Heterogeneity of Biofilm Formation Among Staphylococcus aureus and Coagulase-Negative Staphylococcus Species in Clinically Relevant Intravenous Fat Emulsions
Abstract
1. Introduction
2. Results
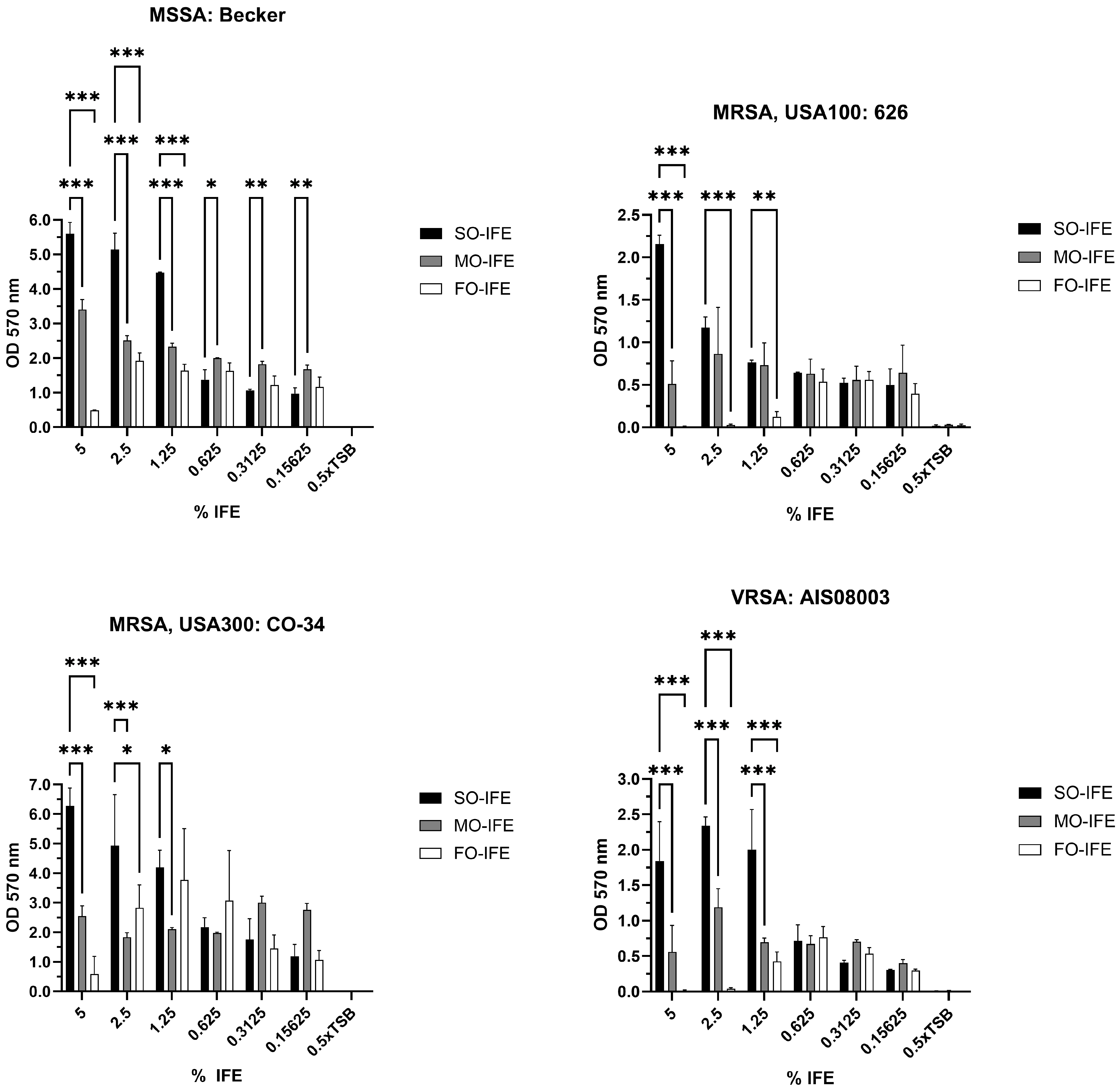
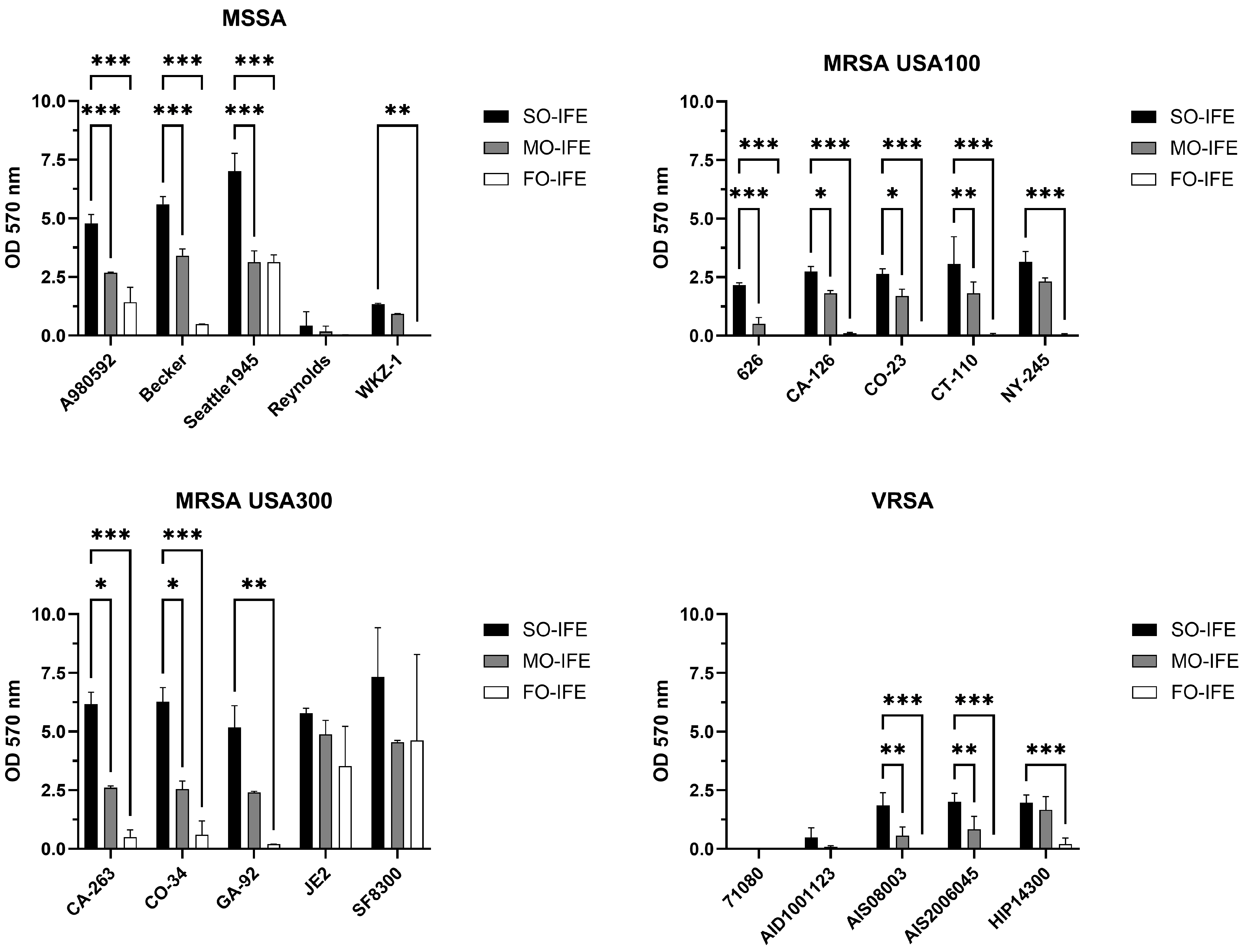
2.1. MO- and FO-IFE Limits Biofilm Formation of S. aureus Isolates Compared to SO-IFE
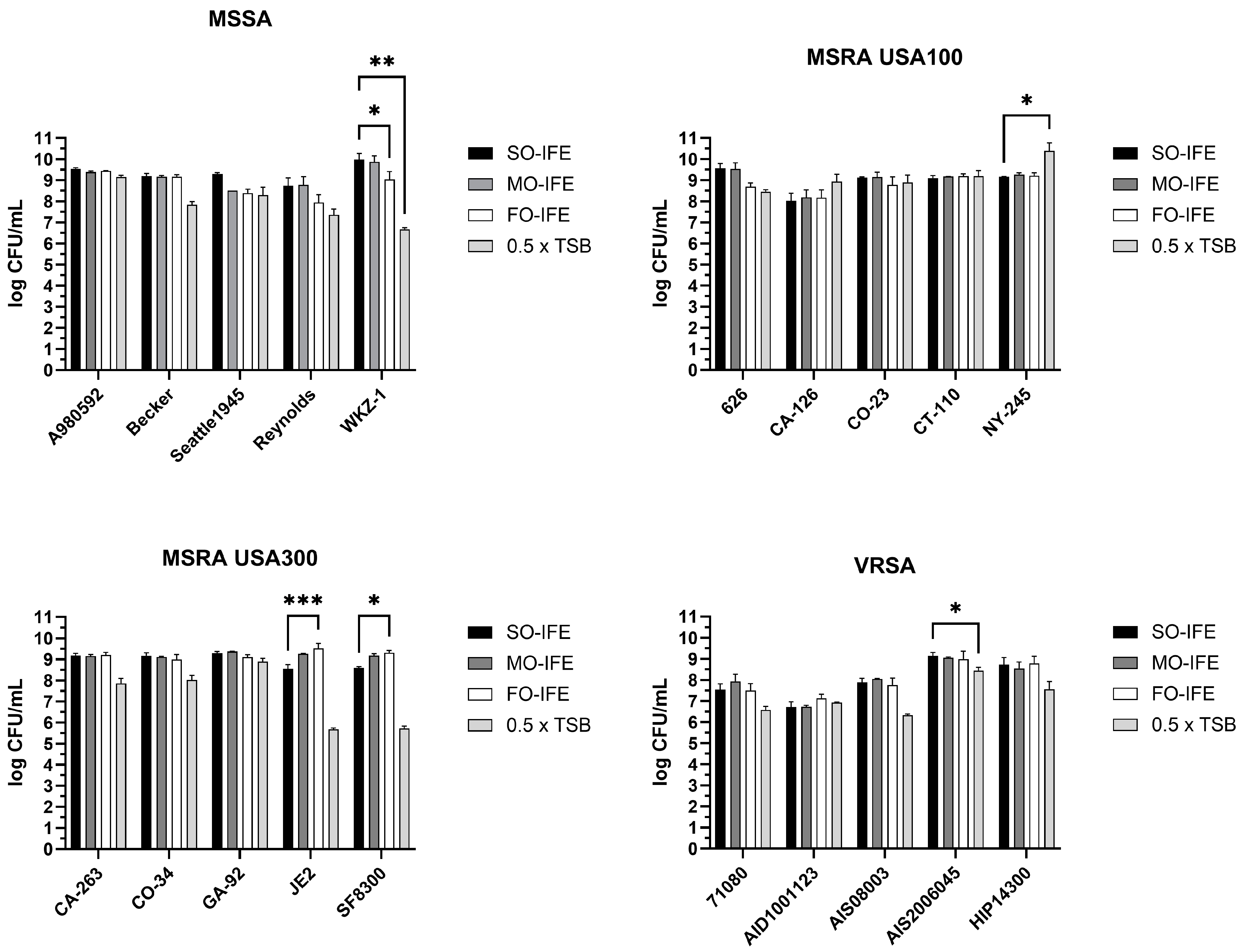
2.2. MO- and FO-IFE Do Not Inhibit Planktonic Growth of S. aureus
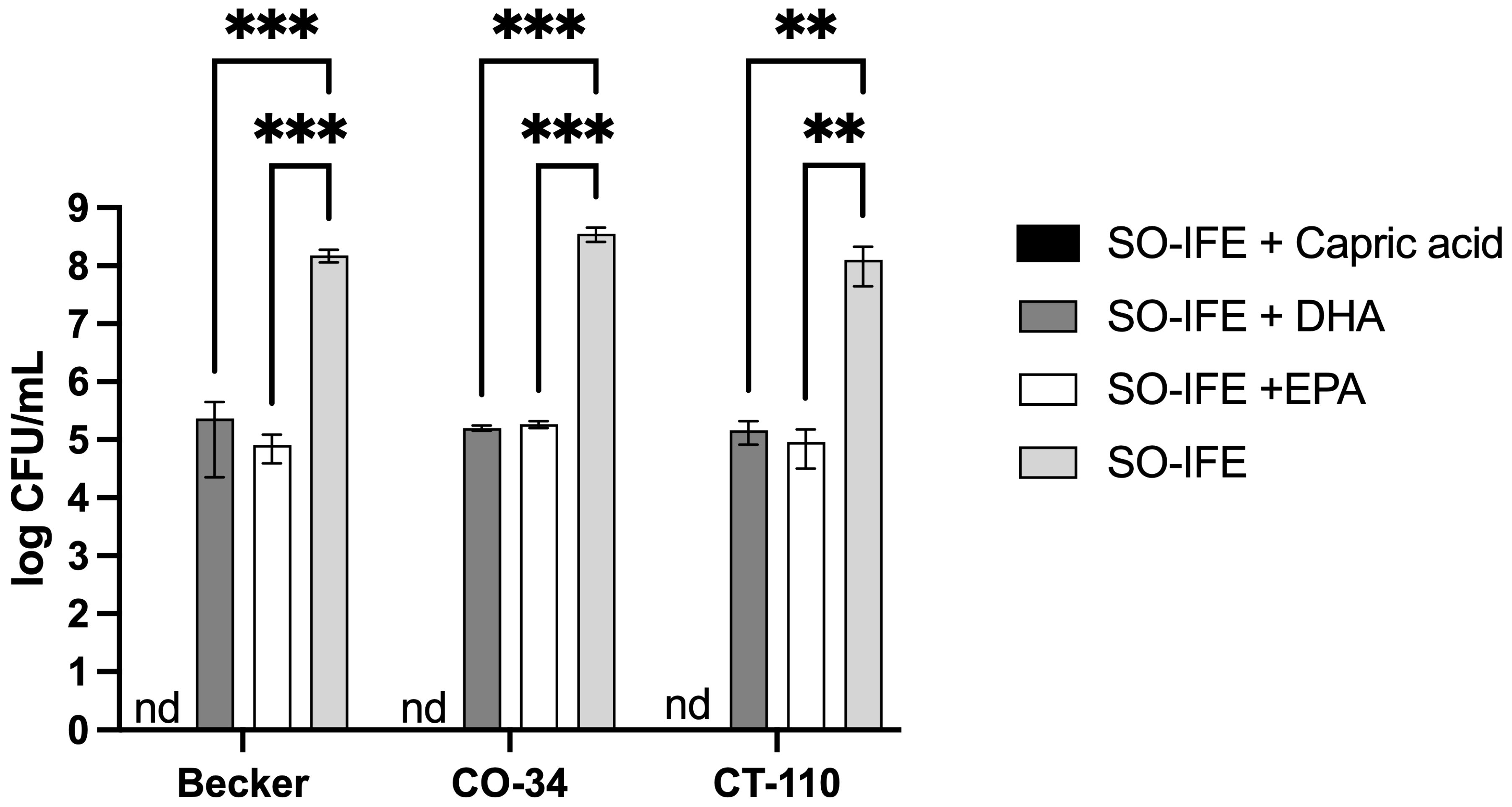
2.3. Fatty Acid Composition May Impact Biofilm Formation of S. aureus
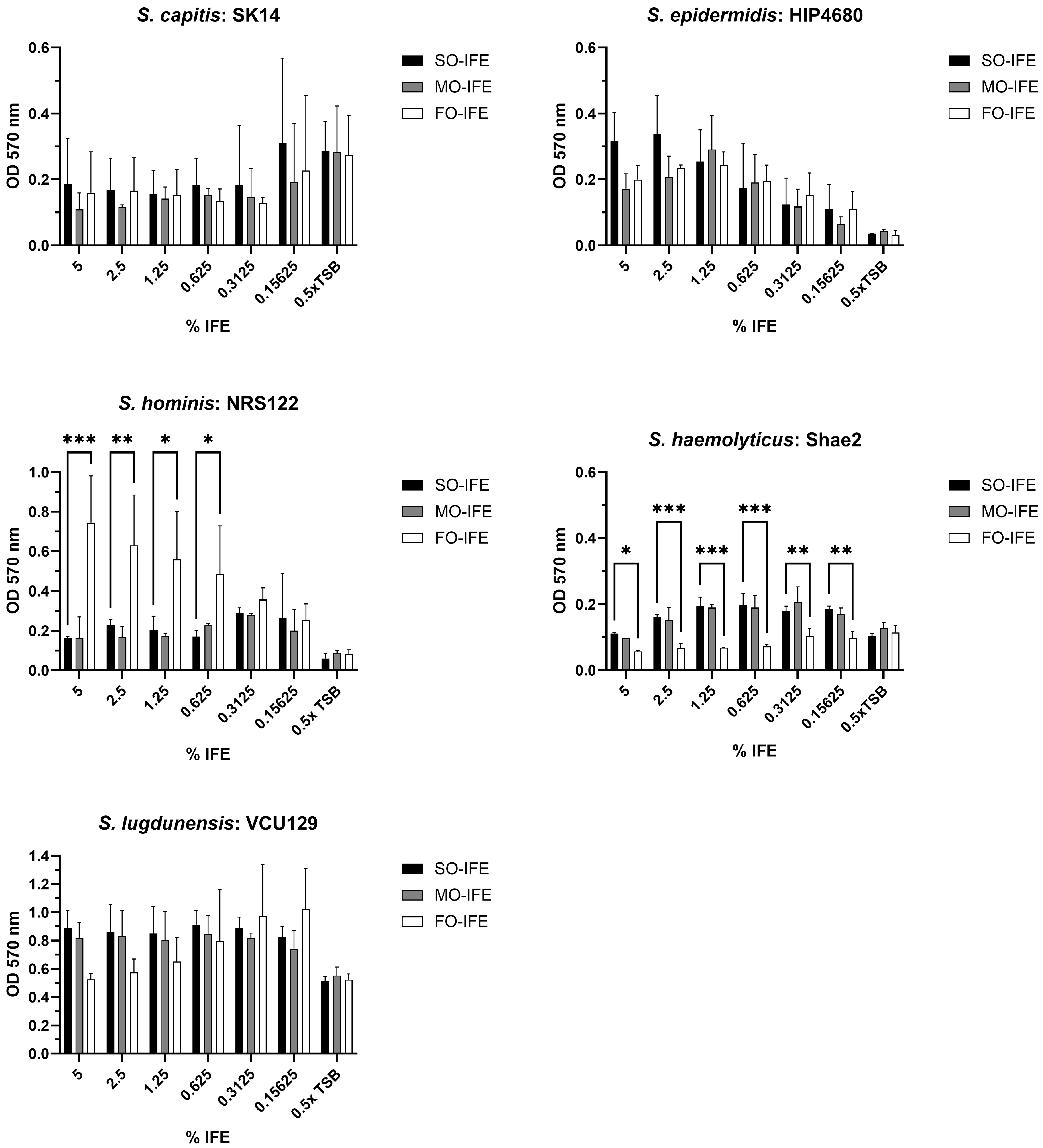
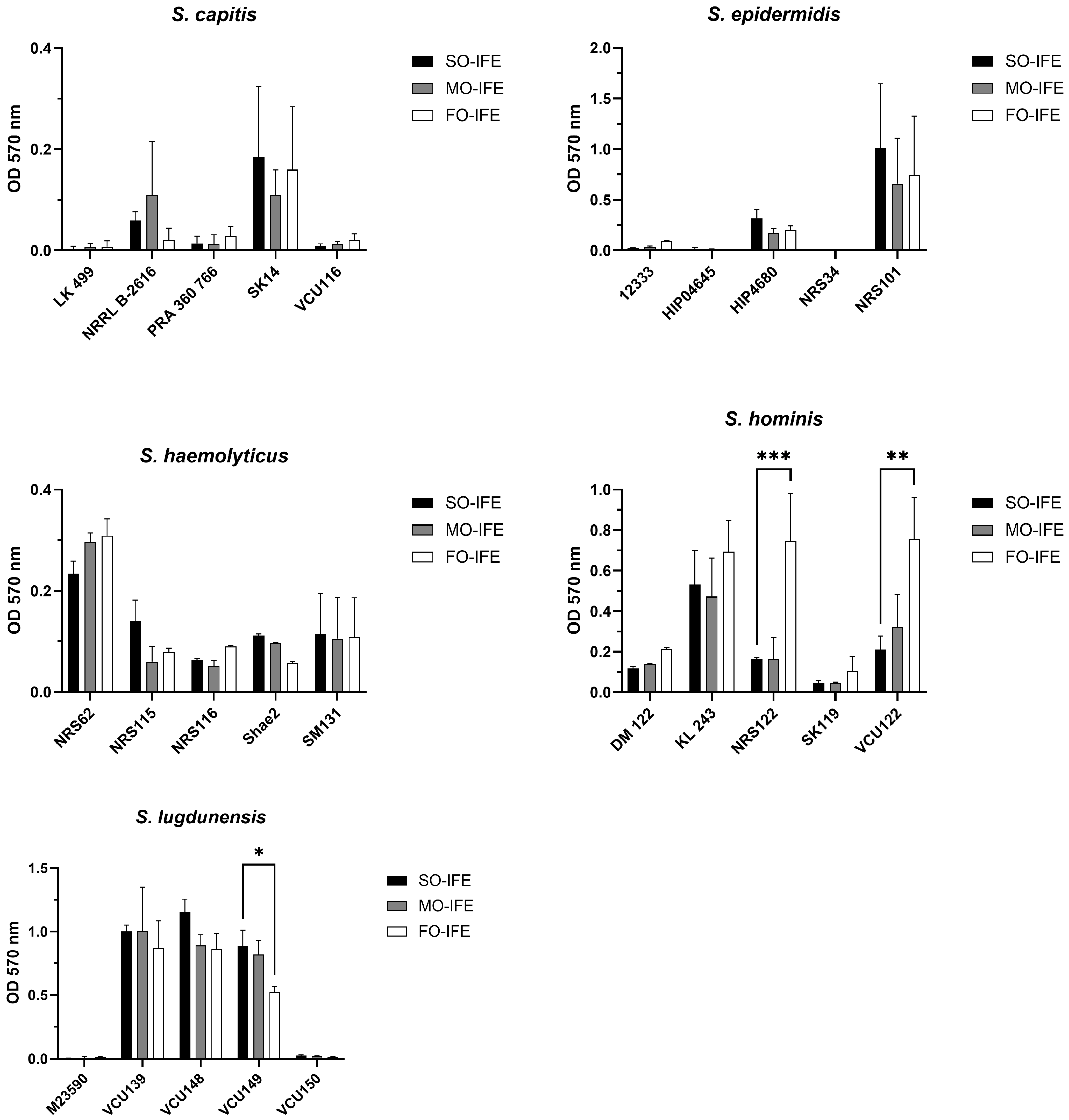
2.4. IFE Does Not Readily Enhance Biofilm Formation of Most CoNS
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture
4.2. Biofilm Formation
4.3. Biofilm Quantification with Crystal Violet Staining
4.4. Planktonic Growth and Quantitative Growth Assay
4.5. Biofilm and Planktonic Growth of S. aureus with Fatty Acid Supplementation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. Clinical Guidelines for the Use of Parenteral and Enteral Nutrition in Adult and Pediatric Patients, 2009. JPEN J. Parenter. Enteral Nutr. 2009, 33, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enteral Nutr. 2017, 41, 706–742. [Google Scholar] [CrossRef]
- Advani, S.; Reich, N.G.; Sengupta, A.; Gosey, L.; Milstone, A.M. Central Line-Associated Bloodstream Infection in Hospitalized Children with Peripherally Inserted Central Venous Catheters: Extending Risk Analyses Outside the Intensive Care Unit. Clin. Infect. Dis. 2011, 52, 1108–1115. [Google Scholar] [CrossRef]
- Fonseca, G.; Burgermaster, M.; Larson, E.; Seres, D.S. The Relationship Between Parenteral Nutrition and Central Line-Associated Bloodstream Infections: 2009–2014. JPEN J. Parenter. Enteral Nutr. 2018, 42, 171–175. [Google Scholar] [CrossRef]
- Netto, R.; Mondini, M.; Pezzella, C.; Romani, L.; Lucignano, B.; Pansani, L.; D’Argenio, P.; Cogo, P. Parenteral nutrition is one of the most significant risk factors for nosocomial infections in a pediatric cardiac intensive care unit. JPEN J. Parenter. Enteral Nutr. 2017, 41, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.A.; Sherertz, R.J.; Warren, D.K. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Novosad, S.A.; Fike, L.; Dudeck, M.A.; Allen-Bridson, K.; Edwards, J.R.; Edens, C.; Sinkowitz-Cochran, R.; Powell, K.; Kuhar, D. Pathogens causing central-line-associated bloodstream infections in acute-care hospitals-United States, 2011–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 313–319. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Freeman, J.; Goldman, D.A.; Smith, N.E.; Sidebottom, D.G.; Epstein, M.F.; Platt, R. Association of Intravenous Lipid Emulsion and Coagulase-Negative Bacteremia in Neonatal Intensive Care Units. N. Engl. J. Med. 1990, 323, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Langevin, P.B.; Gravenstein, N.; Doyle, T.J.; Roberts, S.A.; Skinner, S.; Langevin, S.O.; Gulig, P.A. Growth of Staphylococcus aureus in Diprivan and Intralipid. Anesthesiology 1991, 91, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Saiman, L.; Ludington, E.; Pfaller, M.; Rangel-Frausto, S.; Wiblin, T.R.; Dawson, J.; Blumberg, H.M.; Patterson, J.E.; Rinaldi, M.; Edwards, J.E.; et al. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr. Infect. Dis. J. 2000, 19, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Swindell, K.; Lattif, A.A.; Chandra, J.; Mukherjee, P.K.; Ghannoun, M.A. Parenteral Lipid Emulsion Induces Germination of Candida albicans and Increases Biofilm Formation on Medical Catheters Surfaces. Infect. Dis. J. 2009, 200, 473–480. [Google Scholar] [CrossRef]
- Benjamin, D.K., Jr.; Stoll, B.J.; Gantz, M.G.; Walsh, M.C.; Sánchez, P.J.; Das, A.; Shankaran, S.; Higgins, R.D.; Auten, K.J.; Miller, N.A.; et al. Neonatal candidiasis: Epidemiology, risk factors, and clinical judgment. Pediatrics 2010, 126, e865–e873. [Google Scholar] [CrossRef]
- Fell, G.L.; Nandivada, P.; Gura, K.M.; Puder, M. Intravenous Lipid Emulsions in Parenteral Nutrition. Adv. Nutr. 2015, 6, 600–610. [Google Scholar] [CrossRef]
- Anez-Bustillos, L.; Dao, D.T.; Fell, G.L.; Puder, M.; Gura, K.M. Intravenous Fat Emulsions for the Adult and Pediatric Patient: Understanding the Differences. Nutr. Clin. Pract. 2016, 31, 596–609. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Stultz, J.S.; Coltrane, M.E.; Fortwendel, J.P.; Peters, B.M. Disparate Candida albicans biofilm formation in clinical lipid emulsions due to capric acid mediated inhibition. Antimicrob. Agents Chemother. 2019, 63, e01394-19. [Google Scholar] [CrossRef]
- Alvira-Arill, G.R.; Willems, H.M.E.; Fortwendel, J.P.; Yarbrough, A.; Tansmore, J.; Sierra, C.M.; Bashqoy, F.; Stultz, J.S.; Peters, B.M. Impact of Intravenous Fat Emulsion Choice on Candida Biofilm, Hyphal Growth, and Catheter-Related Bloodstream Infections in Pediatric Patients. Infect. Dis. J. 2024, 229, 588–598. [Google Scholar] [CrossRef]
- Alvira-Arill, G.R.; Herrera, O.R.; Tsang, C.C.S.; Wang, J.; Peters, B.M.; Stultz, J.S. Comparison of catheter-related bloodstream infection rates in pediatric patients receiving parenteral nutrition with soybean oil-based intravenous fat emulsion versus a mixed oil fat emulsion. Pharmacotherapy 2022, 42, 898–904. [Google Scholar] [CrossRef]
- Noble, R.M.N.; Salim, S.Y.; Walker, B.; Khadaroo, R.G.; Chiarella, A.B.; Gragasin, F.S.; Bourque, S.L. Survival of Staphylococcus epidermidis in Propofol and Intralipid in the Dead Space of Intravenous Injection Ports. Anesth. Analg. 2019, 129, e20–e22. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, J.H.; Raorane, C.J.; Oh, S.T.; Park, J.G.; Lee, J. Herring Oil and Omega Fatty Acids Inhibit Staphylococcus aureus Biofilm Formation and Virulence. Front. Microbiol. 2018, 9, 1241. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Rohde, M.; Molinari, G.; Stadler, M.; Abraham, W.R. Unsaturated Fatty Acids Control Biofilm Formation of Staphylococcus aureus and Other Gram-Positive Bacteria. Antibiotics 2020, 9, 788. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Lee, J. Inhibition of Staphylococcus aureus Biofilm Formation and Virulence Factor Production by Petroselinic Acid and Other Unsaturated C18 Fatty Acids. Microbiol. Spectr. 2022, 10, e0133022. [Google Scholar] [CrossRef]
- Trautner, B.W.; Darouiche, R.O. Catheter-associated infections: Pathogenesis affects prevention. Arch. Intern. Med. 2004, 164, 842–850. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.V.; Kanagaraja, A.; Sakthivelu, M.; Devadasan, V.; Gopinath, S.C.B.; Raman, P. Role of fatty acids in modulating quorum sensing in Pseudomonas aeruginosa and Chromobacterium violaceum: An integrated experimental and computational analysis. Int. Microbiol. 2024. [Google Scholar] [CrossRef]
- Nicol, M.; Alexandre, S.; Luizet, J.B.; Skogman, M.; Jouenne, T.; Salcedo, S.P.; Dé, E. Unsaturated Fatty Acids Affect Quorum Sensing Communication System and Inhibit Motility and Biofilm Formation of Acinetobacter baumannii. Int. J. Mol. Sci. 2018, 19, 214. [Google Scholar] [CrossRef]
- Romero, L.C.; Silva, L.P.; Teixeira, N.B.; de Camargo, K.V.; Del Masso Pereira, M.A.; Corrente, J.E.; Pereira, V.C.; de Lourdes Ribeiro de Souza da Cunha, M. Staphylococcus capitis Bloodstream Isolates: Investigation of Clonal Relationship, Resistance Profile, Virulence and Biofilm Formation. Antibiotics 2024, 13, 147. [Google Scholar] [CrossRef]
- Chavignon, M.; Coignet, L.; Bonhomme, M.; Bergot, M.; Tristan, A.; Verhoeven, P.; Josse, J.; Laurent, F.; Butin, M. Environmental Persistence of Staphylococcus capitis NRCS-A in Neonatal Intensive Care Units: Role of Biofilm Formation, Desiccation, and Disinfectant Tolerance. Microbiol. Spectr. 2022, 10, e0421522. [Google Scholar] [CrossRef]
- Fredheim, E.G.; Klingenberg, C.; Rohde, H.; Frankenberger, S.; Gaustad, P.; Flægstad, T.; Sollid, J.E. Biofilm formation by Staphylococcus haemolyticus. J. Clin. Microbiol. 2009, 47, 1172–1180. [Google Scholar] [CrossRef]
- Silva, P.V.; Cruz, R.S.; Keim, L.S.; de Paula, G.R.; Carvalho, B.T.F.; Coelho, L.R.; Carvalho, M.C.d.S.; da Rosa, J.M.C.; Figueiredo, A.M.S.; Teixeira, L.A. The antimicrobial susceptibility, biofilm formation and genotypic profiles of Staphylococcus haemolyticus from bloodstream infections. Mem. Inst. Oswaldo Cruz 2013, 108, 812–813. [Google Scholar] [CrossRef]
- Mendoza-Olazarán, S.; Morfin-Otero, R.; Rodríguez-Noriega, E.; Llaca-Díaz, J.; Flores-Treviño, S.; González-González, G.M.; Villarreal-Treviño, L.; Garza-González, E. Microbiological and molecular characterization of Staphylococcus hominis isolates from blood. PLoS ONE 2013, 8, e61161. [Google Scholar] [CrossRef] [PubMed]
- Szczuka, E.; Telega, K.; Kaznowski, A. Biofilm formation by Staphylococcus hominis strains isolated from human clinical specimens. Folia Microbiol. 2015, 60, 1–5. [Google Scholar] [CrossRef]
- Tseng, S.P.; Lin, Y.T.; Tsai, J.C.; Hung, W.-C.; Chen, H.-J.; Chen, P.-F.; Hsueh, P.-R.; Teng, L.-J. Genotypes and phenotypes of Staphylococcus lugdunensis isolates recovered from bacteremia. J. Microbiol. Immunol. Infect. 2015, 48, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Fouts, D.E.; Archer, G.L.; Mongodin, E.F.; DeBoy, R.T.; Ravel, J.; Paulsen, I.T.; Kolonay, J.F.; Brinkac, L.; Beanan, M.; et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 2005, 187, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef]
- Shanks, R.M.; Sargent, J.L.; Martinez, R.M.; Graber, M.L.; O’Toole, G.A. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol. Dial. Transplant. 2006, 21, 2247–2255. [Google Scholar] [CrossRef]
- Peters, B.M.; Ward, R.M.; Rane, H.S.; Lee, S.A.; Noverr, M.C. Efficacy of ethanol against Candida albicans and Staphylococcus aureus polymicrobial biofilms. Antimicrob. Agents Chemother. 2013, 57, 74–82. [Google Scholar] [CrossRef]
- Sieuwerts, S.; de Bok, F.A.; Mols, E.; de vos, W.M.; Vlieg, J.E. A simple and fast method for determining colony forming units. Lett. Appl. Microbiol. 2008, 47, 275–278. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvira-Arill, G.R.; Herrera, O.R.; Stultz, J.S.; Peters, B.M. Heterogeneity of Biofilm Formation Among Staphylococcus aureus and Coagulase-Negative Staphylococcus Species in Clinically Relevant Intravenous Fat Emulsions. Antibiotics 2025, 14, 484. https://doi.org/10.3390/antibiotics14050484
Alvira-Arill GR, Herrera OR, Stultz JS, Peters BM. Heterogeneity of Biofilm Formation Among Staphylococcus aureus and Coagulase-Negative Staphylococcus Species in Clinically Relevant Intravenous Fat Emulsions. Antibiotics. 2025; 14(5):484. https://doi.org/10.3390/antibiotics14050484
Chicago/Turabian StyleAlvira-Arill, Gustavo R., Oscar R. Herrera, Jeremy S. Stultz, and Brian M. Peters. 2025. "Heterogeneity of Biofilm Formation Among Staphylococcus aureus and Coagulase-Negative Staphylococcus Species in Clinically Relevant Intravenous Fat Emulsions" Antibiotics 14, no. 5: 484. https://doi.org/10.3390/antibiotics14050484
APA StyleAlvira-Arill, G. R., Herrera, O. R., Stultz, J. S., & Peters, B. M. (2025). Heterogeneity of Biofilm Formation Among Staphylococcus aureus and Coagulase-Negative Staphylococcus Species in Clinically Relevant Intravenous Fat Emulsions. Antibiotics, 14(5), 484. https://doi.org/10.3390/antibiotics14050484





