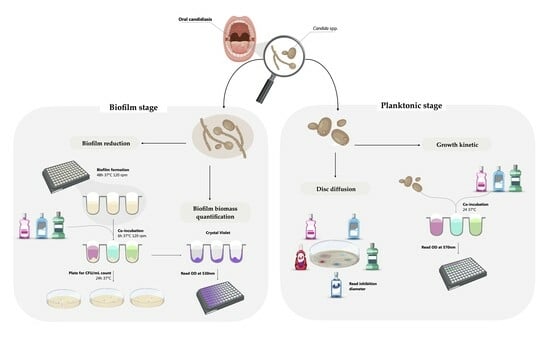
Repurposing Mouthwashes: Antifungal and Antibiofilm Abilities of Commercially Available Mouthwashes Against Candida spp.
Abstract
1. Introduction
2. Results
2.1. Mouthwash Composition
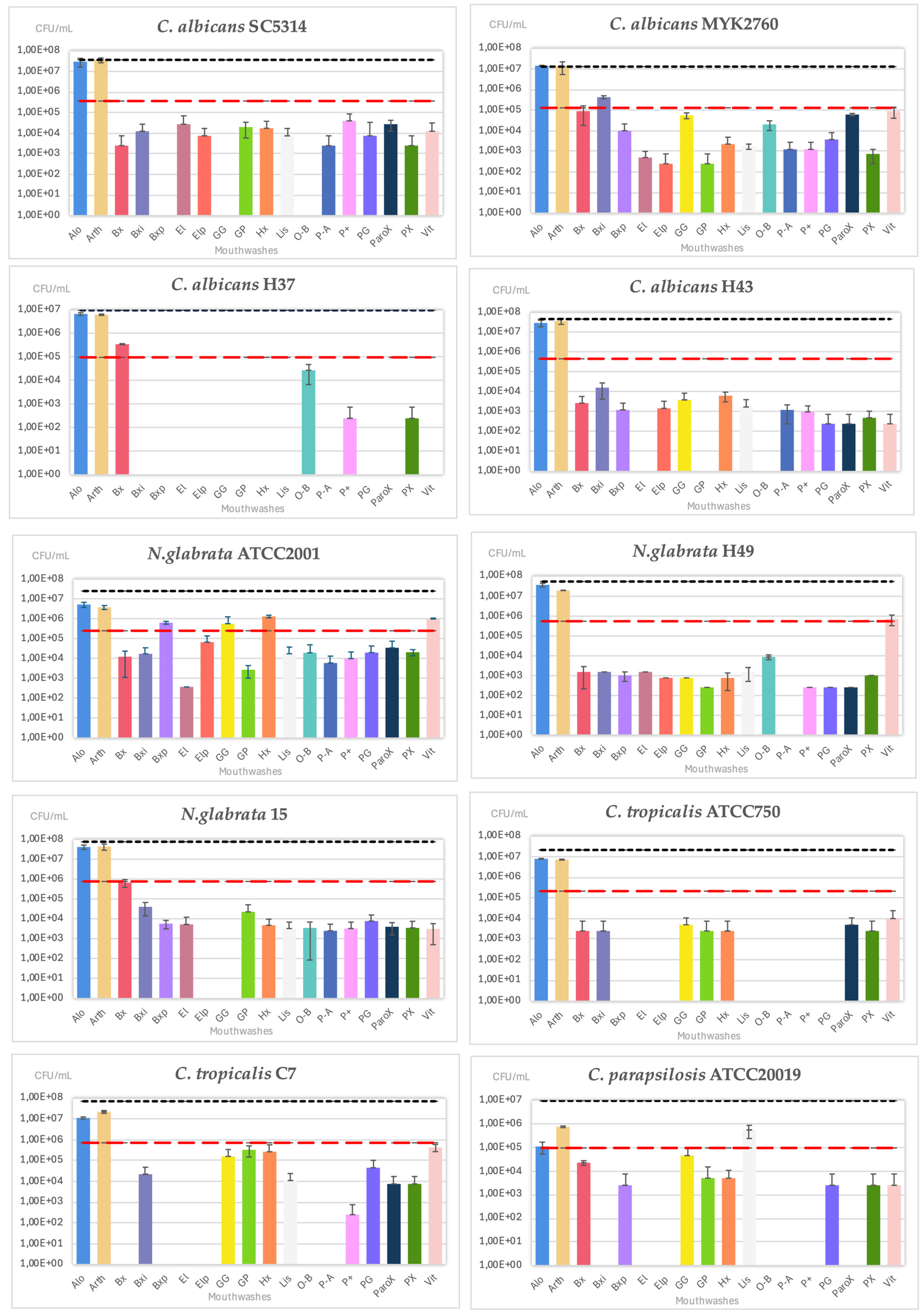
2.2. Antifungal Susceptibility
2.2.1. Disc Diffusion Assay
2.2.2. Growth Kinetics Assay
2.3. Biofilm Assays
2.3.1. Minimal Biofilm Eradication Concentration
2.3.2. Biofilm Biomass Quantification
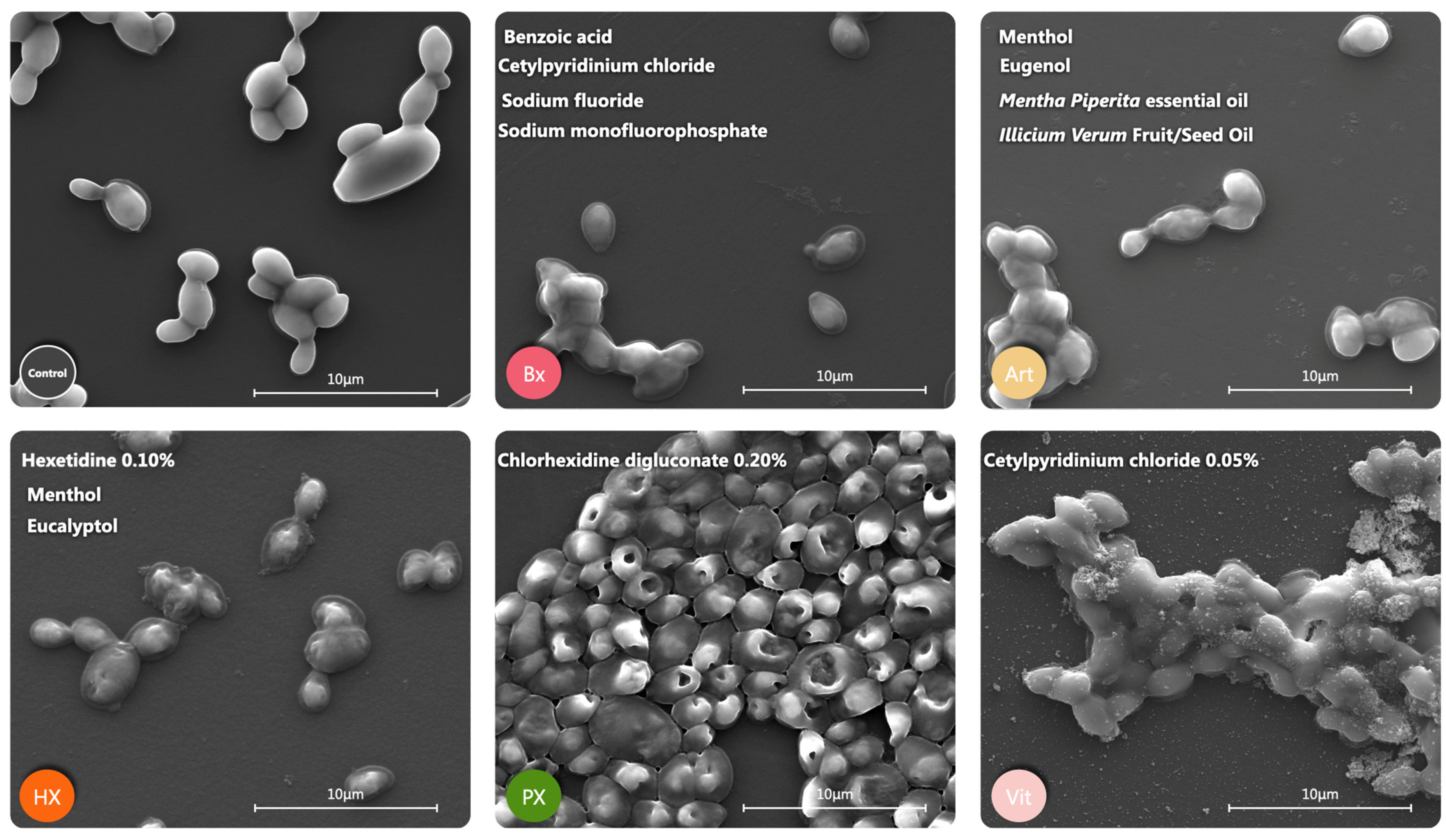
2.4. Morphological Alterations
3. Discussion
4. Materials and Methods
4.1. Organisms and Growth Conditions
4.2. Mouthwash Selection
4.3. Antifungal Susceptibility Testing (AFST)
4.3.1. Disc Diffusion Assay
4.3.2. Growth Kinetics in Planktonic Candida spp.
4.4. Biofilm Assays
4.4.1. Biofilm Reduction
4.4.2. Biofilm Biomass Quantification (Cristal Violet Assay)
4.5. Morphological Alterations
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alo | Alodont |
| Art | Arthrodont |
| BSE | Backscattered Electron |
| Bx | Bexident Gums Daily use |
| Bxi | Bexident intensive gums 0.12% |
| Bxp | Bexident Post 0.20% |
| CHX | Chlorhexidine digluconate |
| CPC | Cetylpyridinium chloride |
| CV | Crystal violet |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| El | Eludril Classic |
| Elp | EluPerio |
| GG | Gum Gingidex 0.06% + CPC |
| GP | Gum Paroex 0.12% + CPC |
| Hx | Hextril 0.10% |
| Lis | Listerine Cool Mint |
| MBEC | Minimal Biofilm Eradication Concentration |
| OD | Optical Density |
| O-B | Oral-B Pro-expert Professional Protection |
| PX | Parodontax Extra 0.20% |
| ParoX | Paroex 0.12% |
| P-A | Perio-Aid Intensive Care |
| P+ | PerioPlus Curaprox |
| PG | Periogard |
| SE | Secondary Electron |
| SEM | Scanning electron microscopy |
| Vit | Vitis Gingival Mouthwash |
Appendix A
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 113.2% | 46.4% | 17.2% | 50% | No |
 | 14.7% | 71.8% | 20.6% | 12.5% | Yes |
 | 21.0% | 14.4% | 15.0% | 25% | No |
 | 8.0% | 23.3% | 21.4% | 12.5% | Yes |
 | −72.0% | 8.7% | 12.5% | 12.5% | Yes |
 | −17.0% | −59.6% | 4.1% | 25% | Yes |
 | 14.7% | −8.3% | −20.5% | 50% | No |
 | 0.8% | 4.0% | 4.7% | 12.5% | Yes |
 | −19.8% | −7.9% | −6.4% | 12.5% | Yes |
 | 37.8% | −23.1% | −14.1% | 25% | Yes |
 | 4.8% | 6.6% | 3.2% | 50% | No |
 | 11.5% | 2.7% | 7.8% | 25% | No |
 | 14.1% | 1.9% | 0.4% | 50% | No |
 | 14.4% | 2.4% | 0.9% | 50% | No |
 | −2.2% | 1.8% | 0.5% | 12.5% | Yes |
 | 11.7% | 1.1% | −13.3% | 50% | No |
 | 5.2% | 4.4% | 1.3% | 50% | No |
 | 0.0% | 7.6% | 3.7% | 12.5% | Yes |
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 97.0% | 45.4% | 12.6% | 50.0% | No |
 | 89.2% | 53.4% | 12.0% | 50.0% | No |
 | 11.4% | 12.9% | 12.0% | 12.5% | Yes |
 | 22.2% | 17.3% | 16.8% | 50.0% | No |
 | 5.0% | 6.2% | 8.6% | 12.5% | Yes |
 | −52.0% | −43.5% | 4.2% | 12.5% | Yes |
 | −10.8% | −9.2% | −16.6% | 50.0% | No |
 | 16.0% | 7.7% | 1.0% | 50.0% | No |
 | 5.6% | 1.5% | 0.7% | 50.0% | No |
 | −14.5% | −12.6% | −11.7% | 12.5% | Yes |
 | 30.3% | 5.8% | 10.6% | 25.0% | No |
 | 2.3% | 1.9% | 8.1% | 25.0% | Yes |
 | 7.2% | 5.7% | 0.8% | 50.0% | No |
 | 15.3% | 10.9% | 0.1% | 50.0% | No |
 | 15.9% | 6.6% | 0.3% | 50.0% | No |
 | 6.0% | 3.3% | −9.1% | 50.0% | No |
 | 10.5% | 7.6% | 5.7% | 50.0% | No |
 | 10.3% | 2.1% | −1.3% | 50.0% | No |
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 35.1% | 9.2% | 2.6% | 50.0% | No |
 | 143.2% | 115.8% | 10.7% | 50.0% | No |
 | 9.8% | 11.6% | 22.4% | 12.5% | Yes |
 | 15.5% | 12.8% | 21.9% | 25.0% | Yes |
 | 7.6% | 10.7% | 8.5% | 12.5% | Yes |
 | −77.3% | −63.2% | −16.4% | 12.5% | Yes |
 | −37.4% | −31.6% | −28.4% | 12.5% | Yes |
 | 10.0% | 5.3% | 2.0% | 50.0% | No |
 | −10.6% | −9.2% | −7.8% | 12.5% | Yes |
 | −42.3% | −34.0% | −26.2% | 12.5% | Yes |
 | 45.2% | 6.6% | 2.2% | 50.0% | No |
 | 4.7% | 5.2% | 1.6% | 50.0% | No |
 | 5.5% | 5.8% | 0.6% | 50.0% | No |
 | 6.4% | 5.6% | 0.5% | 50.0% | No |
 | 5.9% | 5.3% | 1.9% | 50.0% | No |
 | −22.3% | −18.0% | −16.2% | 12.5% | Yes |
 | 6.3% | 4.7% | 9.5% | 25.0% | Yes |
 | 1.7% | 1.9% | 11.3% | 12.5% | Yes |
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 9.7% | 8.4% | 2.4% | 50.0% | No |
 | 140.7% | 103.0% | 10.1% | 50.0% | No |
 | 7.5% | 7.5% | 18.3% | 25.0% | Yes |
 | 14.7% | 15.6% | 19.9% | 12.5% | Yes |
 | 9.3% | 10.3% | 7.8% | 50.0% | No |
 | −80.5% | −72.1% | −16.8% | 12.5% | Yes |
 | −33.4% | −26.8% | −21.5% | 12.5% | Yes |
 | 4.6% | 3.8% | 0.9% | 50.0% | No |
 | −11.7% | −10.5% | −6.7% | 12.5% | Yes |
 | −44.4% | −37.2% | −29.8% | 12.5% | Yes |
 | 35.1% | 14.7% | 25.3% | 25.0% | No |
 | −33.5% | −34.4% | 28.2% | 25.0% | Yes |
 | 7.5% | 5.4% | 1.9% | 50.0% | No |
 | 7.2% | 5.8% | 1.5% | 50.0% | No |
 | 5.4% | 4.4% | 1.6% | 50.0% | No |
 | −23.0% | −16.3% | −15.2% | 12.5% | Yes |
 | 9.2% | 4.9% | 2.4% | 50.0% | No |
 | 0.9% | 1.0% | −0.9% | 50.0% | No |
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 241.5% | 101.9% | 3.1% | 50.0% | No |
 | 70.7% | 13.6% | 0.0% | 50.0% | No |
 | 16.4% | 5.3% | 3.4% | 50.0% | No |
 | 8.3% | 7.4% | 5.1% | 50.0% | No |
 | 15.1% | 16.8% | 18.4% | 12.5% | Yes |
 | −162.6% | −141.7% | 82.8% | 12.5% | Yes |
 | −83.8% | −72.3% | −51.7% | 12.5% | Yes |
 | 12.0% | 12.8% | 4.3% | 50.0% | No |
 | −18.3% | −17.8% | −14.0% | 12.5% | Yes |
 | −81.1% | −71.4% | −49.8% | NAO | Yes |
 | 42.2% | 19.0% | 1.9% | 50.0% | No |
 | 10.3% | 9.2% | 2.8% | 50.0% | No |
 | 10.2% | 8.1% | 3.8% | 50.0% | No |
 | 7.3% | 7.8% | 1.9% | 50.0% | No |
 | 8.6% | 6.4% | 2.8% | 50.0% | No |
 | −54.5% | −45.4% | −32.2% | 12.5% | Yes |
 | 10.1% | 8.7% | 3.7% | 50.0% | No |
 | 3.7% | 3.1% | −2.3% | 50.0% | No |
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 5.9% | 4.4% | 3.5% | 50.0% | No |
 | 136.8% | 66.3% | 0.1% | 50.0% | No |
 | 4.8% | 3.5% | 3.8% | 25.0% | No |
 | 5.3% | 5.4% | 4.8% | 50.0% | No |
 | 12.5% | 13.5% | 8.6% | 50.0% | No |
 | −80.7% | −72.8% | 40.3% | 12.5% | Yes |
 | −67.6% | −56.7% | −40.1% | 12.5% | Yes |
 | 6.2% | 6.0% | 3.6% | 50.0% | No |
 | −18.3% | −14.1% | −11.6% | 12.5% | Yes |
 | −64.9% | −55.3% | −36.9% | 12.5% | Yes |
 | 48.3% | 12.0% | 1.2% | 50.0% | No |
 | 7.3% | 7.1% | 2.7% | 50.0% | No |
 | 8.8% | 21.8% | 2.3% | 50.0% | No |
 | 4.8% | 75.2% | 2.4% | 50.0% | No |
 | 5.4% | 4.3% | 0.9% | 50.0% | No |
 | −38.5% | −32.4% | −22.5% | 12.5% | Yes |
 | 7.5% | 5.1% | 2.8% | 50.0% | No |
 | 1.5% | 0.2% | −2.2% | 50.0% | No |
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 6.2% | 2.4% | −0.7% | 50.0% | No |
 | 49.0% | 24.1% | 0.7% | 50.0% | No |
 | 1.7% | 0.7% | −0.3% | 50.0% | No |
 | 4.0% | 3.6% | 2.7% | 50.0% | No |
 | 5.3% | 6.9% | 4.3% | 50.0% | No |
 | −6.1% | 3.0% | 51.4% | 12.5% | Yes |
 | −21.6% | −13.5% | 4.2% | 12.5% | Yes |
 | 4.4% | 5.0% | 2.4% | 50.0% | No |
 | −3.4% | 4.3% | 16.7% | 12.5% | Yes |
 | −19.8% | −11.5% | 5.3% | 12.5% | Yes |
 | 30.6% | 9.4% | 2.2% | 50.0% | No |
 | 5.5% | 5.4% | 2.4% | 50.0% | No |
 | 3.3% | 3.0% | −0.7% | 50.0% | No |
 | 2.8% | 3.4% | −0.5% | 50.0% | No |
 | 1.7% | 1.2% | 0.4% | 50.0% | No |
 | −12.4% | −7.8% | 4.3% | 12.5% | Yes |
 | 4.8% | 3.9% | 2.9% | 50.0% | No |
 | 4.2% | 7.7% | 0.7% | 50.0% | No |
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 73.1% | 22.2% | 31.7% | 25.0% | No |
 | 114.3% | 93.2% | 29.1% | 50.0% | No |
 | 13.1% | 15.4% | 18.7% | 12.5% | Yes |
 | 10.5% | 17.0% | 6.3% | 50.0% | No |
 | 8.4% | 9.1% | 19.0% | 12.5% | Yes |
 | −87.4% | −59.7% | −5.5% | 12.5% | Yes |
 | −50.0% | −43.1% | −32.2% | 12.5% | Yes |
 | 6.7% | 3.3% | 2.0% | 50.0% | No |
 | −9.6% | −11.3% | −6.5% | 25.0% | Yes |
 | −38.8% | −44.2% | −22.7% | 25.0% | Yes |
 | 60.2% | 11.1% | 7.9% | 50.0% | No |
 | 5.1% | 2.7% | 4.4% | 25.0% | No |
 | 4.4% | 4.0% | 14.9% | 25.0% | Yes |
 | 1.4% | 2.8% | 1.3% | 50.0% | No |
 | 8.0% | 2.9% | 1.4% | 50.0% | No |
 | −22.0% | −24.7% | −5.8% | 25.0% | Yes |
 | 6.0% | 3.9% | 3.1% | 50.0% | No |
 | 3.9% | −1.0% | 4.2% | 25.0% | Yes |
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 10.0% | 16.0% | 1.4% | 50.0% | No |
 | 92.2% | 65.0% | 2.2% | 50.0% | No |
 | 2.2% | 1.3% | 1.3% | 25.0% | No |
 | 3.0% | 1.9% | 1.8% | 50.0% | No |
 | 4.8% | 5.5% | 3.0% | 50.0% | No |
 | −55.4% | −47.6% | 34.9% | 12.5% | Yes |
 | −38.0% | −33.4% | −22.9% | 12.5% | Yes |
 | 3.9% | 3.6% | 1.8% | 50.0% | No |
 | −10.4% | −8.4% | −5.8% | 12.5% | Yes |
 | −36.7% | −32.3% | −21.6% | 12.5% | Yes |
 | 30.6% | 8.3% | 0.5% | 50.0% | No |
 | 2.7% | 2.6% | 0.8% | 50.0% | No |
 | 4.2% | 2.7% | 0.8% | 50.0% | No |
 | 2.9% | 4.3% | 0.8% | 50.0% | No |
 | 4.0% | 1.8% | 0.8% | 50.0% | No |
 | −26.6% | −23.8% | −17.6% | 12.5% | Yes |
 | 4.7% | 2.5% | 35.7% | 25.0% | Yes |
 | −0.5% | −0.2% | 29.3% | 12.5% | Yes |
References
- Porta, E.O.J.; Kalesh, K.; Steel, P.G. Navigating Drug Repurposing for Chagas Disease: Advances, Challenges, and Opportunities. Front. Pharmacol. 2023, 14, 1233253. [Google Scholar] [CrossRef]
- Sleigh, S.H.; Barton, C.L. Repurposing Strategies for Therapeutics. Pharm. Med. 2010, 24, 151–159. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Sansonetty, F.; Rodrigues, A.G.; Martinez De Oliveira, J.; Fonseca, A.F.; Mårdh, P.A. Antifungal Activity of Ibuprofen Alone and in Combination with Fluconazole against Candida Species. J. Med. Microbiol. 2000, 49, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Babaei, F.; Mirzababaei, M.; Tavakkoli, A.; Nassiri-Asl, M.; Hosseinzadeh, H. Can Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Be Repurposed for Fungal Infection? Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 59–75. [Google Scholar] [CrossRef]
- Dolan, K.; Montgomery, S.; Buchheit, B.; DiDone, L.; Wellington, M.; Krysan, D.J. Antifungal Activity of Tamoxifen: In Vitro and In Vivo Activities and Mechanistic Characterization. Antimicrob. Agents Chemother. 2009, 53, 3337–3346. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, M.; Konarzewska, P.; Roberge, J.Y.; Han, G.-S.; Wang, Y.; Carman, G.M.; Xue, C. The Anticancer Drug Bleomycin Shows Potent Antifungal Activity by Altering Phospholipid Biosynthesis. Microbiol. Spectr. 2022, 10, e0086222. [Google Scholar] [CrossRef]
- Kaur, J.; Nobile, C.J. Antifungal Drug-Resistance Mechanisms in Candida Biofilms. Curr. Opin. Microbiol. 2023, 71, 102237. [Google Scholar] [CrossRef]
- Andrade, J.C.; Kumar, S.; Kumar, A.; Černáková, L.; Rodrigues, C.F. Application of Probiotics in Candidiasis Management. Crit. Rev. Food Sci. Nutr. 2022, 62, 8249–8264. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 24 April 2024).
- Muzyka, B.C.; Epifanio, R.N. Update on Oral Fungal Infections. Dent. Clin. N. Am. 2013, 57, 561–581. [Google Scholar] [CrossRef]
- Lu, S.-Y. Oral Candidosis: Pathophysiology and Best Practice for Diagnosis, Classification, and Successful Management. J. Fungi 2021, 7, 555. [Google Scholar] [CrossRef]
- Borman, A.M.; Johnson, E.M. Name Changes for Fungi of Medical Importance, 2018 to 2019. J. Clin. Microbiol. 2021, 59, e01811-20. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.M.C.V.; Lopes, B.O.; de Leite, A.C.R.M.; Cruz, G.S.; de Brito, É.H.S.; de Lima, L.F.; Černáková, L.; Azevedo, N.F.; Rodrigues, C.F. Characterization of Oral Candida spp. Biofilms in Children and Adults Carriers from Eastern Europe and South America. Antibiotics 2023, 12, 797. [Google Scholar] [CrossRef]
- Rodrigues, C.; Alves, D.; Henriques, M. Combination of Posaconazole and Amphotericin B in the Treatment of Candida Glabrata Biofilms. Microorganisms 2018, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, S.R.; Parker, J.K.; Khutoryanskiy, V.V. Oral Care Product Formulations, Properties and Challenges. Colloids Surf. B Biointerfaces 2021, 200, 111567. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; McCullough, M.; Kumar, P.; McGrath, C. Mouthwashes: Implications for Practice. Int. Dent. J. 2023, 73, S98–S101. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Dotta, T.C.; Cellini, L.; Iezzi, G.; D’Ercole, S.; Petrini, M. The Antibacterial and Antifungal Capacity of Eight Commercially Available Types of Mouthwash against Oral Microorganisms: An In Vitro Study. Antibiotics 2023, 12, 675. [Google Scholar] [CrossRef]
- Aoun, G.; Cassia, A.; Berberi, A. Effectiveness of a Chlorhexidine Digluconate 0.12% and Cetylpyridinium Chloride 0.05% Solution in Eliminating Candida Albicans Colonizing Dentures: A Randomized Clinical in Vivo Study. J. Contemp. Dent. Pract. 2015, 16, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Susceptibility of Candida Glabrata Biofilms to Echinocandins: Alterations in the Matrix Composition. Biofouling 2018, 34, 569–578. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Perlin, D.S. Echinocandin Resistance, Susceptibility Testing and Prophylaxis: Implications for Patient Management. Drugs 2014, 74, 1573–1585. [Google Scholar] [CrossRef]
- Gil-Alonso, S.; Jauregizar, N.; Ortega, I.; Eraso, E.; Suárez, E.; Quindós, G. In Vitro Pharmacodynamic Modelling of Anidulafungin against Candida spp. Int. J. Antimicrob. Agents 2016, 47, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Maziere, M.; Rompante, P.; Andrade, J.C.; Rodrigues, C.F. Are Mouthwashes Really Effective against Candida spp.? J. Fungi 2024, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020, 64, 8. [Google Scholar] [CrossRef]
- da Nóbrega Alves, D.; Monteiro, A.F.M.; Andrade, P.N.; Lazarini, J.G.; Abílio, G.M.F.; Guerra, F.Q.S.; Scotti, M.T.; Scotti, L.; Rosalen, P.L.; de Castro, R.D. Docking Prediction, Antifungal Activity, Anti-Biofilm Effects on Candida spp., and Toxicity against Human Cells of Cinnamaldehyde. Molecules 2020, 25, 5969. [Google Scholar] [CrossRef]
- Rachel, R.; Anuradha, M.; Leela, K.V. Evaluating the Antifungal Potential of Cinnamaldehyde: A Study of Its Efficacy against Candida Species. J. Pure Appl. Microbiol. 2024, 18, 2438–2445. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Carnero-Gregorio, M.; López-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; González-Cespón, J.L. Pathogenesis and Clinical Relevance of Candida Biofilms in Vulvovaginal Candidiasis. Front. Microbiol. 2020, 11, 544480. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.; Vila, T.; Romo, J.; Montelongo-Jauregui, D.; Wall, G.; Ramasubramanian, A.; Lopez-Ribot, J. The Candida Albicans Biofilm Matrix: Composition, Structure and Function. J. Fungi 2017, 3, 14. [Google Scholar] [CrossRef]
- Olejnik, E.; Biernasiuk, A.; Malm, A.; Szymanska, J. Evaluation of Antibacterial and Antifungal Properties of Selected Mouthwashes: In Vitro Studies. Curr. Issues Pharm. Med. Sci. 2021, 34, 164–168. [Google Scholar] [CrossRef]
- Korbecka-Paczkowska, M.; Karpinski, T.M. In Vitro Assessment of Antifungal and Antibiofilm Efficacy of Commercial Mouthwashes against Candida Albicans. Antibiotics 2024, 13, 117. [Google Scholar] [CrossRef]
- Gómez-Moreno, G.; Valerón-Rodríguez, F. Pseudomembranous Oral Candidiasis Resolved with a Mouthwash Containing 0.05% Chlorhexidine + 0.05% Cetylpyridinium Chloride. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5725–5728. [Google Scholar] [CrossRef]
- Duggan, S.; Usher, J. Candida Glabrata: A Powerhouse of Resistance. PLoS Pathog. 2023, 19, e1011651. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Rodrigues, M.; Silva, S.; Henriques, M. Candida Glabrata Biofilms: How Far Have We Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef]
- Kucharíková, S.; Tournu, H.; Lagrou, K.; Van Dijck, P.; Bujdáková, H. Detailed Comparison of Candida Albicans and Candida Glabrata Biofilms under Different Conditions and Their Susceptibility to Caspofungin and Anidulafungin. J. Med. Microbiol. 2011, 60, 1261–1269. [Google Scholar] [CrossRef]
- Franconi, I.; Lupetti, A. In Vitro Susceptibility Tests in the Context of Antifungal Resistance: Beyond Minimum Inhibitory Concentration in Candida spp. J. Fungi 2023, 9, 1188. [Google Scholar] [CrossRef] [PubMed]
- Edlind, M.P.; Smith, W.L.; Edlind, T.D. Effects of Cetylpyridinium Chloride Resistance and Treatment on Fluconazole Activity versus Candida Albicans. Antimicrob. Agents Chemother. 2005, 49, 843–845. [Google Scholar] [CrossRef]
- Zhang, H.; Yi, X.; Chen, M.; Shi, H.; Tan, L.; Lu, H.; Sun, Y.; Yang, F. Synergistic Effect of Chlorhexidine and Azoles on Candida Biofilm on Titanium Surface. J. Med. Mycol. 2023, 33, 101417. [Google Scholar] [CrossRef] [PubMed]
- Alvendal, C.; Mohanty, S.; Bohm-Starke, N.; Brauner, A. Anti-Biofilm Activity of Chlorhexidine Digluconate against Candida Albicans Vaginal Isolates. PLoS ONE 2020, 15, e0238428. [Google Scholar] [CrossRef]
- Chandra, J.; Mukherjee, P.K.; Leidich, S.D.; Faddoul, F.F.; Hoyer, L.L.; Douglas, L.J.; Ghannoum, M.A. Antifungal Resistance of Candidal Biofilms Formed on Denture Acrylic in Vitro. J. Dent. Res. 2001, 80, 903–908. [Google Scholar] [CrossRef]
- Nemes, D.; Kovács, R.; Nagy, F.; Mező, M.; Poczok, N.; Ujhelyi, Z.; Pető, Á.; Fehér, P.; Fenyvesi, F.; Váradi, J.; et al. Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms—Assessment of Cytotoxicity and Antimicrobial Activity. Molecules 2018, 23, 1827. [Google Scholar] [CrossRef]
- Coenye, T. Biofilm Antimicrobial Susceptibility Testing: Where Are We and Where Could We Be Going? Clin. Microbiol. Rev. 2023, 36, 4. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing Disk Diffusion Methodology. Available online: https://www.eucast.org/ast_of_bacteria/disk_diffusion_methodology (accessed on 9 December 2024).
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Definitive Document E.Def 7.4: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Fermentative Yeasts (Revised Version). 2023. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E.Def_7.4_Yeast_definitive_revised_2023.pdf (accessed on 1 April 2025).
- Rodrigues, C.; Henriques, M. Liposomal and Deoxycholate Amphotericin B Formulations: Effectiveness against Biofilm Infections of Candida spp. Pathogens 2017, 6, 62. [Google Scholar] [CrossRef] [PubMed]
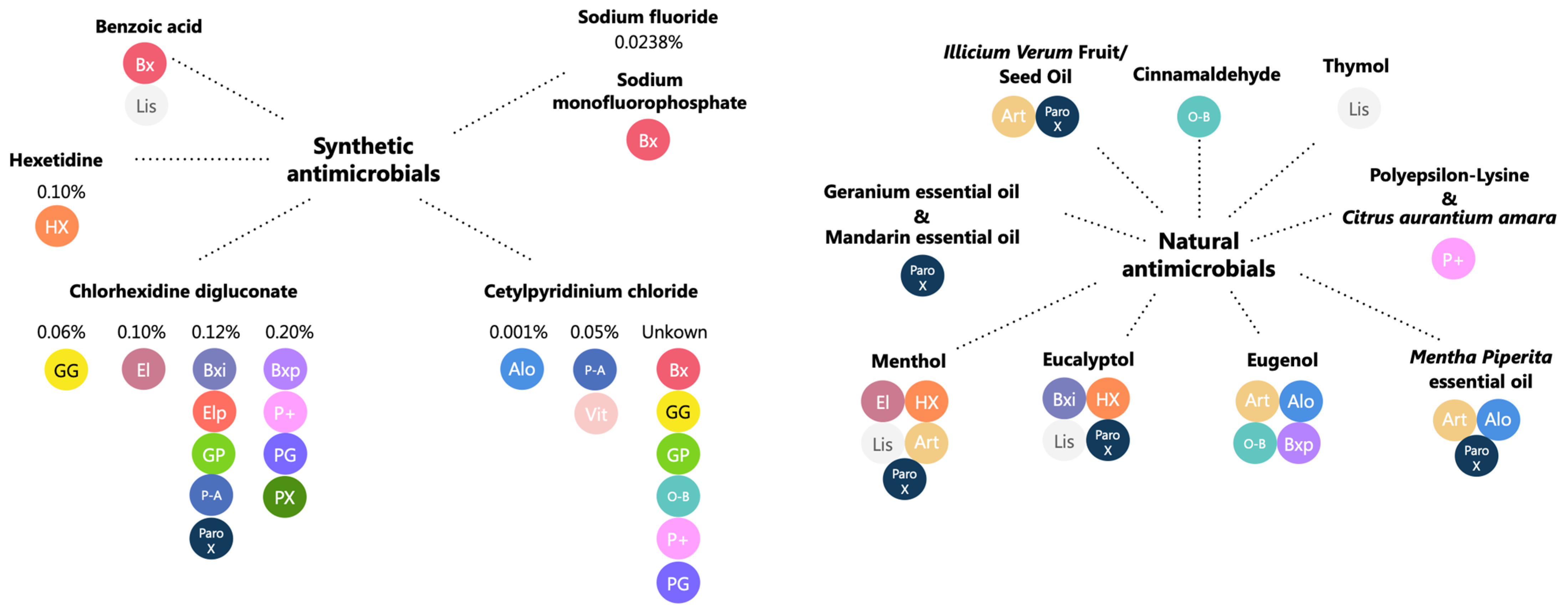

| MW |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | ||||||||||||||||||||
| Alcohol | + | + | + | + | 22% | |||||||||||||||
| Allantoin | + | + | + | + | 22% | |||||||||||||||
| Anethol | + | 6% | ||||||||||||||||||
| Aroma | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 89% | |||
| Benzoic acid | + | + | 11% | |||||||||||||||||
| Benzyl alcohol | + | 6% | ||||||||||||||||||
| Calcium acetate sodium | + | 6% | ||||||||||||||||||
| Chitosan | + | 6% | ||||||||||||||||||
| Chlorobutanol | 0.01 | 0.5 | 6% | |||||||||||||||||
| CHX | 0.12 | 0.2 | 0.1 | 0.12 | 0.06 | 0.12 | 0.12 | 0.2 | 0.2 | 0.12 | 0.2 | 61% | ||||||||
| CI 14720 (Azorubine) | + | + | + | 17% | ||||||||||||||||
| CI 16035 | + | 6% | ||||||||||||||||||
| CI 16255 | + | + | 11% | |||||||||||||||||
| CI 42051 | + | + | + | 17% | ||||||||||||||||
| CI 42053 | + | 6% | ||||||||||||||||||
| CI 42090 | + | + | 11% | |||||||||||||||||
| CI 47005 | + | 6% | ||||||||||||||||||
| Cinnamal | + | 6% | ||||||||||||||||||
| Citric acid | + | + | + | + | + | + | + | + | 44% | |||||||||||
| Citrus aurantium amara | + | 6% | ||||||||||||||||||
| Cocamidopropyl Betaine | + | 6% | ||||||||||||||||||
| CPC | 0.001 | + | + | + | + | 0.05 | + | + | 0.05 | 39% | ||||||||||
| D-Panthenol | + | + | + | + | 22% | |||||||||||||||
| Diethylhexyl sodium sulfosuccinate | + | 6% | ||||||||||||||||||
| Dipotassium Glycyrrhizate | + | 6% | ||||||||||||||||||
| Ethanol | 96 | + | 11% | |||||||||||||||||
| Eucalyptol | + | + | + | + | 22% | |||||||||||||||
| Eugenol | 0.001 | + | + | + | 17% | |||||||||||||||
| Gellan Gum | + | 6% | ||||||||||||||||||
| Geranium essential oil | + | 6% | ||||||||||||||||||
| Glycerin/Glycerol | + | + | + | + | + | + | + | + | + | 50% | ||||||||||
| Hexetidine | 0.1 | 6% | ||||||||||||||||||
| Hydroxyethyl cellulose | + | 6% | ||||||||||||||||||
| Illicium verum Fruit/Seed Oil | + | + | 11% | |||||||||||||||||
| Lactic acid | + | + | + | 17% | ||||||||||||||||
| Laureth-9 | + | 6% | ||||||||||||||||||
| Limonene | + | + | + | + | + | + | 33% | |||||||||||||
| Maltol | + | 6% | ||||||||||||||||||
| Mandarin essential oil | + | 6% | ||||||||||||||||||
| Mentha piperita oil | + | + | + | 17% | ||||||||||||||||
| Menthol | + | + | + | + | + | 28% | ||||||||||||||
| Menthone | + | 6% | ||||||||||||||||||
| Menthyl acetate | + | 6% | ||||||||||||||||||
| Methylparaben | + | + | 11% | |||||||||||||||||
| Metyl salicylate | + | 6% | ||||||||||||||||||
| Neohesperin Dihydrchalone | + | 6% | ||||||||||||||||||
| o-cymen-5-ol | + | 6% | ||||||||||||||||||
| PEG-25 | + | 6% | ||||||||||||||||||
| PEG-40 | + | + | + | + | + | + | + | + | + | 50% | ||||||||||
| PEG-60 hydrogenated castor oil | + | + | 11% | |||||||||||||||||
| Phenoxyethanol | + | 6% | ||||||||||||||||||
| Poloxamer 188 | + | 6% | ||||||||||||||||||
| Poloxamer 407 | + | + | + | 17% | ||||||||||||||||
| Polyepsilon-Lysine | + | 6% | ||||||||||||||||||
| Polysorbate 20 | + | 6% | ||||||||||||||||||
| Polysorbate 80 | + | 6% | ||||||||||||||||||
| Potassium acesulfame | + | + | 11% | |||||||||||||||||
| Potassium nitrate | 0% | |||||||||||||||||||
| Potassium sorbate | + | 6% | ||||||||||||||||||
| Propolys extracts | 0% | |||||||||||||||||||
| Propylene Glycol | + | + | + | + | + | + | + | + | 44% | |||||||||||
| Propylparaben | + | 6% | ||||||||||||||||||
| Sodium benzoate | + | + | + | + | 22% | |||||||||||||||
| Sodium chloride | + | 6% | ||||||||||||||||||
| Sodium citrate | + | + | + | + | 22% | |||||||||||||||
| Sodium fluoride | 0.024 | 0.03 | 11% | |||||||||||||||||
| Sodium gluconate | + | 6% | ||||||||||||||||||
| Sodium hydroxyide | + | + | + | 17% | ||||||||||||||||
| Sodium lactate | + | 6% | ||||||||||||||||||
| Sodium monofluorophosphate | + | 6% | ||||||||||||||||||
| Sodium saccharin | + | + | + | + | + | + | + | + | + | 50% | ||||||||||
| Sorbitol | + | + | + | + | + | + | 33% | |||||||||||||
| Sucralose | + | + | + | + | 22% | |||||||||||||||
| Thymol | + | 6% | ||||||||||||||||||
| Triacetin | + | 6% | ||||||||||||||||||
| Vaniline | + | 6% | ||||||||||||||||||
| VP/VA Copolymer | + | 6% | ||||||||||||||||||
| Water | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 100% | |
| Xylitol | + | + | + | 17% | ||||||||||||||||
| Zinc Chloride | + | 6% | ||||||||||||||||||
| Zinc Lactate | + | 6% | ||||||||||||||||||
| Strains | C. albicans SC 5314 | C. albicans MYK2760 | C. albicans H37 | C. albicans H43 | N. glabrata ATCC2001 | N. glabrata H49 | N. glabrata 15 | C. tropicalis ATCC750 | C. tropicalis C7 | C. parapsilosis ATCC 20019 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MW | |||||||||||
 | 2.25 ± 0.35 | 0 ± 0.0 | 0 ± 0.0 | 3.75 ± 0.35 | 3.75 ± 0.35 | 0 ± 0.0 | 3.5 ± 0.71 | 1.5 ± 0.71 | 0 ± 0.0 | 0.25 ± 0.35 | |
 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0.5 ± 0.71 | 0 ± 0.0 | |
 | 6 ± 1.41 | 4 ± 0.0 | 4.25 ± 0.35 | 6.75 ± 0.35 | 6.75 ± 1.77 | 0 ± 0.0 | 6.75 ± 0.35 | 4.5 ± 0.71 | 4 ± 0.0 | 4.25 ± 0.35 | |
 | 3.25 ± 0.35 | 6 ± 0.0 | 3 ± 0.71 | 3.25 ± 0.35 | 4 ± 0.71 | 8.25 ± 1.77 | 4 ± 0.0 | 7.75 ± 1.06 | 7 ± 0 | 5.5 ± 0.0 | |
 | 6 ± 0.0 | 6.5 ± 2.12 | 5.75 ± 1.06 | 5.75 ± 1.77 | 5 ± 1.41 | 9.75 ± 1.77 | 5.25 ± 0.35 | 7.75 ± 1.06 | 9.5 ± 2.12 | 6 ± 0.71 | |
 | 3.75 ± 2.47 | 5 ± 1.41 | 5.75 ± 1.77 | 7 ± 5.66 | 4 ± 1.41 | 10.75 ± 3.89 | 4 ± 1.41 | 10 ± 7.07 | 11 ± 2.83 | 6 ± 1.41 | |
 | 4 ± 0.0 | 5.75 ± 0.35 | 4 ± 0.0 | 3.25 ± 0.35 | 2.75 ± 0.35 | 9.75 ± 0.35 | 3.5 ± 0.71 | 8.5 ± 0.71 | 7.5 ± 0.71 | 4 ± 0.0 | |
 | 7.75 ± 0.35 | 3.75 ± 0.35 | 4 ± 0.71 | 7.5 ± 1.41 | 7 ± 0.71 | 7.75 ± 0.35 | 6.75 ± 0.35 | 7.25 ± 0.35 | 5 ± 1.41 | 5.75 ± 0.35 | |
 | 4.5 ± 3.54 | 4.5 ± 2.12 | 3.5 ± 0.71 | 7 ± 0 | 8.5 ± 0.71 | 10.5 ± 0.71 | 8.5 ± 0.71 | 8.75 ± 0.35 | 7.75 ± 1.06 | 7.75 ± 0.35 | |
 | 1.75 ± 2.47 | 4 ± 1.41 | 3.5 ± 0.71 | 0.25 ± 0.35 | 2 ± 1.41 | 5.5 ± 2.83 | 4.75 ± 1.06 | 1 ± 0.0 | 3 ± 1.41 | 1.75 ± 1.77 | |
 | 1 ± 0.0 | 0.5 ± 0.71 | 1 ± 0.0 | 2 ± 0.71 | 0.75 ± 0.35 | 0.75 ± 0.35 | 1 ± 0.0 | 0 ± 0.0 | 0.5 ± 0.71 | 0 ± 0.0 | |
 | 5.5 ± 1.41 | 3 ± 0.0 | 3.5 ± 0.71 | 6 ± 1.41 | 5.5 ± 0.71 | 1.5 ± 0.71 | 6 ± 1.41 | 5 ± 1.41 | 3.75 ± 0.35 | 6 ± 0.0 | |
 | 5.5 ± 2.12 | 2.75 ± 0.35 | 2.25 ± 1.06 | 4.25 ± 0.35 | 3.75 ± 0.35 | 7 ± 1.41 | 6 ± 0.0 | 6 ± 1.41 | 5.75 ± 0.35 | 5.75 ± 1.06 | |
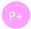 | 6 ± 0.0 | 5.25 ± 1.77 | 4.5 ± 2.12 | 6.25 ± 1.06 | 5.75 ± 1.77 | 10 ± 2.83 | 6 ± 1.41 | 8.5 ± 0.71 | 7.25 ± 1.06 | 6.75 ± 1.06 | |
 | 4.75 ± 0.35 | 7.25 ± 1.06 | 6.25 ± 1.06 | 4.25 ± 0.35 | 5 ± 1.41 | 10.5 ± 2.12 | 5.75 ± 1.06 | 9 ± 1.41 | 8.5 ± 3.55 | 5.75 ± 0.35 | |
 | 3.75 ± 0.35 | 5.75 ± 0.35 | 4.75 ± 0.35 | 3.75 ± 0.35 | 4 ± 1.41 | 9.25 ± 1.06 | 3.75 ± 0.35 | 9.25 ± 0.35 | 7.5 ± 0.71 | 5.25 ± 1.06 | |
 | 4.25 ± 0.35 | 6.75 ± 1.06 | 6.5 ± 0.71 | 4.5 ± 0.71 | 4.5 ± 0.71 | 9.5 ± 1.41 | 4 ± 2.83 | 8.5 ± 0.71 | 7 ± 0 | 6.5 ± 0.71 | |
 | 6 ± 0.71 | 4.25 ± 1.06 | 4.5 ± 0.71 | 6 ± 1.41 | 5.75 ± 1.06 | 1.5 ± 0.71 | 5.75 ± 1.77 | 6 ± 1.41 | 3.75 ± 0.35 | 5 ± 1.41 | |
| Mouthwash Concentration | MFC | Increased Growth | |||
|---|---|---|---|---|---|
| 12.5% | 25% | 50% | |||
 | 9.6% | 4.4% | −0.9% | 50% | No |
 | 119.0% | 91.7% | 3.9% | 50% | No |
 | 5.1% | 6.0% | 14.3% | 12.5% | Yes |
 | 10.4% | 10.6% | 14.2% | 12.5% | Yes |
 | 6.6% | 8.4% | 6.7% | 12.5% | Yes |
 | −5.2% | 11.6% | 124.9% | 12.5% | Yes |
 | −11.9% | −4.2% | 1.4% | 12.5% | Yes |
 | 6.1% | 4.5% | 1.7% | 50% | No |
 | −2.6% | 0.4% | 13.7% | 12.5% | Yes |
 | −15.8% | −8.6% | 6.8% | 12.5% | Yes |
 | 55.3% | 9.9% | 1.6% | 50% | No |
 | 4.9% | 5.4% | 2.3% | 50% | No |
 | 3.2% | 2.8% | −1.7% | 50% | No |
 | 5.3% | 4.2% | −0.3% | 50% | No |
 | 4.2% | 2.9% | 4.9% | 25% | Yes |
 | −8.2% | −4.9% | −1.1% | 12.5% | Yes |
 | 8.3% | 6.1% | 5.5% | 50% | No |
 | 5.6% | 7.1% | 0.9% | 50% | No |
| Strains | C. albicans SC 5314 | C. albicans MYK2760 | C. albicans H37 | C. albicans H43 | N. glabrata ATCC2001 | N. glabrata H49 | N. glabrata 15 | C. tropicalis ATCC750 | C. tropicalis C7 | C. parapsilosis ATCC 20019 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MW | |||||||||||
 | 9% | −152% | −147% | 3% | 52% | −126% | 24% | −14% | 15% | −39% | |
 | −3% | −82% | −54% | 23% | 36% | −158% | 71% | 17% | 24% | −48% | |
 | 31% | −13% | 33% | 65% | 64% | 15% | 81% | 25% | 24% | 36% | |
 | 24% | −32% | 50% | 52% | 58% | 25% | 62% | 5% | 32% | 47% | |
 | −78% | −263% | −112% | −17% | 28% | −200% | 43% | −6% | −115% | −35% | |
 | 90% | −16% | 20% | 59% | 11% | 30% | 89% | 42% | −26% | 49% | |
 | 32% | −25% | −4% | 54% | 63% | 37% | 35% | 37% | 34% | 39% | |
 | 51% | −29% | −17% | 76% | 80% | 16% | 46% | 35% | 31% | 45% | |
 | 50% | −72% | −11% | 67% | 78% | 37% | 59% | 29% | 18% | 28% | |
 | 62% | −2% | −40% | 50% | 80% | 48% | 76% | −42% | 6% | −49% | |
 | 11% | 26% | 74% | 30% | 58% | 57% | 67% | −29% | 64% | 18% | |
 | 31% | 3% | 32% | 62% | 76% | −31% | 46% | −31% | −61% | −41% | |
 | 49% | 39% | 45% | 50% | 64% | 38% | 49% | 1% | −3% | −63% | |
 | 58% | 44% | 54% | 59% | 55% | 44% | 73% | 1% | −3% | 6% | |
 | 52% | 39% | 5% | 64% | 32% | 53% | 68% | 9% | 25% | −47% | |
 | 50% | 28% | 4% | 62% | 66% | 63% | 55% | 2% | 18% | 2% | |
 | 61% | 16% | 52% | 95% | 74% | 64% | 77% | 20% | 14% | −1% | |
 | 18% | 2% | 2% | −24% | 44% | 29% | 63% | −16% | 10% | −23% | |
| Symbol | Mouthwash |
|---|---|
 | Alodont |
 | Arthrodont |
 | Bexident Gums Daily use |
 | Bexident Intensive gums 0.12% |
 | Bexident Post 0.20% |
 | Eludril Classic |
 | Eluperio |
 | Gum Gingidex 0.06% + CPC |
 | Gum Paroex 0.12% + CPC |
 | Hextril 0.10% |
 | Listerine Cool Mint |
 | Oral-B Pro-expert Professional Proctection |
 | Parodontax Extra 0.20% |
 | Paroex 0.12% |
 | Perioplus Curaprox |
 | Perio·Aid Intensive Care |
 | Periogard |
 | Vitis Gingival Mouthwash |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maziere, M.; Rompante, P.; Andrade, J.C.; De Oliveira, B.S.F.; Alves, M.C.; Rodrigues, C.F. Repurposing Mouthwashes: Antifungal and Antibiofilm Abilities of Commercially Available Mouthwashes Against Candida spp. Antibiotics 2025, 14, 483. https://doi.org/10.3390/antibiotics14050483
Maziere M, Rompante P, Andrade JC, De Oliveira BSF, Alves MC, Rodrigues CF. Repurposing Mouthwashes: Antifungal and Antibiofilm Abilities of Commercially Available Mouthwashes Against Candida spp. Antibiotics. 2025; 14(5):483. https://doi.org/10.3390/antibiotics14050483
Chicago/Turabian StyleMaziere, Marie, Paulo Rompante, José Carlos Andrade, Beatriz S. F. De Oliveira, Mariana C. Alves, and Celia Fortuna Rodrigues. 2025. "Repurposing Mouthwashes: Antifungal and Antibiofilm Abilities of Commercially Available Mouthwashes Against Candida spp." Antibiotics 14, no. 5: 483. https://doi.org/10.3390/antibiotics14050483
APA StyleMaziere, M., Rompante, P., Andrade, J. C., De Oliveira, B. S. F., Alves, M. C., & Rodrigues, C. F. (2025). Repurposing Mouthwashes: Antifungal and Antibiofilm Abilities of Commercially Available Mouthwashes Against Candida spp. Antibiotics, 14(5), 483. https://doi.org/10.3390/antibiotics14050483