Abstract
Staphylococcus hyicus is a significant pathogen in swine, primarily causing exudative epidermitis. Addressing S. hyicus infections requires both the characterization of virulence and antimicrobial resistance (AMR) in farm-recovered isolates. This study aimed to characterize the virulence, AMR, and biofilm formation of S. hyicus isolates from Spanish swine farms. A total of 49 isolates were analyzed, originating from animals with cutaneous, reproductive, and systemic clinical signs. Half of the isolates (49.0%) were positive for at least one virulence factor (VF) gene, with SHETA being the most frequent (28.6%). A high frequency of multidrug resistant (MDR) isolates was observed (83.7%), with significant resistance to commonly used antimicrobials, including lincosamides (83.7%), pleuromutilins (81.6%), penicillins (75.5%), and tetracyclines (73.5%). All isolates exhibited robust in vitro biofilm formation capacity (DC = 15.6 ± 7.0). Significant associations were found between VFs, biofilm formation, and AMR patterns, highlighting the link between the resistance to lincosamides and pleuromutilins (p < 0.001; Φ = 0.57) and macrolides (p < 0.001; Φ = 0.48), and the association of AMR with the ExhC and ExhD VF genes. These findings underscore the need for targeted diagnostics to improve management and therapeutic strategies to mitigate the impact of S. hyicus on swine production.
1. Introduction
Staphylococcus hyicus, a zoonotic pathogen that very rarely causes bacteriemia and sepsis in humans [1], is the causative agent of porcine exudative epidermitis (EE) in pigs, primarily affecting suckling and newly weaned piglets [2]. EE is characterized by a generalized or located skin disorder, including exfoliation, sebaceous secretion, and the formation of a brownish coat of exudate that may cover the entire body [3]. Additionally, S. hyicus has been associated with other clinical conditions such as sow mastitis and metritis [4], as well as systemic infections, including arthritis, in weaning piglets [5].
S. hyicus can be categorized into toxigenic and non-toxigenic strains based on their ability to cause EE in pigs [6]. The pathogenicity of toxigenic S. hyicus is mainly determined by exfoliative toxins, which are the primary virulence factors (VFs) inducing EE [7]. To date, six exfoliative toxins have been identified, including ExhA, ExhB, ExhC, and ExhD, primarily described in Denmark [8], and SHETA and SHETB, firstly described in Japan [9,10]. Another relevant VF that may be considered is biofilm formation. Although no specific information is available on biofilm formation by S. hyicus, other Staphylococcus species are known to possess a considerable number of genes encoding for biofilm production, adhesion factors, and exoenzymes [11]. These structured aggregates of bacterial cells surrounded by an extracellular matrix enhance bacterial survival by impairing both the host immune system and the effectiveness of antimicrobials [12], leading to therapeutic failure.
Addressing clinical conditions caused by S. hyicus requires both the characterization of the virulence of farm-recovered isolates and the evaluation of their antimicrobial resistance (AMR). Accurate AMR characterization is crucial for guiding effective antimicrobial therapy and ensuring antimicrobial stewardship, as it contributes to limiting the development and spread of AMR in food-producing animals, which poses a significant risk to animal, environmental, and public health. This underscores the need for implementing more restrictive legislation on antimicrobial use in food-producing animals, such as that adopted by the European Union, particularly in relation to the restriction of prophylactic and metaphylactic use [13]. However, updated information on AMR in S. hyicus is sparse and largely limited to non-European regions [2,14], with no recent studies from Spain. Therefore, to expand and update the characterization of S. hyicus, this study aimed to characterize the virulence and antimicrobial susceptibility profiles of a selection of S. hyicus isolates recovered from Spanish pig farms, together with the first description of S. hyicus in vitro biofilm formation ability.
2. Results
2.1. Isolation and Characterization of Staphylococcus hyicus
A selection of 49 S. hyicus isolates were recovered from Spanish swine farms across ten regions from May to December 2023. A detailed summary of these isolates is available in Table 1. Most of the isolates originated from the skin (51.0%) and from pigs showing clinical signs compatible with epidermitis (57.1%). Notably, approximately a quarter of the isolates were from reproductive exudates (26.5%) and cases of metritis in sows (24.5%). Additionally, another quarter of the isolates had a visceral origin (joint, 14.3%; brain, 8.2%) from piglets with systemic clinical signs (18.4%) such as polyserositis. Furthermore, the isolates were not limited to clinical cases in suckling or weaning piglets (73.5%), but also included those from adult animals, particularly sows (26.5%). Finally, the isolates were obtained from both white crossbred (44.9%) and Iberian pigs (55.1%), indicating a broad distribution across two different breeds.

Table 1.
Characteristics of the 49 Staphylococcus hyicus recovered from Spanish swine farms.
2.2. Characterization of Virulence Factors
Among the 49 S. hyicus isolates, 49.0% (n = 24) harbored at least one of the VFs evaluated. The most frequently detected VF was SHETA (28.6%; n = 14), followed by ExhC (16.3%; n = 8), ExhB (12.2%; n = 6), and both ExhA and ExhD (10.2%; n = 5). Only one isolate (2.0%) carried SHETB (Figure 1A; Figure 2A). When assessing the presence of multiple VFs within the same isolate, we observed that 24.5% (n = 12) of the isolates contained only one VF gene, 20.4% (n = 10) harbored two VFs, and two isolates (4.1%) carried three and four VF genes, respectively. Notably, 51.0% of isolates (n = 25) were negative for all evaluated VFs.
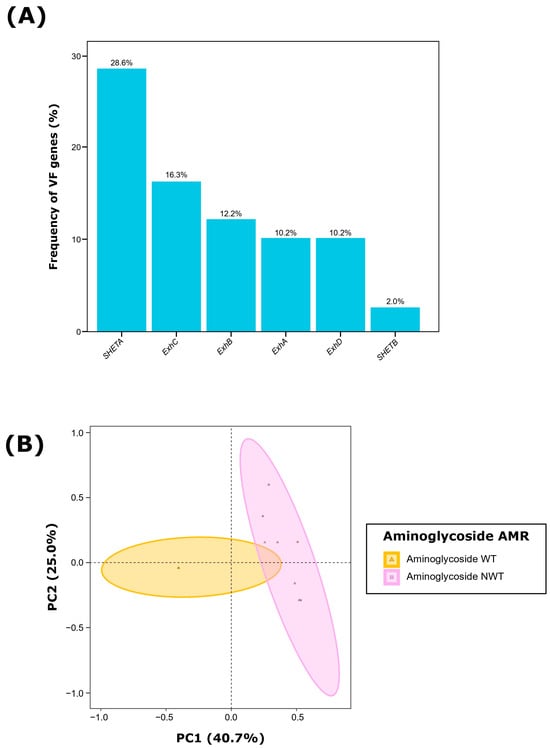
Figure 1.
Virulence factor (VF) characterization of 49 Staphylococcus hyicus isolates from Spanish swine farms. (A) Frequency (%) of each VF gene and (B) Principal component analysis (PCA) of the six evaluated VF genes, showing grouping based on aminoglycoside antimicrobial resistance (AMR) of each S. hyicus isolate. WT: wild type, susceptible; NWT: non-wild type, resistant.
Regarding specific VF combinations, we identified nine different patterns, with two of them involving three or four VFs (Supplementary Table S1). The most common pattern was the association of ExhC-SHETA (12.2%; p < 0.01), followed by the combinations of ExhB-SHETA and ExhD-SHETA, each at 4.1% (n = 2). Notably, the only isolate harboring four VFs carried the combination ExhA-ExhC-ExhD-SHETB.
The analysis of S. hyicus isolates based on their VFs revealed that the first two dimensions of the PCA captured 65.7% of the total variability (Figure 1B). Dimension 1 accounted for 40.7% of the variability, primarily influenced by SHETA (68.0%) and ExhC (29.8%). In contrast, Dimension 2 explained 25.0% of the variability, with significant contributions from ExhA (33.7%), and ExhB (20.3%). Additionally, when evaluating the effect of AMR susceptibility profiling, we observed that the AMR to aminoglycosides accounted for 14.9% of the observe variability among S. hyicus isolates (PERMANOVA, p < 0.01), with most isolates non-wild type (NWT, resistant) to aminoglycosides being positive to both SHETA and ExhC (Figure 2A). There was no discernible impact from other antimicrobial classes, clinical signs, type of sample, production phase, pig breed or geographic origin.
2.3. Antimicrobial Susceptibility Profiling
Among the S. hyicus isolates evaluated in this study, 93.9% (n = 46) were resistant to at least one antimicrobial class, with 83.7% (n = 41) classified as multidrug resistant (MDR). Most of the isolates showed resistance to six (22.4%; n = 11) or seven (20.4%; n = 10) antimicrobial classes, with four isolates resistant to eight classes. The AMR patterns exhibited considerable heterogeneity, with 29 different combinations identified, none of which were predominant (Supplementary Table S2). The most common pattern was resistance to phenicols–pleuromutilins–tetracyclines–penicillins–aminoglycosides–macrolides–lincosamides (12.2%; n = 6), followed by phenicols–pleuromutilins–tetracyclines–penicillins–quinolones–macrolides–lincosamides and pleuromutilins–tetracyclines–penicillins–lincosamides (8.2%; n = 4).
Minimum inhibitory concentration (MIC) values for all evaluated antimicrobials are shown in Table 2. We observed that the NWT phenotype exceeded 50% for 9 of the 18 antimicrobials tested. NWT was particularly relevant for clindamycin (83.7%), tiamulin (81.6%), penicillin (75.5%), and chlortetracycline (73.5%). In contrast, the NWT phenotype was below 10% for ceftiofur and trimethoprim–sulfamethoxazole (4.1%).
Due to the absence of the specific antimicrobial concentrations that include the epidemiological cut-off (ECOFF) values for neomycin and spectinomycin on the microdilution plates used (Table 2), conclusive results for these antibiotics could not be obtained. For neomycin, seven isolates had MICs above the breakpoint, confirming the NWT phenotype, but it was not possible to determine the resistance status for the remaining 42 isolates. Conversely, for spectinomycin, 14 isolates had MICs below the breakpoint, indicating WT, but the resistance status for the remaining 35 isolates could not be verified.
AMR clustering at the class level demonstrated three main clusters (Figure 2A). The first one included those isolates with no or very few resistances. The other two included MDR S. hyicus, which were mainly divided by macrolide and, to a lesser extent, phenicol resistance. Notably, most aminoglycoside resistant S. hyicus exhibited a very similar MDR pattern and were mainly recovered from weaning piglets with epidermitis. It is remarkable that all S. hyicus isolates resistant to macrolides (59.1%; n = 29) were resistant to all evaluated members of this class, including tylosin, tilmicosin, and tulathromycin. This consistency was also observed for tetracyclines (NWT, 73.5%; n = 36), with only three isolates being wild type (WT, susceptible) for oxytetracycline but NWT to chlortetracycline. For aminoglycosides, despite notable differences among NWT phenotypes for gentamicin, neomycin and spectinomycin, no conclusive results could be drawn for the latter two due to the limitations in the antimicrobial concentration ranges, as discussed in the previous paragraph.
Principal component analysis (PCA) of S. hyicus isolates based on their AMR phenotype at the class level showed that the first two dimensions accounted for 57.5% of the variability. Dimension 1 represented 40.3% of the variability, mainly determined by macrolides (26.0%) and phenicols (21.9%). Dimension 2 accounted for 17.2% of the variability, predominantly determined by quinolones (66.8%) and aminoglycosides (17.3%). Further ordination analyses revealed that the type of sample, along with ExhC and ExhD VFs, collectively explained 26.7% of the AMR variability (PERMANOVA, p < 0.05). Specifically, the type of sample accounted for 13.2% (p < 0.01) (Figure 2B), showing similar AMR patterns in isolates from reproductive exudates and skin when compared to joints and brain. Within VFs, ExhD contributed 8.1% (p < 0.01) and ExhC contributed 5.4% (p < 0.05) to the variability. Although the clinical signs and the production phase also showed significant effects (PERMANOVA, p < 0.05), these were omitted due to the close association with the type of sample.

Table 2.
Minimum inhibitory concentrations (MICs) of 18 antimicrobials against 49 Staphylococcus hyicus isolates from swine farms. The thick line represents the ECOFF used for each antimicrobial to classify isolates into wild type (WT) and non-wild type (NWT). Areas in gray represent values outside the concentrations included in the broth microdilution method.
Table 2.
Minimum inhibitory concentrations (MICs) of 18 antimicrobials against 49 Staphylococcus hyicus isolates from swine farms. The thick line represents the ECOFF used for each antimicrobial to classify isolates into wild type (WT) and non-wild type (NWT). Areas in gray represent values outside the concentrations included in the broth microdilution method.
| Antimicrobial | Nº of Isolates with MIC (µg/mL) | MIC | WT | NWT | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | MIC50 | MIC90 | n | % | n | % | |
| c Penicillin | 12 | 3 | 6 | 2 | 2 | 1 | 2 | 23 | 4 | >8 | 12 | 24.5 | 37 | 75.5 | |||||
| b Ampicillin | 17 | 7 | 7 | 6 | 8 | 3 | 0 | 1 | 1 | 4 | 17 | 39.7 | 32 | 30.3 | |||||
| c Ceftiofur | 2 | 11 | 31 | 3 | 1 | 1 | 1 | 2 | 47 | 95.9 | 2 | 4.1 | |||||||
| b Gentamicin | 38 | 1 | 1 | 2 | 3 | 4 | ≤1 | 16 | 39 | 79.6 | 10 | 20.4 | |||||||
| c Spectinomycin | 0 | 0 | 5 | 9 | 35 | >64 | >64 | 14 | 28.6 | - | - | ||||||||
| b Neomycin | 42 | 6 | 0 | 1 | ≤4 | 8 | - | - | 7 | 14.3 | |||||||||
| b Tulathromycin | 1 | 8 | 10 | 2 | 1 | 0 | 0 | 27 | >64 | >64 | 21 | 42.9 | 28 | 51.1 | |||||
| b Tilmicosin | 20 | 0 | 0 | 1 | 0 | 28 | ≤4 | ≤4 | 20 | 40.8 | 29 | 59.2 | |||||||
| b Tylosin | 2 | 10 | 8 | 0 | 0 | 0 | 1 | 28 | >32 | >32 | 20 | 40.8 | 29 | 59.2 | |||||
| b Chlortetracycline | 6 | 7 | 3 | 0 | 1 | 32 | >8 | >8 | 13 | 26.5 | 36 | 73.5 | |||||||
| b Oxytetracycline | 12 | 4 | 0 | 0 | 0 | 33 | >8 | >8 | 16 | 32.7 | 33 | 67.3 | |||||||
| d Danofloxacin | 7 | 23 | 2 | 3 | 14 | 0.25 | >1 | - | - | - | - | ||||||||
| b Enrofloxacin | 19 | 10 | 2 | 3 | 2 | 13 | 0.25 | >2 | 29 | 59.2 | 20 | 40.8 | |||||||
| c Clindamycin | 8 | 0 | 2 | 0 | 2 | 3 | 0 | 36 | >16 | >16 | 8 | 16.3 | 41 | 83.7 | |||||
| a SXT | 47 | 2 | ≤2/38 | ≤2/38 | 47 | 95.9 | 2 | 4.1 | |||||||||||
| d Sulfadimethoxine | 43 | 6 | ≤256 | >256 | - | - | - | - | |||||||||||
| b Tiamulin | 1 | 7 | 1 | 1 | 0 | 1 | 1 | 37 | >32 | >32 | 9 | 18.4 | 40 | 81.6 | |||||
| b Florfenicol | 0 | 0 | 0 | 8 | 17 | 15 | 9 | 8 | >8 | 25 | 51 | 24 | 49 | ||||||
a SXT: sulfamethoxazole–trimethoprim. b ECOFF defined by the EUCAST for Staphylococcus hyicus. c ECOFF defined by the EUCAST for Staphylococcus aureus. d ECOFF not defined.
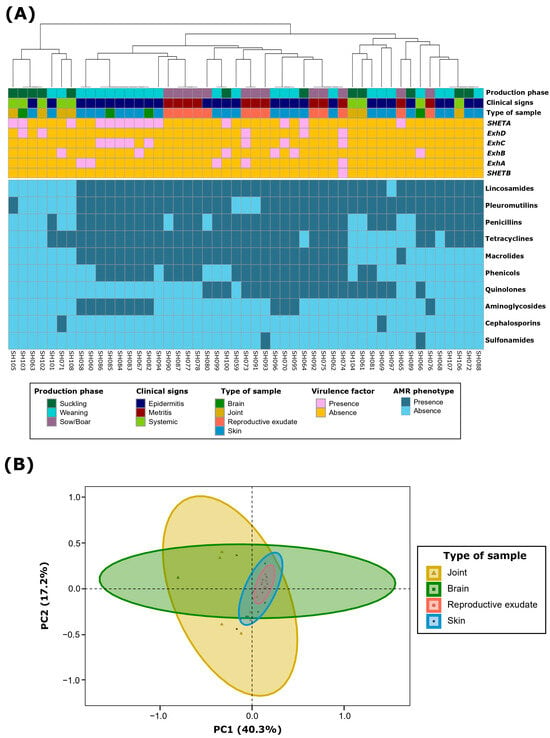
Figure 2.
Antimicrobial resistance (AMR) characterization at the class level of 49 Staphylococcus hyicus isolates from Spanish swine farms. (A) AMR phenotype clustering based on antimicrobial classes, using the unweighted pair group method with arithmetic mean (UPGMA) as the hierarchical clustering method. (B) Principal component analysis (PCA) of the AMR patterns, showing grouping based on the type of sample of each S. hyicus isolate.
2.4. Cooccurrence among Virulence Factors and Antimicrobial Susceptibility Profiling
Several associations were observed between the VFs and AMR phenotypes at the AMR class level (Figure 3, Table 3). The most frequent interaction was the very strong co-occurrence of ExhC with three AMR classes: aminoglycosides (p < 0.01; Φ = 0.45), phenicols (p < 0.01; Φ = 0.40), and macrolides (p < 0.05; Φ = 0.31). Additionally, there was a significant association between SHETA and aminoglycosides (p < 0.05; Φ = 0.32), and between ExhD and pleuromutilins (p < 0.05; Φ = 0.28).
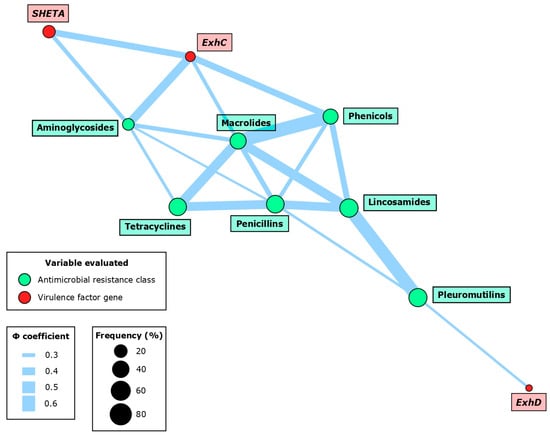
Figure 3.
Network associations between virulence factor (VF) genes and antimicrobial resistance (AMR) at the class level in 49 Staphylococcus hyicus isolates from Spanish swine farms. Node size is determined by the percentage occurrence of the VF gene or AMR class. Edge size is proportional to the magnitude of the association based on the Φ coefficient. The network was constructed using significant associations (p < 0.05).

Table 3.
Significant associations (p < 0.05) between virulence factors and phenotypic antimicrobial resistance (AMR) at the class level in 49 Staphylococcus hyicus isolates from Spanish swine farms.
Within the VFs, we only detected the previously mentioned interaction between ExhC and SHETA (p < 0.01; Φ = 0.39). Among the AMR classes, 14 different associations were described involving seven different AMR classes: aminoglycosides, phenicols, macrolides, tetracyclines, penicillins, lincosamides, and pleuromutilins. Notably, there was a very strong co-occurrence between antimicrobials that share AMR mechanisms, such as macrolides with phenicols (p < 0.001; Φ = 0.61) and lincosamides (p < 0.001; Φ = 0.48), or the latter also with pleuromutilins (p < 0.001; Φ = 0.57).
2.5. Biofilm Formation
All evaluated S. hyicus isolates exhibited a very high biofilm formation capacity (DC = 15.6 ± 7.0), with wide differences that ranged from 6.6 to 31.6. Significant differences in biofilm formation were observed in relation to metadata, VFs, and AMR phenotypes (Figure 4).
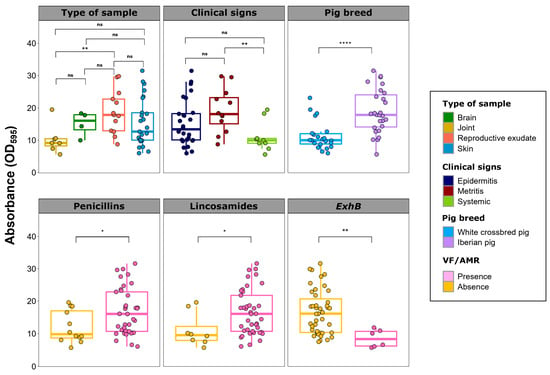
Figure 4.
Biofilm formation of Staphylococcus hyicus isolates from Spanish swine farms. Boxplots illustrating the quantitative biofilm formation of S. hyicus, comparing the type of sample and the clinical signs of the animals, the pig breed, the antimicrobial resistance to penicillins and lincosamides, or the presence of the ExhB virulence factor (VF) gene. Quantification was performed as the difference from the negative control (DC) in absorbance (OD595). Each S. hyicus isolate is represented by a dot with a horizontal jitter for visibility. The horizontal box lines represent the first quartile, the median, and the third quartile. Whiskers extend to 1.5 times the interquartile range. Differences between groups were evaluated using the Wilcoxon rank-sum test. The level of statistical significance was represented with asterisks: four asterisks (****) indicated a p-value less than 0.0001; two asterisks (**) indicated a p-value between 0.001 and 0.01; one asterisk (*) indicated a p-value between 0.01 and 0.05; non-significance (ns) indicated a p-value higher than 0.05.
Isolates from animal with metritis showed significantly higher biofilm formation than those from piglets with systemic clinical signs (p < 0.01). This is reflected in lower biofilm formation in isolates from joints compared to those from reproductive exudates (p < 0.01). Regardless of the region, isolates from Iberian pigs had higher biofilm formation than those from white crossbred pigs (p < 0.001). Regarding VFs, isolates harboring ExhB showed lower biofilm formation capacity than those without this VF gene (p < 0.01). Conversely, resistance to penicillins and lincosamides was consistently associated with significantly higher biofilm formation (p < 0.05).
3. Discussion
Current limitations on antimicrobial use in the European Union [13] have led to increasing concerns about the potential reemergence of pathogens in swine production, such as S. hyicus. The lack of updated information on this pathogen, particularly in Europe, makes it necessary to further characterize clinical S. hyicus isolates. Here, by establishing associations among pathogenicity markers, biofilm formation capacity, and AMR patterns, we enhanced the understanding and management of clinical S. hyicus recovered from Spanish swine farms, contributing to more targeted and effective therapeutic strategies.
S. hyicus is the primary causative agent of EE, particularly in suckling and weaning piglets [2]. In this study, over half of the isolates were recovered from animals exhibiting clinical signs of epidermitis. Additionally, we isolated S. hyicus from sows and boars primarily affected by reproductive signs, particularly sows with metritis. Although not extensively studied, prior research has reported the potential role of S. hyicus in reproductive diseases in sows, especially inducing mastitis and metritis [4], as observed in this study. About 25% of clinical cases were associated with systemic infections, with isolates recovered from joints and the brain. While not frequently described, a previous study reported the involvement of S. hyicus in causing arthritis in suckling to weaning piglets [4] and suppurative pneumonia and pericarditis in piglets [15]. However, none have characterized its presence in brain samples. This systemic dissemination may be explained by the bacteriemia caused by hematogenous spread or external inoculation following skin wounds [16]. Indeed, this has been previously reported in systemic infection in humans with endocarditis [17] and spondylodiscitis [16]. These findings demonstrate that S. hyicus should be considered not only as a cutaneous pathogen but also as a potential systemic agent primarily affecting piglets.
The characterization of the pathogenicity of S. hyicus requires evaluating the presence of exfoliative toxins, particularly in toxigenic strains causing EE. In this study, nearly half of the isolates (49.0%) were positive for one or more of the six characterized toxins, consistent with previous findings [18]. Although previous studies have examined the prevalence of these toxins [14,19,20], it may be challenging to provide a detailed comparative analysis due to geographical differences and the potential changes in prevalence over time. We observed that SHETA was the most frequently detected VF gene (28.6%). SHETA is a toxigenic VF that has been described to contribute to the clinical signs of EE, although its toxigenic mechanism remains unknown [19]. In contrast to the plasmid location of the SHETB gene, SHETA is chromosomally encoded [9] and genetically related to the ExhB gene, with 98.6% DNA homology [21]. Additionally, we observed a significant association of SHETA with ExhC, a gene encoding one the four Exh toxins, which was the second most prevalent VF (16.3%) in this study. Exh toxins, encoded by the ExhA, ExhB, ExhC and ExhD genes, are characteristic of toxigenic S. hyicus involved in EE. These toxins, which have been detected at a low frequency in this study, cause a loss of cell adhesion in the epidermis of porcine skin by cleaving desmoglein-1 [22]. Therefore, evaluating exfoliative toxins may be essential for identifying and studying S. hyicus isolates from clinical cases, especially EE. However, more than half of the isolates (51.0%) were negative for the six characterized toxins, regardless of the sample type or the clinical signs. This highlights the need for further characterization of other potential pathogenic mechanisms in S. hyicus.
Biofilm formation has been identified as an essential pathogenic mechanism in related bacteria, such as S. aureus and coagulase-negative staphylococci [11]. However, until now, to the best of our knowledge, no previous study has specifically addressed biofilm formation in S. hyicus and its underlying mechanisms. Here, we demonstrate for the first time the robust in vitro biofilm formation of S. hyicus, regardless of sample type. This suggests a high survival capacity in certain environments, which could contribute to the persistence of other bacterial pathogens in coinfection processes. Notably, a significantly lower biofilm formation was observed among S. hyicus from systemic locations compared to those from reproductive exudates. Previous studies have shown the importance of biofilm formation for survival in reproductive environments, such as S. aureus in bovine mastitis [23] or Trueperella pyogenes in cattle endometritis [24]. The lower biofilm formation in joint-related S. hyicus contrasts with previous studies suggesting strong biofilm aggregates in synovial fluid caused by S. aureus [25] or Staphylococcus lugdunensis [26]. However, it should be noted that, despite the significantly lower biofilm formation, the levels are still exceptionally high compared to other porcine pathogens [27]. Additionally, there is an association between biofilm formation and resistance to certain antimicrobials, such as penicillins and lincosamides. A recent study reported the association between biofilm formation and AMR in S. aureus [28], highlighting the complexity and significance of biofilm formation not only in pathogenicity but also in therapeutic success and the survival of S. hyicus in its biological niche.
Antimicrobials, along with biosecurity and management practices such as optimizing animal density, nutrition, hygiene, and measures aimed at reducing skin injuries in piglets, remain essential for controlling S. hyicus outbreaks, particularly in the absence of a commercial vaccine [29]. However, antimicrobial use should be guided by responsible stewardship principles to limit the development and spread of AMR. Therefore, evaluating the phenotypic AMR profile of S. hyicus recovered from clinical samples is crucial. Here, we observed a remarkably high prevalence of MDR S. hyicus (83.7%) on Spanish swine farms, consistent with previous studies from Japan and Brazil [14,30]. Notably, 51% of S. hyicus were resistant to six to eight different antimicrobial classes, highlighting the particularly high AMR observed in most isolates. When evaluating AMR by specific antimicrobials, we found the highest AMR frequencies against those most frequently used in Spanish swine production [31], such as lincosamides (83.7%), pleuromutilins (81.6%), penicillins (75.5%), and tetracyclines (73.5%). Resistance to last-resort antimicrobials was remarkably lower, particularly for ceftiofur (4.1%) and, to a lesser extent, for enrofloxacin (40.8%). This contrasts with higher enrofloxacin resistance reported in Brazil [14], likely due to less restrictive legislation on antimicrobial use in food-producing animals. These findings underscore the impact of antimicrobial use on the AMR phenotype of S. hyicus and emphasize the need for targeted diagnosis to promote responsible antimicrobial use and therapeutic success.
Several factors influence the selection of specific bacterial strains, with antibiotic use being one of the primary factors [32]. AMR selective pressure can also lead to cross-selection and co-selection events. Cross-selection occurs when a single antimicrobial resistance gene (ARG) confers resistance to multiple antimicrobial classes, while co-selection occurs when two genes are physically linked on a piece of DNA so are inherited together, either chromosomally encoded or located in potentially mobilizable genomic regions [33]. In this study, we observed strong associations between certain antimicrobial classes, such as lincosamides with pleuromutilins (p < 0.001; Φ = 0.57) or macrolides (p < 0.001; Φ = 0.48). This may be explained by the cross-resistance conferred by certain ARGs such as lsa(E) or erm(T), previously reported in S. hyicus isolates from Spanish swine farms [34], which confer resistance to lincosamides and pleuromutilins, and macrolides and lincosamides, respectively. The co-selection of certain AMR phenotypes with ExhC and ExhD may be due to the possible association of these VFs and ARGs in certain potentially mobile genomic regions. Despite extensive characterization [35], little is known about the genomic location of these Exh genes. Furthermore, we observed a significant association between the AMR phenotype and the sample type, indicating potential adaptations of certain pathogenic S. hyicus strains or lineages to specific biological niches, as has been reported for S. aureus due to its genomic plasticity [36]. This is demonstrated by the differential presence of the chromosomally encoded SHETA. Therefore, further genomic characterization of S. hyicus is needed to clearly confirm the associations observed in this study.
In conclusion, here we provide significant insight into the wide diversity and complexity of virulence, AMR, and biofilm formation in S. hyicus isolates from Spanish swine farms, recovered from animals with cutaneous, reproductive and systemic clinical signs. A high prevalence of MDR isolates was observed, with particularly high resistance to antimicrobials commonly used in Spanish swine production. Additionally, only about half of the isolates harbored at least one VF gene, despite their isolation from clinical cases, suggesting the presence of other virulence mechanisms. Notably, all isolates demonstrated robust biofilm formation capacity, indicating a potential for survival and persistence in various biological niches. The associations between virulence factors, biofilm formation, and AMR patterns indicate the possible mechanisms of adaptation and selection in S. hyicus. These findings underscore the need for targeted diagnostics and further genomic characterization to improve the management and therapeutic strategies against S. hyicus in swine production.
4. Materials and Methods
4.1. Sampling and Bacterial Isolation and Characterization
Clinical samples were collected from the skin, reproductive exudates, joints, and brain of pigs displaying clinical signs compatible with epidermitis, metritis and systemic clinical signs such as polyserositis. These samples were obtained from both Iberian and white crossbred pigs from Spanish swine farms, with collections occurring from May to December 2023.
The collected swabs were cultured on blood agar plates (Oxoid, Madrid, Spain) for 24 h at 37 °C under aerobic conditions. Presumptive white, non-hemolytic colonies were subsequently subcultured on tryptic soy agar (TSA) (Condalab, Madrid, Spain) and incubated for 24 h at 37 °C. These colonies were confirmed as S. hyicus using MALDI-TOF mass spectrometry employing the IVD MALDI Biotyper (Bruker Daltonik, Bremen, Germany) according to the manufacturer’s standard protocols.
4.2. Molecular Characterization of Virulence Factors
For molecular characterization of the VFs, DNA extraction of S. hyicus isolates was performed. A single colony was inoculated in 100 µL sterile distilled water and boiled for 10 min at 100 °C. Subsequently, it was centrifuged at 12,000 rpm for 10 min and the supernatant with the DNA extracted was transferred to a new sterile microtube for further analyses. The purified supernatant was stored at −20 °C until further use.
The molecular characterization of the VSs was performed using simplex PCRs for the SHETA and SHETB genes and a multiplex PCR for the ExhA, ExhB, ExhC, and ExhD genes. The primers (20 µM) and the amplicon size (in base pairs, bp) are available in Supplementary Table S3.
The amplification protocol for the SHETA simplex PCR included a denaturization step at 94 °C for 3 min, followed by 30 amplification cycles that included a 30 s denaturization at 94 °C, 30 s, annealing at 58 °C, and a 1 min and 10 s extension at 72 °C. It finalized with a final extension at 72 °C for 10 min. The SHETB simplex PCR consisted of a denaturization step at 94 °C for 2 min, followed by 35 amplification cycles that included a 30 s denaturization at 94 °C, 30 s, annealing at 50 °C, and a 1 min and 10 s extension at 72 °C. It finalized with a final extension at 72 °C for 10 min.
For its part, the multiplex PCR included a denaturization step at 94 °C for 2 min, followed by 35 amplification cycles with a 30 s denaturization at 94 °C, 30 s annealing at 50 °C, and a 1 min and 10 s extension at 72 °C. It finalized with a final extension at 72 °C for 10 min.
The mixture for both multiplex and simplex PCR reactions consisted of 3 μL of extracted DNA, 0.5 μL of DNA polymerase (5 U/μL) (Biotools, Madrid, Spain), 6.5 μL of 10X amplification PCR buffer with MgCl2 (Biotools, Madrid, Spain), 0.5 μL of each primer (20 μM) (Roche Diagnostics, Basel, Switzerland), 1 μL of dNTPs (25 mM each) (Biotools, Madrid, Spain), and nuclease-free water (Invitrogen, Carlsbad, CA, USA) to reach a final volume of 50 μL.
4.3. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing followed the procedures outlined by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [37]. The MIC of the tested antimicrobials was determined using the broth microdilution method. It established the MIC50 and MIC90, which are the concentrations that inhibit the growth of 50% and 90% of the isolates, respectively. The microbiological resistance was determined in accordance with the ECOFF value, thus dividing the microorganisms depending on whether they have (non-wild type, NWT) or not (wild type, WT) acquired resistance mechanisms to each antimicrobial [38]. Non-wild type and resistant phenotype are indistinctly used throughout this study. MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial classes [39]. A microorganism susceptible to all antimicrobials tested was defined as pansusceptible (PNS).
AMR was evaluated with BOPO6F Sensititre plates (TREK Diagnostic Systems, East Grinstead, UK). The antimicrobials evaluated and their ECOFFs are shown in Table 2. Staphylococcus aureus ATCC 29213 was used as the control strain. The ECOFFs were primarily selected for S. hyicus and if no information was available, they were extrapolated from S. aureus (e.g., penicillin, ceftiofur, spectinomycin, and clindamycin). No ECOFF could be established for sulfadimethoxine and danofloxacin.
S. hyicus isolates were cultured on TSA at 37 °C for 24 h under aerophilic conditions. After its growth, a single colony was resuspended in 5 mL of 0.9% sterile saline solution to reach a turbidity of McFarland 0.5. Fifty microliters of the bacterial suspension were transferred to 11 mL of Mueller-Hinton broth (TREK Diagnostic Systems, East Grinstead, UK) and 50 µL per well was dispensed with the Sensititre AIM Automated Inoculation Delivery System (TREK Diagnostic Systems, East Grinstead, UK). Plates were sealed and incubated in an aerophilic atmosphere at 37 °C for 24 h.
4.4. Biofilm Formation Assay
Biofilm formation of S. hyicus was quantified by crystal violet staining, following a biofilm formation protocol previously described for S. aureus [40] with slight modifications. Briefly, a single colony was inoculated into 96-well polystyrene microfiber cell culture-treated plates (Corning Incorporated, Corning, NY, USA) containing 200 µL of tryptic soy broth (TSB) (Condalab, Madrid, Spain) supplemented with 0.1% glucose (VWR, Leuven, Belgium) and incubated for 24 h at 37 °C under aerobic conditions. Sterile TSB was used as the negative control.
Following incubation, the culture medium and unattached bacteria were removed by aspiration. The formed biofilms were stained with 100 μL of 2% crystal violet for 30 min, washed three times with distilled water, and dried at 37 °C for 15 min. To release the dye, 100 μL of 95% ethanol was added and the plates were briefly agitated. The absorbance of the biofilm biomass was quantified at 595 nm (A595). All assays were conducted in triplicate to ensure reliability of the results. The final optical density (OD) value of each isolate was expressed as the mean of the three measurements subtracting the average OD of the negative control (difference from the control, DC), to lessen the possible unevenness in absorbance quantification.
4.5. Data Analysis and Results Visualization
A database was created in an Excel sheet (Microsoft Office 365) to include metadata, VFs (presence or absence), antimicrobials tested (WT or NWT) and biofilm formation. The metadata were the Spanish region from which the clinical sample was recovered, clinical signs (epidermitis, metritis, and systemic), type of sample (skin, joint, brain or reproductive exudate), production phase (suckling, weaning, and sow/boar), and pig breed (Iberian breed or white crossbred pig). AMR analyses were performed at the antimicrobial and antimicrobial class level. Biofilm formation was expressed numerically as DC and categorized based on the DC value into low (DC ≤ 2), medium (2 > DC ≤ 3), and high (DC > 3), as previously described [41]. All analyses were conducted using R version 4.3.2 (31 October 2023 ucrt) [42]. Plots were produced using the ggplot2 version 3.5.1 [43], igraph version 2.0.3 [44] and ggraph version 2.2.1 [45] packages and further modified using the software Inkscape version 1.3.2 (https://inkscape.org/, accessed on 11 July 2024).
A clustering of S. hyicus isolates was performed according to their AMR phenotype using the unweighted pair group method with arithmetic mean (UPGMA) as the hierarchical clustering method. The pheatmap package [46] was used for the representation of the clustered heatmaps of isolates. Comparisons among isolates for metadata, VFs, and AMR phenotype were carried out with Fisher’s exact test. Comparisons for DC biofilm formation was carried out using the Wilcoxon rank-sum test. p-values were adjusted following the Benjamini and Hochberg method [47]. Significance was established at p < 0.05.
Ordination of S. hyicus isolates based on their AMR phenotype and VFs was estimated using a Jaccard distance matrix and analyzed by principal component analysis (PCA), and the two main dimensions for the principal components were characterized. The effect of the metadata, AMR phenotypes, and VFs was determined by permutational multivariate analysis of variance (PERMANOVA) using distance matrices with adonis2 function.
Associations between VF genes and the AMR phenotype consisted of a primary approach involving the execution of Fisher’s exact test to identify significant associations between the VFs and AMR profiles. It was further complemented by calculation of the Phi coefficient (Φ) to measure the strength of these associations. Subsequently, the percentage of occurrence for each VF and antimicrobial was computed. Significant associations were visualized in a network graph, where node sizes were determined by their percentage occurrence and edge sizes reflected the magnitude of Φ. Φ was categorized as very strong (Φ > 0.25), strong (Φ > 0.15), moderate (Φ > 0.10) or weak (Φ > 0.05).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13090871/s1, Table S1: Combination of virulence factor genes in 49 Staphylococcus hyicus isolates from Spanish swine farms; Table S2: Combination of phenotypic antimicrobial resistance at the class level in 49 Staphylococcus hyicus isolates from Spanish swine farms; Table S3: Primers used for molecular characterization of Staphylococcus hyicus virulence factors.
Author Contributions
Conceptualization, S.M.-M., O.M.-A., C.B.G.-M. and Á.A.-T.; methodology, E.R.-C., A.G.-F. and R.M.-P.; software, O.M.-A.; validation, S.M.-M., O.M.-A. and C.B.G.-M.; formal analysis, O.M.-A. and E.R.-C.; investigation, E.R.-C.; A.G.-F., O.M.-A. and A.I.P.-C.; resources, O.M.-A., E.R.-C., Á.A.-T. and S.M.-M.; data curation, O.M.-A.; writing—original draft preparation, O.M.-A. and E.R.-C.; writing—review and editing, O.M.-A., E.R.-C., A.I.P.-C. and S.M.-M.; visualization, O.M.-A.; supervision, S.M.-M. and C.B.G.-M.; project administration, S.M.-M.; funding acquisition, S.M.-M. All authors have read and agreed to the published version of the manuscript.
Funding
Alba González-Fernández holds a grant from the University of León. Rubén Miguélez-Pérez holds a grant from Junta de Castilla y León co-financed by the European Social Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author/s.
Acknowledgments
We acknowledge the excellent technical assistance provided by María Mediavilla and the contribution in some parts by Mario Delgado and Carmen Arenas.
Conflicts of Interest
Author Álvaro Aguarón-Turrientes was employed by the company Laboratorios SYVA. The remaining authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Casanova, C.; Iselin, L.; Von Steiger, N.; Droz, S.; Sendi, P. Staphylococcus hyicus Bacteremia in a Farmer. J. Clin. Microbiol. 2011, 49, 4377–4378. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Friendship, R.M.; Poljak, Z.; Weese, J.S.; Dewey, C.E. An Investigation of Exudative Epidermitis (Greasy Pig Disease) and Antimicrobial Resistance Patterns of Staphylococcus hyicus and Staphylococcus aureus Isolated from Clinical Cases. Can. Vet. J. 2013, 54, 139. [Google Scholar] [PubMed]
- Wegener, H.C.; Skov-Jensen, E.W. Exudative epidermitis. In Diseases of Swine, 9th ed.; Straw, B.E., Zimmerman, J.J., D’Allaire, S., Taylor, D.J., Eds.; Blackwell Pub: Ames, IA, USA, 2006; pp. 675–679. [Google Scholar]
- de Winter, P.J.J.; Verdonck, M.; de Kruif, A.; Devriese, L.A.; Haesebrouck, F. Bacterial Endometritis and Vaginal Discharge in the Sow: Prevalence of Different Bacterial Species and Experimental Reproduction of the Syndrome. Anim. Reprod. Sci. 1995, 37, 325–335. [Google Scholar] [CrossRef]
- Hill, B.D.; Corney, B.G.; Wagner, T.M. Importance of Staphylococcus hyicus as a Cause of Arthritis in Pigs up to 12 Weeks of Age. Aust. Vet. J. 1996, 73, 179–181. [Google Scholar] [CrossRef]
- Tanabe, T.; Sato, H.; Sato, H.; Watanabe, K.; Hirano, M.; Hirose, K.; Kurokawa, S.; Nakano, K.; Saito, H.; Maehara, N. Correlation between Occurrence of Exudative Epidermitis and Exfoliative Toxin-Producing Ability of Staphylococcus hyicus. Vet. Microbiol. 1996, 48, 9–17. [Google Scholar] [CrossRef]
- Leekitcharoenphon, P.; Pamp, S.J.; Andresen, L.O.; Aarestrup, F.M. Comparative Genomics of Toxigenic and Non-Toxigenic Staphylococcus hyicus. Vet. Microbiol. 2016, 185, 34–40. [Google Scholar] [CrossRef]
- Andresen, L.O.; Ahrens, P. A Multiplex PCR for Detection of Genes Encoding Exfoliative Toxins from Staphylococcus hyicus. J. Appl. Microbiol. 2004, 96, 1265–1270. [Google Scholar] [CrossRef]
- Sato, H.; Watanabe, T.; Higuchi, K.; Teruya, K.; Ohtake, A.; Murata, Y.; Saito, H.; Aizawa, C.; Danbara, H.; Maehara, N. Chromosomal and Extrachromosomal Synthesis of Exfoliative Toxin from Staphylococcus hyicus. J. Bacteriol. 2000, 182, 4096. [Google Scholar] [CrossRef]
- Watanabe, T.; Sato, H.; Hatakeyama, Y.; Matsuzawa, T.; Kawai, M.; Aizawa, C.; Danbara, H.; Maehara, N. Cloning of the Gene Coding for Staphylococcus hyicus Exfoliative Toxin B and Its Expression in Escherichia coli. J. Bacteriol. 2000, 182, 4101. [Google Scholar] [CrossRef]
- Argemi, X.; Hansmann, Y.; Prola, K.; Prévost, G. Coagulase-Negative Staphylococci Pathogenomics. Int. J. Mol. Sci. 2019, 20, 1215. [Google Scholar] [CrossRef]
- Silva, V.; Correia, E.; Pereira, J.E.; González-Machado, C.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Exploring the Biofilm Formation Capacity in S. pseudintermedius and Coagulase-Negative Staphylococci Species. Pathogens 2022, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Regulation—2019/6—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2019/6/oj (accessed on 20 July 2024).
- Moreno, A.M.; Moreno, L.Z.; Poor, A.P.; Matajira, C.E.C.; Moreno, M.; Gomes, V.T.d.M.; da Silva, G.F.R.; Takeuti, K.L.; Barcellos, D.E. Antimicrobial Resistance Profile of Staphylococcus hyicus Strains Isolated from Brazilian Swine Herds. Antibiotics 2022, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, J.; Zhu, L.; Guo, C.; Lu, H.; Guo, C.; Li, X.; Wang, X. A Fatal Suppurative Pneumonia in Piglets Caused by a Pathogenic Coagulase-Positive Strain of Staphylococcus hyicus. Vet. Res. Commun. 2017, 41, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Foissac, M.; Lekaditi, M.; Loutfi, B.; Ehrhart, A.; Dauchy, F.A. Spondylodiscitis and Bacteremia Due to Staphylococcus hyicus in an Immunocompetent Man. Germs 2016, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Kirk, F.; Mashicharan, M.; Braddick, M.; Saxena, P. Staphylococcus hyicus, a Novel Pathogen Causing Destructive Infective Endocarditis Requiring Mitral Annular Reconstruction. JTCVS Tech. 2022, 13, 70. [Google Scholar] [CrossRef]
- Andresen, L.O. Production of Exfoliative Toxin by Isolates of Staphylococcus hyicus from Different Countries. Vet. Rec. 2005, 157, 376–378. [Google Scholar] [CrossRef]
- Kanbar, T.; Voytenko, A.V.; Alber, J.; Lämmler, C.; Weiss, R.; Skvortzov, V.N. Distribution of the Putative Virulence Factor Encoding Gene Sheta in Staphylococcus hyicus Strains of Various Origins. J. Vet. Sci. 2008, 9, 327. [Google Scholar] [CrossRef]
- Futagawa-Saito, K.; Ba-Thein, W.; Higuchi, T.; Sakurai, N.; Fukuyasu, T. Nationwide Molecular Surveillance of Exfoliative Toxigenic Staphylococcus hyicus on Pig Farms across Japan. Vet. Microbiol. 2007, 124, 370–374. [Google Scholar] [CrossRef]
- Onuma, K.; Uoya, Y.; Koide, T.; Shibata, A.; Tanabe, T.; Sato, H. Detection of Staphylococcus hyicus Exfoliative Toxin Genes by Dot Blot Hybridization and Multiplex Polymerase Chain Reaction. Microbiol. Immunol. 2011, 55, 168–173. [Google Scholar] [CrossRef]
- Fudaba, Y.; Nishifuji, K.; Andresen, L.O.; Yamaguchi, T.; Komatsuzawa, H.; Amagai, M.; Sugai, M. Staphylococcus hyicus Exfoliative Toxins Selectively Digest Porcine Desmoglein 1. Microb. Pathog. 2005, 39, 171–176. [Google Scholar] [CrossRef]
- Király, J.; Hajdučková, V.; Gregová, G.; Szabóová, T.; Pilipčinec, E. Resistant S. aureus Isolates Capable of Producing Biofilm from the Milk of Dairy Cows with Subclinical Mastitis in Slovakia. Agriculture 2024, 14, 571. [Google Scholar] [CrossRef]
- Araújo, D.; Silva, A.R.; Fernandes, R.; Serra, P.; Barros, M.M.; Campos, A.M.; Oliveira, R.; Silva, S.; Almeida, C.; Castro, J. Emerging Approaches for Mitigating Biofilm-Formation-Associated Infections in Farm, Wild, and Companion Animals. Pathogens 2024, 13, 320. [Google Scholar] [CrossRef]
- Dastghey, S.; Parvizi, J.; Shapiro, I.M.; Hickok, N.J.; Otto, M. Effect of Biofilms on Recalcitrance of Staphylococcal Joint Infection to Antibiotic Treatment. J. Infect. Dis. 2015, 211, 641. [Google Scholar] [CrossRef] [PubMed]
- Hagstrand Aldman, M.; Thompson, O.; Påhlman, L.I. Biofilm Formation Is Associated with Poor Outcome in Prosthetic Joint Infections Caused by Staphylococcus lugdunensis. Infect. Dis. 2023, 55, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Miguelez-Perez, R.; Mencia-Ares, O.; Gutierrez-Martin, C.B.; Gonzalez-Fernandez, A.; Petrocchi-Rilo, M.; Delgado-Garcia, M.; Martinez-Martinez, S. Biofilm Formation in Streptococcus suis: In Vitro Impact of Serovar and Assessment of Coinfections with Other Porcine Respiratory Disease Complex Bacterial Pathogens. bioRxiv 2024. [Google Scholar] [CrossRef]
- Silva, V.; Correia, E.; Pereira, J.E.; González-Machado, C.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Biofilm Formation of Staphylococcus aureus from Pets, Livestock, and Wild Animals: Relationship with Clonal Lineages and Antimicrobial Resistance. Antibiotics 2022, 11, 772. [Google Scholar] [CrossRef]
- Arsenakis, I.; Boyen, F.; Haesebrouck, F.; Maes, D.G.D. Autogenous Vaccination Reduces Antimicrobial Usage and Mortality Rates in a Herd Facing Severe Exudative Epidermitis Outbreaks in Weaned Pigs. Vet. Rec. 2018, 182, 744. [Google Scholar] [CrossRef]
- Futagawa-Saito, K.; Ba-Thein, W.; Fukuyasu, T. Antimicrobial Susceptibilities of Exfoliative Toxigenic and Non-Toxigenic Staphylococcus hyicus Strains in Japan. J. Vet. Med. Sci. 2009, 71, 681–684. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2022—Trends from 2010 to 2022—Thirteenth ESVAC Report. 2023. Available online: https://op.europa.eu/en/publication-detail/-/publication/59eda847-b429-11ee-b164-01aa75ed71a1/language-en (accessed on 23 July 2024).
- Larsson, D.G.J.; Flach, C.F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2021, 20, 257–269. [Google Scholar] [CrossRef]
- Murray, L.M.; Hayes, A.; Snape, J.; Kasprzyk-Hordern, B.; Gaze, W.H.; Murray, A.K. Co-Selection for Antibiotic Resistance by Environmental Contaminants. NPJ Antimicrob. Resist. 2024, 2, 9. [Google Scholar] [CrossRef]
- Mencía-Ares, O.; Borowiak, M.; Argüello, H.; Cobo-Díaz, J.F.; Malorny, B.; Álvarez-Ordóñez, A.; Carvajal, A.; Deneke, C. Genomic Insights into the Mobilome and Resistome of Sentinel Microorganisms Originating from Farms of Two Different Swine Production Systems. Microbiol. Spectr. 2022, 10, e02896-22. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, P.; Andresen, L.O. Cloning and Sequence Analysis of Genes Encoding Staphylococcus hyicus Exfoliative Toxin Types A, B, C, and D. J. Bacteriol. 2004, 186, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus Host Interactions and Adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- EUCAST The European Committee on Antimicrobial Susceptibility Testing—EUCAST. Available online: http://www.eucast.org/ (accessed on 15 May 2024).
- EUCAST MIC and Inhibition Zone Diameter Distributions of Microorganisms without and with Phenotypically Evident Resistance Mechanisms. Available online: https://mic.eucast.org/ (accessed on 17 May 2024).
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Croes, S.; Deurenberg, R.H.; Boumans, M.L.L.; Beisser, P.S.; Neef, C.; Stobberingh, E.E. Staphylococcus aureus Biofilm Formation at the Physiologic Glucose Concentration Depends on the S. aureus Lineage. BMC Microbiol. 2009, 9, 229. [Google Scholar] [CrossRef]
- Dong, C.L.; Che, R.X.; Wu, T.; Qu, Q.W.; Chen, M.; Zheng, S.D.; Cai, X.H.; Wang, G.; Li, Y.H. New Characterization of Multi-Drug Resistance of Streptococcus suis and Biofilm Formation from Swine in Heilongjiang Province of China. Antibiotics 2023, 12, 132. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Wickham, H. Ggplot2. 2016. Available online: https://link.springer.com/book/10.1007/978-3-319-24277-4 (accessed on 17 June 2024).
- Network Analysis and Visualization [R Package Igraph Version 2.0.3]. 2024. Available online: https://cran.r-project.org/web/packages/igraph/index.html (accessed on 17 June 2024).
- Pedersen, T.L. An Implementation of Grammar of Graphics for Graphs and Networks [R Package Ggraph Version 2.2.1]. 2024. Available online: https://cran.r-project.org/web/packages/ggraph/index.html (accessed on 17 June 2024).
- CRAN: Package Pheatmap. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 24 July 2024).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).