Administration. Pharmaceuticals 2021, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Khazani Asforooshani, M.; Elikaei, A.; Abed, S.; Shafiei, M.; Barzi, S.M.; Solgi, H.; Badmasti, F.; Sohrabi, A. A Novel Enterococcus Faecium Phage EF-M80: Unveiling the Effects of Hydrogel-Encapsulated Phage on Wound Infection Healing. Front. Microbiol. 2024, 15, 1416971. [Google Scholar] [CrossRef]
- Samananda Singh, L. Nano-Emulsion Encapsulation for the Efficient Delivery of Bacteriophage Therapeutics. Biologicals 2024, 85, 101725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panagiotopoulos, A.-P.; Sagona, A.P.; Tsakri, D.; Ferous, S.; Anastassopoulou, C.; Tsakris, A. Virological and Pharmaceutical Properties of Clinically Relevant Phages. Antibiotics 2025, 14, 487. https://doi.org/10.3390/antibiotics14050487
Panagiotopoulos A-P, Sagona AP, Tsakri D, Ferous S, Anastassopoulou C, Tsakris A. Virological and Pharmaceutical Properties of Clinically Relevant Phages. Antibiotics. 2025; 14(5):487. https://doi.org/10.3390/antibiotics14050487
Chicago/Turabian StylePanagiotopoulos, Antonios-Periklis, Antonia P. Sagona, Deny Tsakri, Stefanos Ferous, Cleo Anastassopoulou, and Athanasios Tsakris. 2025. "Virological and Pharmaceutical Properties of Clinically Relevant Phages" Antibiotics 14, no. 5: 487. https://doi.org/10.3390/antibiotics14050487
APA StylePanagiotopoulos, A.-P., Sagona, A. P., Tsakri, D., Ferous, S., Anastassopoulou, C., & Tsakris, A. (2025). Virological and Pharmaceutical Properties of Clinically Relevant Phages. Antibiotics, 14(5), 487. https://doi.org/10.3390/antibiotics14050487








