Antimicrobial Susceptibility Profiles of Erysipelothrix rhusiopathiae and Riemerella anatipestifer Isolates from Clinical Cases of Waterfowl in Hungary Between 2022 and 2023
Abstract
:1. Introduction
2. Results
2.1. Susceptibility of Riemerella anatipestifer Strains
2.2. Susceptibility of Erysipelothrix rhusiopathiae Strains
3. Discussion
Conclusions
4. Materials and Methods
4.1. Origin of the Strains
4.2. Preparation of Antimicrobial Stock Solutions
4.3. Determination of Minimum Inhibitory Concentrations
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| CLSI | Clinical Laboratory Standards Institute |
| CFU | Colony-forming unit |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| MHB | Mueller–Hinton broth |
| MIC | Minimum inhibitory concentration |
| R. anatipestifer | Riemerella anatipestifer |
| E. rhusiopathiae | Erysipelothrix rhusiopathiae |
References
- Sheikh, B.A.; Bhat, B.A.; Mir, M.A. Antimicrobial Resistance: New Insights and Therapeutic Implications. Appl. Microbiol. Biotechnol. 2022, 106, 6427–6440. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Batacan, R.; Bajagai, Y.S. Rapid Growth of Antimicrobial Resistance: The Role of Agriculture in the Problem and the Solutions. Appl. Microbiol. Biotechnol. 2022, 106, 6953–6962. [Google Scholar] [CrossRef]
- Singhal, T. Antimicrobial Resistance: The “Other” Pandemic! Indian. J. Pediatr. 2022, 89, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Bjerke, L. Antibiotic Geographies and Access to Medicines: Tracing the Role of India’s Pharmaceutical Industry in Global Trade. Soc. Sci. Med. 2022, 312, 115386. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Rehman, M.U.; Yang, H.; Yang, Z.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhu, D.; et al. Distribution and Association of Antimicrobial Resistance and Virulence Traits in Escherichia Coli Isolates from Healthy Waterfowls in Hainan, China. Ecotoxicol. Environ. Saf. 2021, 220, 112317. [Google Scholar] [CrossRef] [PubMed]
- KSH Baromfiállomány (19.1.1.29). Available online: https://www.ksh.hu/stadat_files/mez/hu/mez0029.html (accessed on 18 May 2023).
- Yassin, A.K.; Gong, J.; Kelly, P.; Lu, G.; Guardabassi, L.; Wei, L.; Han, X.; Qiu, H.; Price, S.; Cheng, D.; et al. Antimicrobial Resistance in Clinical Escherichia Coli Isolates from Poultry and Livestock, China. PLoS ONE 2017, 12, e0185326. [Google Scholar] [CrossRef]
- Essősy, M.; Fodor, I.; Ihnáth, Z.; Karancsi, Z.; Kovács, D.; Szalai, K.V.; Szentmiklósi, D.; Jerzsele, Á. The Possibilities of Antibiotic-Free Broiler-Hen Fattening, with Special Reference to the Use of Pre- and Probiotics. Magy. Állatorvosok Lapja 2020, 142, 397–407. [Google Scholar]
- Kovács, D.; Palkovicsné Pézsa, N.; Farkas, O.; Jerzsele, Á. Usage of Antibiotic Alternatives in Pig Farming: Literature Review. Magy. Állatorvosok Lapja 2021, 143, 281–282. [Google Scholar]
- Zhu, D.; Yang, Z.; Xu, J.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Pan-Genome Analysis of Riemerella anatipestifer Reveals Its Genomic Diversity and Acquired Antibiotic Resistance Associated with Genomic Islands. Funct. Integr. Genom. 2020, 20, 307–320. [Google Scholar] [CrossRef]
- Guan, Q.; Yang, H.; Liao, C.; Zhao, J.; Wang, J.; Liu, Y.; Han, Q.; Zhang, H. In Silico Analysis and Immune Response of YaeT Protein Against Riemerella anatipestifer in Ducks. Appl. Biochem. Biotechnol. 2023, 195, 7483–7501. [Google Scholar] [CrossRef]
- Cammayo, P.L.T.; Fernandez-Colorado, C.P.; Flores, R.A.; Roy, A.; Kim, S.; Lillehoj, H.S.; Kim, W.H.; Min, W. IL-17A Treatment Influences Murine Susceptibility to Experimental Riemerella anatipestifer Infection. Dev. Comp. Immunol. 2020, 106, 103633. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; Zhang, S.; et al. Genome-Wide Association Study Reveals Serovar-Associated Genetic Loci in Riemerella anatipestifer. BMC Genom. 2024, 25, 57. [Google Scholar] [CrossRef]
- Vo, T.-T.; Dang, V.-T.; Le, D.-H.; Nguyen, T.-H. Identification, Serotyping, and Antimicrobial Susceptibility of Riemerella anatipestifer Isolated from Ducks in Vietnam. Open Vet. J. 2022, 12, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gong, X.; Chen, Q.; Zheng, F.; Ji, G.; Liu, Y. Threshold Level of Riemerella anatipestifer Crossing Blood-Brain Barrier and Expression Profiles of Immune-Related Proteins in Blood and Brain Tissue from Infected Ducks. Vet. Immunol. Immunopathol. 2018, 200, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zheng, M.; Xu, J.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Prevalence of Fluoroquinolone Resistance and Mutations in the gyrA, parC and parE Genes of Riemerella anatipestifer Isolated from Ducks in China. BMC Microbiol. 2019, 19, 271. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; He, X.; Du, X.; Gao, Y.; Shan, X.; Hu, Z.; Hu, Q. PaR1 Secreted by the Type IX Secretion System Is a Protective Antigen of Riemerella anatipestifer. Front. Microbiol. 2022, 13, 1082712. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Durkalec, A.; Tomczyk, G.; Gerilovych, I.; Kursa, O. Molecular Detection and Phylogenetic Analysis of Riemerella anatipestifer in Poultry and Wild Geese in Poland. Pathogens 2023, 12, 256. [Google Scholar] [CrossRef]
- Cha, S.-Y.; Seo, H.-S.; Wei, B.; Kang, M.; Roh, J.-H.; Yoon, R.-H.; Kim, J.-H.; Jang, H.-K. Surveillance and Characterization of Riemerella anatipestifer from Wild Birds in South Korea. J. Wildl. Dis. 2015, 51, 341–347. [Google Scholar] [CrossRef]
- Benmazouz, I.; Kövér, L.; Kardos, G. The Rise of Antimicrobial Resistance in Wild Birds: Potential AMR Sources and Wild Birds as AMR Reservoirs and Disseminators: Literature Review. Magy. Állatorvosok Lapja 2024, 146, 91–105. [Google Scholar] [CrossRef]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity Situation of Large-Scale Poultry Farms in Hungary According to the Databases of National Food Chain Safety Office Centre for Disease Control and Biosecurity Audit System of Poultry Product Board of Hungary in the Period of 2021–2022. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Nowaczek, A.; Dec, M.; Stępień-Pyśniak, D.; Wilczyński, J.; Urban-Chmiel, R. Characterization of Riemerella anatipestifer Strains Isolated from Various Poultry Species in Poland. Antibiotics 2023, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Such, N.; Molnár, A.; Pál, L.; Farkas, V.; Menyhárt, L.; Husvéth, F.; Dublecz, K. The Effect of Pre- and Probiotic Treatment on the Gumboro-Titer Values of Broilers. Magy. Állatorvosok Lapja 2021, 143, 119–127. [Google Scholar]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antiprotozoal and Antifungal Efficiency of Propolis—Part 2. Magy. Állatorvosok Lapja 2022, 144, 691–704. [Google Scholar]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils—Animal Health Aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Jerzsele, Á.; Somogyi, Z.; Szalai, M.; Kovács, D. Effects of Fermented Wheat Germ Extract on Artificial Salmonella Typhimurium Infection in Broiler Chickens. Magy. Állatorvosok Lapja 2020, 142, 77–85. [Google Scholar]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Hetényi, N.; Bersényi, A.; Hullár, I. Physiological Effects of Medium-Chain Fatty Acids and Triglycerides, and Their Potential Use in Poultry and Swine Nutrition: A Literature Review. Magy. Állatorvosok Lapja 2024, 146, 651–659. [Google Scholar] [CrossRef]
- Eriksson, H.; Brännström, S.; Skarin, H.; Chirico, J. Characterization of Erysipelothrix rhusiopathiae Isolates from Laying Hens and Poultry Red Mites (Dermanyssus Gallinae) from an Outbreak of Erysipelas. Avian Pathol. 2010, 39, 505–509. [Google Scholar] [CrossRef]
- Bobrek, K.; Gaweł, A. Erysipelas Outbreaks in Flocks of Geese in Poland—Biochemical and Genetic Analyses of the Isolates. Avian Dis. 2015, 59, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chang, B.J.; Riley, T.V. Erysipelothrix rhusiopathiae. Vet. Microbiol. 2010, 140, 405–417. [Google Scholar] [CrossRef]
- Meier, S.M.; Kottwitz, J.; Keller, D.I.; Albini, S. Erysipelothrix rhusiopathiae Infection by Geese to Human Transmission. BMJ Case Rep. 2021, 14, e240073. [Google Scholar] [CrossRef]
- Bobrek, K.; Nowak, M.; Borkowska, J.; Bobusia, K.; Gaweł, A. An Outbreak of Erysipelas in Commercial Geese. Pak. Vet. J. 2016, 36, 372. [Google Scholar]
- Mag, P.; Németh, K.; Somogyi, Z.; Jerzsele, Á. Antibacterial therapy based on pharmacokinetic/pharmacodynamic models in small animal medicine-1. Literature review. Magy. Állatorvosok Lapja 2023, 145, 419–438. [Google Scholar] [CrossRef]
- Geda, A.M. Fowl Cholera in Chickens: Current Trends in Diagnosis and Phenotypic Drug Resistance in Gondar City, Ethiopia. Vet. Med. Int. 2024, 2024, 6613019. [Google Scholar] [CrossRef] [PubMed]
- Chibuka, T.; Uehara, H.; Fumikura, S.; Takahashi, K.; Suzuki, Y.; Hoshinoo, K.; Yamamoto, Y. Riemerella anatipestifer Infection in Domestic Ducks in Japan, 2014. J. Vet. Med. Sci. 2016, 78, 1635–1638. [Google Scholar] [CrossRef]
- Chang, F.-F.; Chen, C.-C.; Wang, S.-H.; Chen, C.-L. Epidemiology and Antibiogram of Riemerella anatipestifer Isolated from Waterfowl Slaughterhouses in Taiwan. J. Vet. Res. 2019, 63, 79–86. [Google Scholar] [CrossRef]
- Shousha, A.; Awad, A.; Younis, G. Molecular Characterization, Virulence and Antimicrobial Susceptibility Testing of Riemerella anatipestifer Isolated from Ducklings. Biocontrol Sci. 2021, 26, 181–186. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Ding, H.; Mei, X.; Liu, W.; Zeng, J.; Zeng, Z. In Vitro Susceptibility of Four Antimicrobials against Riemerella anatipestifer Isolates: A Comparison of Minimum Inhibitory Concentrations and Mutant Prevention Concentrations for Ceftiofur, Cefquinome, Florfenicol, and Tilmicosin. BMC Vet. Res. 2016, 12, 250. [Google Scholar] [CrossRef]
- Jacoby, G.A. AmpC β-Lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Hasan, A.; Bose, P.; Aktar, M.T.; Haque, Z.F.; Islam, M.R.; Hossain, M.T.; Siddique, M.P. GroEL Gene-Based Molecular Detection and Antibiogram Profile of Riemerella anatipestifer from Duck in Bangladesh. J. Adv. Vet. Anim. Res. 2022, 9, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Gyuris, É.; Wehmann, E.; Czeibert, K.; Magyar, T. Antimicrobial Susceptibility of Riemerella anatipestifer Strains Isolated from Geese and Ducks in Hungary. Acta Vet. Hung. 2017, 65, 153–165. [Google Scholar] [CrossRef]
- Zhong, C.-Y.; Cheng, A.-C.; Wang, M.-S.; Zhu, D.-K.; Luo, Q.-H.; Chen, S.; Zhang, S.-H.; Chen, X.-Y. Quantitative Real-Time PCR Study of the Expression and Regulation of the Tetracycline Resistance Gene in Riemerella anatipestifer. Poult. Sci. 2013, 92, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C. Mechanisms of Antimicrobial Resistance in Bacteria. Am. J. Med. 2006, 119, S3–S10. discussion S62–S70. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Bacterial Resistance to Antibiotics: Enzymatic Degradation and Modification. Adv. Drug Deliv. Rev. 2005, 57, 1451–1470. [Google Scholar] [CrossRef]
- Bickford, A.A.; Corstvet, R.E.; Rosenwald, A.S. Pathology of Experimental Erysipelas in Turkeys. Avian Dis. 1978, 22, 503–518. [Google Scholar] [CrossRef]
- Bailie, W.E.; Bury, R.J.; Bicknell, E.J.; Knudtson, W.U. Case Report. Erysipelothrix Infection in Goslings. Avian Dis. 1970, 14, 555–556. [Google Scholar]
- Dec, M.; Łagowski, D.; Nowak, T.; Pietras-Ożga, D.; Herman, K. Serotypes, Antibiotic Susceptibility, Genotypic Virulence Profiles and spaA Variants of Erysipelothrix rhusiopathiae Strains Isolated from Pigs in Poland. Pathogens 2023, 12, 409. [Google Scholar] [CrossRef]
- Hess, C.; Bilic, I.; Jandreski-Cvetkovic, D.; Hess, M. Antimicrobial Dilution Susceptibility Testing of Erysipelothrix rhusiopathiae According to CLSI Document VET06 Reveals High Resistance against Penicillin G, Erythromycin and Enrofloxacin. Poultry 2023, 2, 54–62. [Google Scholar] [CrossRef]
- Bobrek, K.; Gaweł, A.; Mazurkiewicz, M. Infections with Erysipelothrix rhusiopathiae in Poultry Flocks. World’s Poult. Sci. J. 2013, 69, 803–812. [Google Scholar] [CrossRef]
- Fidalgo, S.G.; Longbottom, C.J.; Riley, T.V. Susceptibility of Erysipelothrix rhusiopathiae to Antimicrobial Agents and Home Disinfectants. Pathology 2002, 34, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, H.; Jansson, D.S.; Johansson, K.-E.; Båverud, V.; Chirico, J.; Aspán, A. Characterization of Erysipelothrix rhusiopathiae Isolates from Poultry, Pigs, Emus, the Poultry Red Mite and Other Animals. Vet. Microbiol. 2009, 137, 98–104. [Google Scholar] [CrossRef]
- Opriessnig, T.; Hoffman, L.J.; Harris, D.L.; Gaul, S.B.; Halbur, P.G. Erysipelothrix rhusiopathiae: Genetic Characterization of Midwest US Isolates and Live Commercial Vaccines Using Pulsed-Field Gel Electrophoresis. J. Vet. Diagn. Investig. 2004, 16, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C. Fluoroquinolone Resistance among Gram-Positive Cocci. Lancet Infect. Dis. 2002, 2, 530–538. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kijima, M.; Yoshimura, H.; Takahashi, T. Antimicrobial Susceptibilities of Erysipelothrix rhusiopathiae Isolated from Pigs with Swine Erysipelas in Japan, 1988-1998. J. Vet. Med. B Infect. Dis. Vet. Public Health 2001, 48, 115–126. [Google Scholar] [CrossRef]
- Chuma, T.; Kawamoto, T.; Shahada, F.; Fujimoto, H.; Okamoto, K. Antimicrobial Susceptibility of Erysipelothrix rhusiopathiae Isolated from Pigs in Southern Japan with a Modified Agar Dilution Method. J. Vet. Med. Sci. 2010, 72, 643–645. [Google Scholar] [CrossRef]
- Dingle, T.C.; Butler-Wu, S.M. Maldi-Tof Mass Spectrometry for Microorganism Identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef]
- CLSI Standards M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Clinical Lab Standards Institute: Wayne, PA, USA, 2016; Volume 35. [Google Scholar]
- CLSI. Methods for Antimicrobial Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria Isolated from Animals; Clinical Lab Standards Institute: Wayne, PA, USA, 2017; Volume VET06. [Google Scholar]
- Davison, H.C.; Low, J.C.; Woolhouse, M.E. What Is Antibiotic Resistance and How Can We Measure It? Trends Microbiol. 2000, 8, 554–559. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
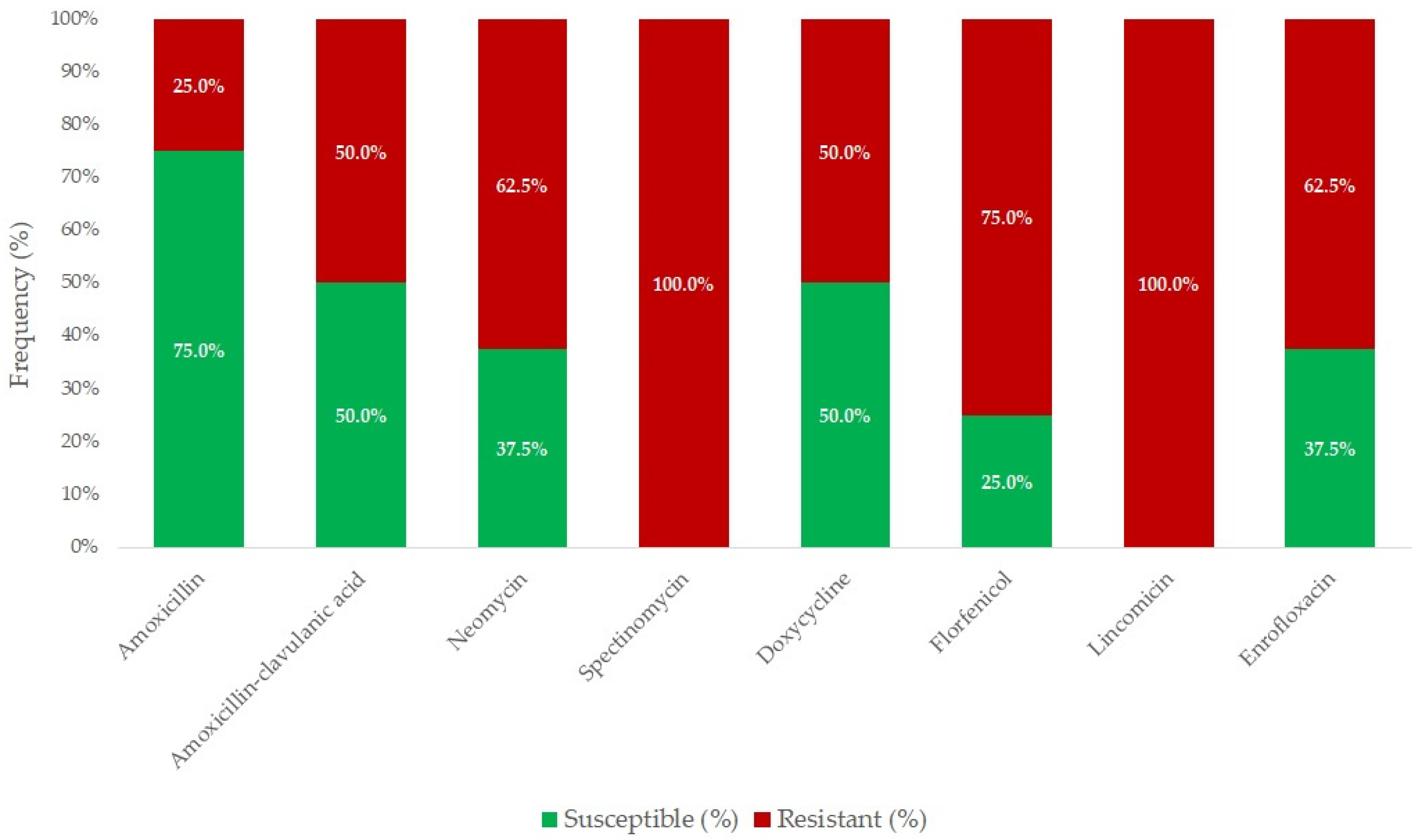


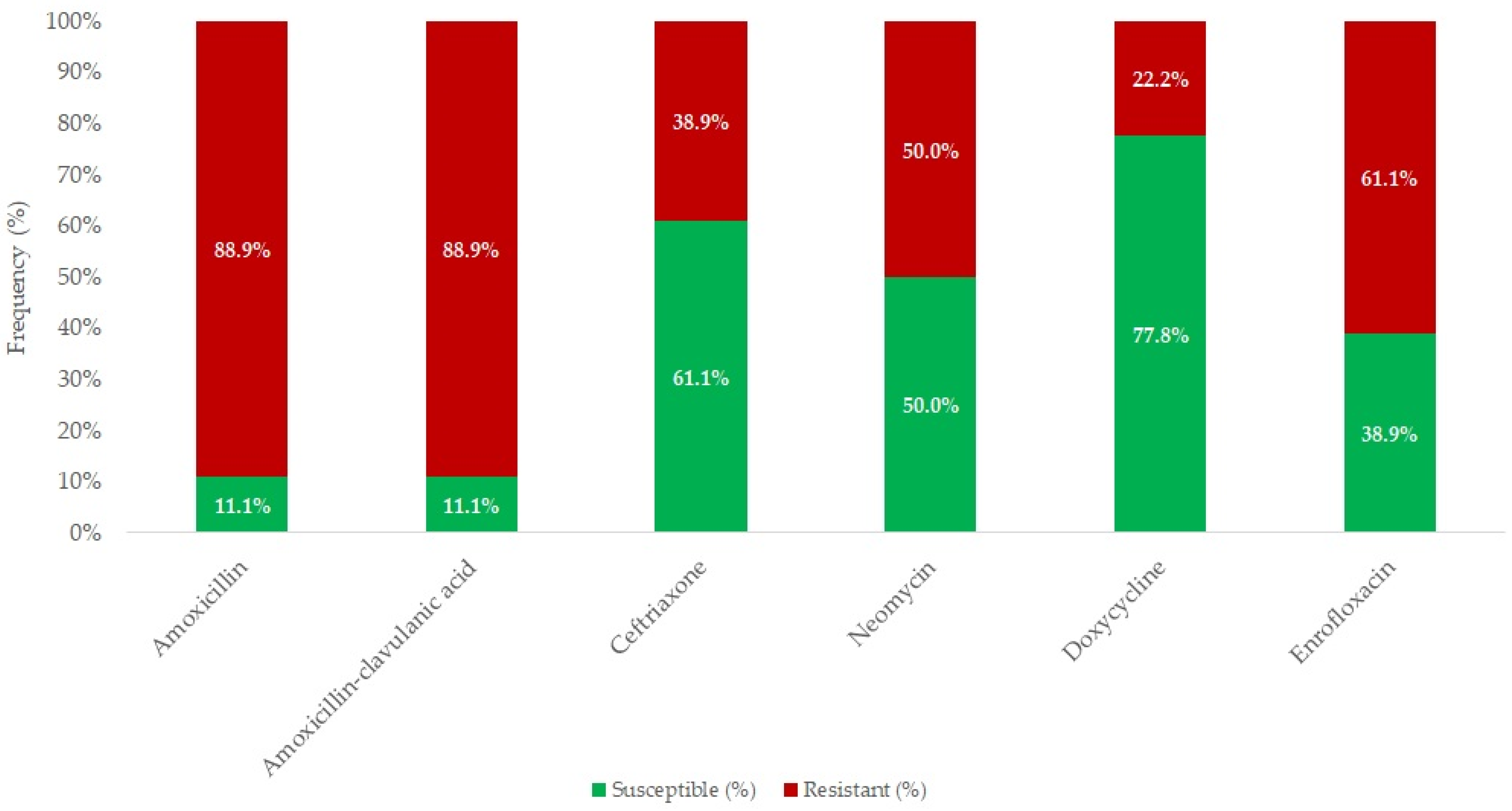

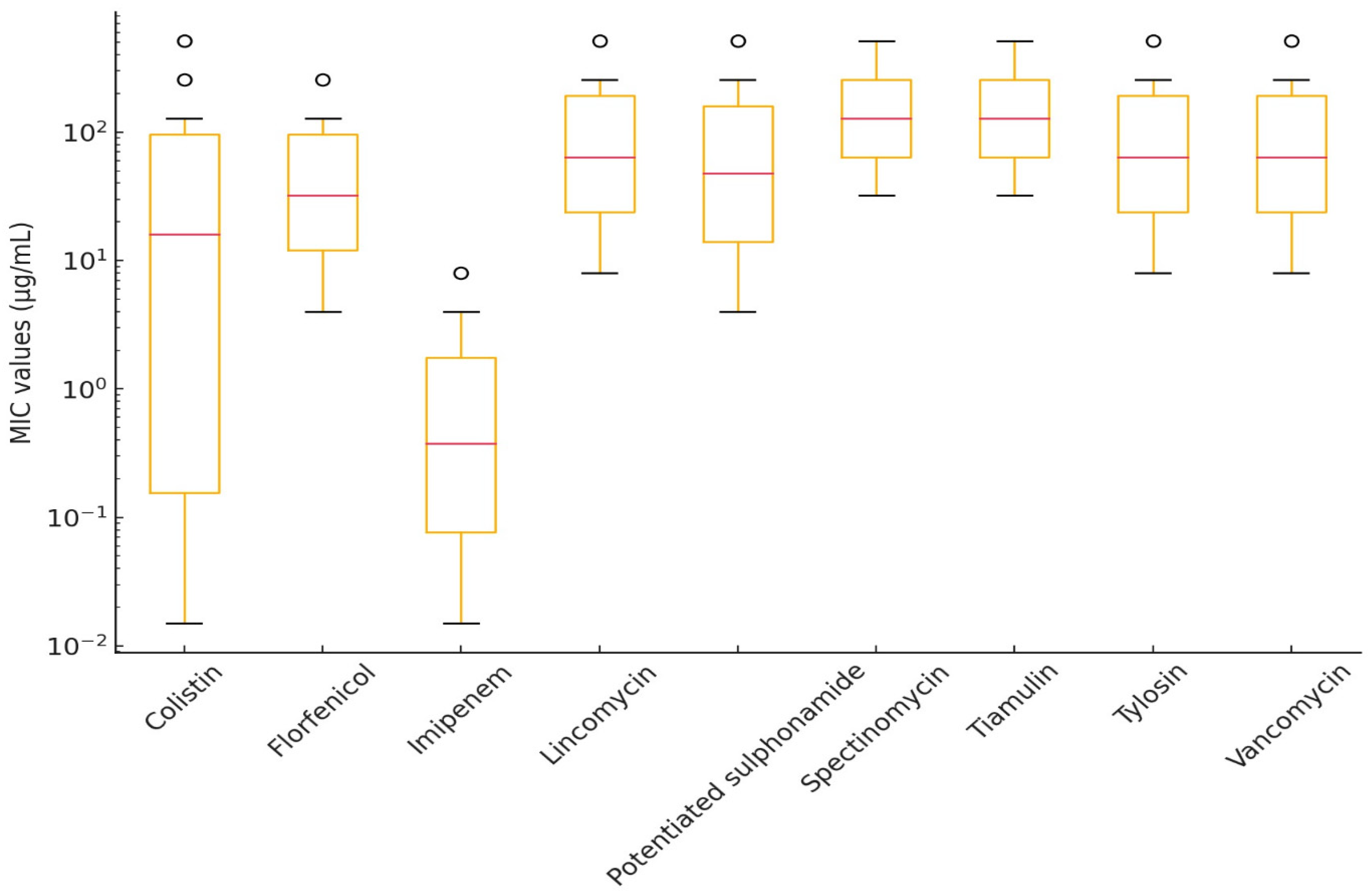
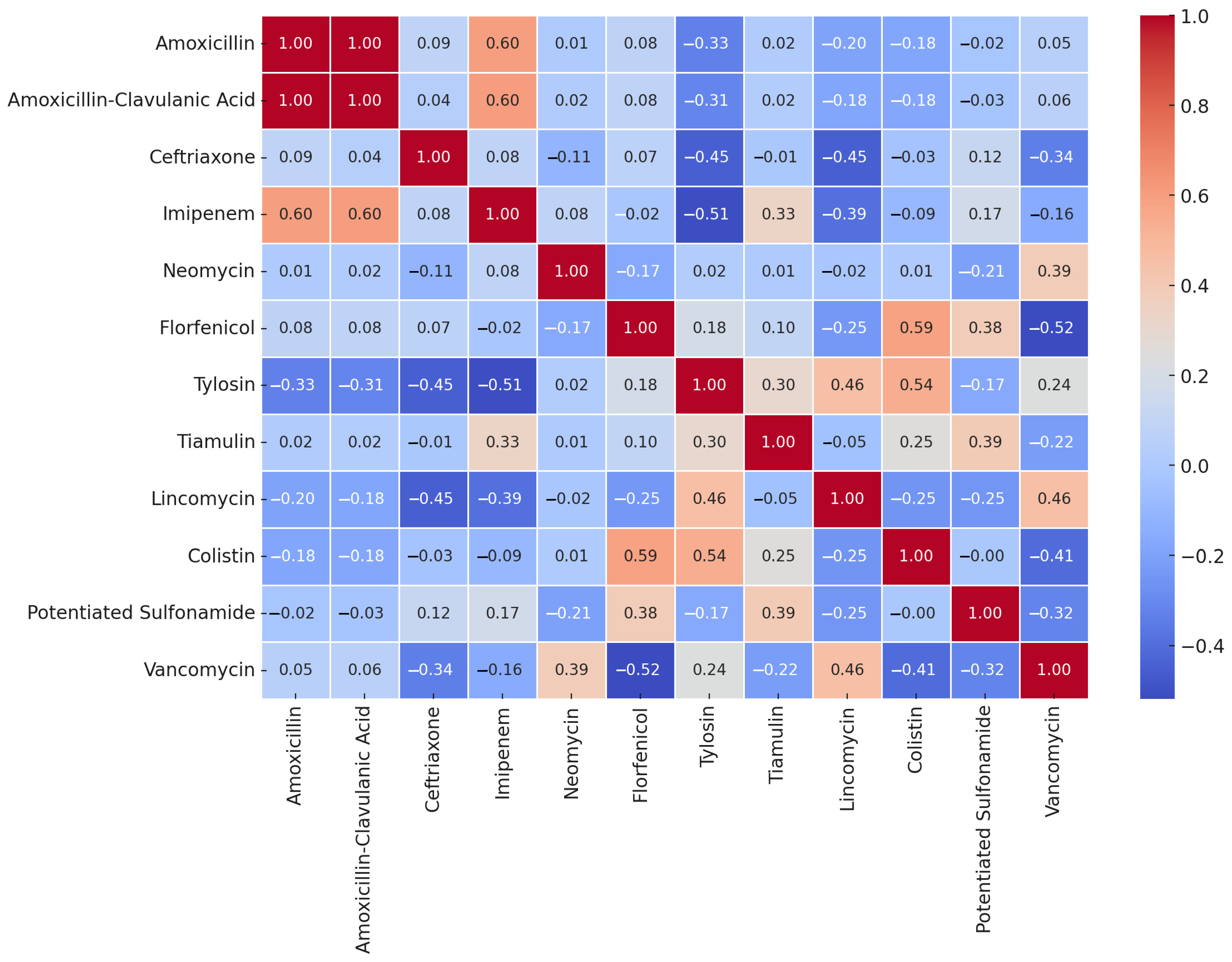
| Antibiotic(s) | Breakpoint (μg/mL) | Range (μg/mL) | Distribution of Strains by MIC (μg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | |||||
| Amoxicillin | 64 | 0.015–1024 | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 2 | 2 | 64 | |||||||||
| Amoxicillin–clavulanic acid 1 | 1 | 0.015–1024 | 4 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0.5 | 16 | |||||||||
| Doxycycline | 32 | 0.015–1024 | 1 | 1 | 0 | 2 | 0 | 0 | 3 | 1 | 4 | 32 | |||||||||
| Enrofloxacin | 1 | 0.015–1024 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 32 | |||||
| Florfenicol | 8 | 0.015–1024 | 1 | 0 | 0 | 1 | 1 | 3 | 1 | 0 | 1 | 16 | 32 | ||||||||
| Lincomycin | 16 | 0.015–1024 | 3 | 2 | 1 | 2 | 256 | 1024 | |||||||||||||
| Neomycin | 16 | 0.015–1024 | 1 | 2 | 0 | 0 | 2 | 0 | 3 | 16 | 64 | ||||||||||
| Spectinomycin | 4 | 0.015–1024 | 6 | 0 | 2 | 64 | 256 | ||||||||||||||
| Antibiotic | Range (μg/mL) | Distribution of Strains by MIC (μg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | ||||
| Ceftriaxone | 0.015–1024 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0.06 | 32 | |||||
| Colistin | 0.015–1024 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0.03 | 0.125 | ||
| Imipenem | 0.015–1024 | 1 | 1 | 2 | 0 | 1 | 0 | 3 | 0.06 | 1 | ||||||||||
| Potentiated sulfonamide 1 | 0.015–1024 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 16 | 32 | ||||||
| Tiamulin | 0.015–1024 | 3 | 3 | 0 | 2 | 256 | 1024 | |||||||||||||
| Tylosin | 0.015–1024 | 5 | 1 | 2 | 256 | 1024 | ||||||||||||||
| Vancomycin | 0.015–1024 | 2 | 1 | 3 | 0 | 2 | 64 | 256 | ||||||||||||
| Antibiotic(s) | Breakpoint (μg/mL) | Range (μg/mL) | Distribution of Strains by MIC (μg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | |||||
| Amoxicillin | 0.5 | 0.015–1024 | 1 | 1 | 3 | 0 | 4 | 2 | 3 | 1 | 1 | 2 | 4 | 32 | |||||||
| Amoxicillin–clavulanic acid 1 | 0.5 | 0.015–1024 | 2 | 3 | 1 | 4 | 2 | 2 | 1 | 1 | 2 | 2 | 32 | ||||||||
| Ceftriaxone | 2 | 0.015–1024 | 3 | 3 | 2 | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 1 | 1 | 0 | 1 | 0.5 | 16 | |||
| Doxycycline | 16 | 0.015–1024 | 3 | 2 | 4 | 4 | 1 | 2 | 1 | 1 | 2 | 16 | |||||||||
| Enrofloxacin | 1 | 0.015–1024 | 1 | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 0 | 1 | 1 | 1 | 32 | ||
| Neomycin | 32 | 0.015–1024 | 1 | 2 | 0 | 2 | 1 | 3 | 5 | 3 | 0 | 0 | 1 | 16 | 64 | ||||||
| Antibiotic | Range (μg/mL) | Distribution of Strains by MIC (μg/mL) | MIC50 (µg/mL) | MIC90 (µg/mL) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | ||||
| Colistin | 0.015–1024 | 3 | 1 | 1 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 1 | 4 | 1 | 0 | 1 | 1 | 0.25 | 64 | |
| Florfenicol | 0.015–1024 | 3 | 3 | 5 | 0 | 1 | 1 | 1 | 4 | 8 | 256 | |||||||||
| Imipenem | 0.015–1024 | 2 | 1 | 1 | 4 | 3 | 2 | 2 | 1 | 2 | 0.25 | 2 | ||||||||
| Lincomycin | 0.015–1024 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 6 | 6 | 512 | 1024 | |||||||
| Potentiated sulfonamide 1 | 0.015–1024 | 1 | 0 | 3 | 3 | 1 | 0 | 0 | 1 | 1 | 1 | 3 | 4 | 64 | 1024 | |||||
| Spectinomycin | 0.015–1024 | 4 | 7 | 2 | 2 | 1 | 64 | 512 | ||||||||||||
| Tiamulin | 0.015–1024 | 2 | 4 | 9 | 3 | 256 | 512 | |||||||||||||
| Tylosin | 0.015–1024 | 1 | 1 | 0 | 0 | 1 | 2 | 10 | 3 | 512 | 1024 | |||||||||
| Vancomycin | 0.015–1024 | 3 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 8 | 4 | 256 | 512 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerek, Á.; Szabó, Á.; Jerzsele, Á. Antimicrobial Susceptibility Profiles of Erysipelothrix rhusiopathiae and Riemerella anatipestifer Isolates from Clinical Cases of Waterfowl in Hungary Between 2022 and 2023. Antibiotics 2025, 14, 478. https://doi.org/10.3390/antibiotics14050478
Kerek Á, Szabó Á, Jerzsele Á. Antimicrobial Susceptibility Profiles of Erysipelothrix rhusiopathiae and Riemerella anatipestifer Isolates from Clinical Cases of Waterfowl in Hungary Between 2022 and 2023. Antibiotics. 2025; 14(5):478. https://doi.org/10.3390/antibiotics14050478
Chicago/Turabian StyleKerek, Ádám, Ábel Szabó, and Ákos Jerzsele. 2025. "Antimicrobial Susceptibility Profiles of Erysipelothrix rhusiopathiae and Riemerella anatipestifer Isolates from Clinical Cases of Waterfowl in Hungary Between 2022 and 2023" Antibiotics 14, no. 5: 478. https://doi.org/10.3390/antibiotics14050478
APA StyleKerek, Á., Szabó, Á., & Jerzsele, Á. (2025). Antimicrobial Susceptibility Profiles of Erysipelothrix rhusiopathiae and Riemerella anatipestifer Isolates from Clinical Cases of Waterfowl in Hungary Between 2022 and 2023. Antibiotics, 14(5), 478. https://doi.org/10.3390/antibiotics14050478








