Antimicrobial Resistance Profile and Biofilm Formation of Listeria monocytogenes Isolated from Meat
Abstract
:1. Introduction
2. Results
2.1. Prevalence and Virulence of L. monocytogenes Strains
2.2. Susceptibility to Antibiotics in L. monocytogenes Strains
2.3. Antimicrobial Resistance Genes Presence in L. monocytogenes Strains
2.4. Biofilm Formation of the Isolates
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Bacterial Isolates
4.2. Detection and Isolation of L. monocytogenes
4.3. Antimicrobial Susceptibility Testing
4.4. DNA Extraction
4.5. Characterization of Resistance Genes and Detection of Virulence Genes
4.6. Assessment of Biofilm-Forming Ability
4.7. Statystical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maung, A.T.; Mohammadi, T.N.; Nakashima, S.; Liu, P.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Antimicrobial Resistance Profiles of Listeria Monocytogenes Isolated from Chicken Meat in Fukuoka, Japan. Int. J. Food Microbiol. 2019, 304, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kayode, A.J.; Okoh, A.I. Antibiotic Resistance Profile of Listeria Monocytogenes Recovered from Ready-to-Eat Foods Surveyed in South Africa. J. Food Prot. 2022, 85, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Uludag, A.A.; Arslan Aydogdu, E.O.; Kimiran, A. The Determination of Presence of Listeria Monocytogenes in Ground Meat Sold in Istanbul. Gazi Univ. J. Sci. 2023, 36, 53–66. [Google Scholar] [CrossRef]
- Anwar, T.M.; Pan, H.; Chai, W.; Ed-Dra, A.; Fang, W.; Li, Y.; Yue, M. Genetic Diversity, Virulence Factors, and Antimicrobial Resistance of Listeria Monocytogenes from Food, Livestock, and Clinical Samples between 2002 and 2019 in China. Int. J. Food Microbiol. 2022, 366, 109572. [Google Scholar] [CrossRef] [PubMed]
- Yushina, Y.K.; Kuznetsova, O.A.; Tutelyan, A.V.; Grudistova, M.A.; Bataeva, D.S.; Reshchikov, M.D.; Tartakovsky, I.S.; Nikolaev, Y.A. Prevalence of Listeria Monocytogenes in Meat Products during 2017–2019 Depending on Technological Factors and Seasons. Theory Pract. Meat Process. 2022, 7, 238–246. [Google Scholar] [CrossRef]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A Review of Listeria Monocytogenes from Meat and Meat Products: Epidemiology, Virulence Factors, Antimicrobial Resistance and Diagnosis. Onderstepoort J. Vet. Res. 2020, 87, e1–e20. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses Report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- Mughini-Gras, L.; Paganini, J.A.; Guo, R.; Coipan, C.E.; Friesema, I.H.M.; van Hoek, A.H.A.M.; van den Beld, M.; Kuiling, S.; Bergval, I.; Wullings, B.; et al. Source Attribution of Listeria Monocytogenes in the Netherlands. Int. J. Food Microbiol. 2025, 427, 110953. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Sun, T.; Gorris, L.G.M.; Wang, X.; Liu, B.; Dong, Q. The Prevalence of Listeria Monocytogenes in Meat Products in China: A Systematic Literature Review and Novel Meta-Analysis Approach. Int. J. Food Microbiol. 2020, 312, 108358. [Google Scholar] [CrossRef]
- Eldin Morshdy, A.M.; Nagaty, E.A.; Abdallah, K.M.; Darwish, W.S.; Mahmoud, A.F. Prevalence of Listeria Monocytogenes in Meat Products Retailed in Egypt and Worldwide: A Review. J. Adv. Vet. Res. 2023, 13, 1487–1490. [Google Scholar]
- Li, W.; Yang, Z.; Hu, J.; Wang, B.; Rong, H.; Li, Z.; Sun, Y.; Wang, Y.; Zhang, X.; Wang, M.; et al. Evaluation of Culturable ‘Last-Resort’ Antibiotic Resistant Pathogens in Hospital Wastewater and Implications on the Risks of Nosocomial Antimicrobial Resistance Prevalence. J. Hazard Mater. 2022, 438, 129477. [Google Scholar] [CrossRef] [PubMed]
- Gana, J.; Gcebe, N.; Moerane, R.; Ngoshe, Y.B.; Moabelo, K.; Adesiyun, A.A. Detection of Pathogenic Serogroups and Virulence Genes in Listeria Monocytogenes Strains Isolated from Beef and Beef Products Retailed in Gauteng Province, South Africa, Using Phenotypic and Polymerase Chain Reaction (PCR)-Based Methods. Int. J. Microbiol. 2024, 2024, 8891963. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, R.A.S.S.; Satharasinghe, D.A.; Tang, J.Y.H.; Rukayadi, Y.; Radu, K.R.; New, C.Y.; Son, R.; Premarathne, J.M.K.J.K. Persistence of Listeria Monocytogenes in Food Commodities: Foodborne Pathogenesis, Virulence Factors, and Implications for Public Health. Food Res. 2021, 5, 1–16. [Google Scholar]
- Milica, P. The Emphasis of Listeria Monocytogenes in Raw Meat. In Proceedings of the 4th International Congress “Food Technology, Quality and Safety”, Novi Sad, Szerbia, 23–25 October 2018. [Google Scholar]
- Silva, V.; Correia, E.; Pereira, J.E.; González-Machado, C.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Exploring the Biofilm Formation Capacity in S. Pseudintermedius and Coagulase-Negative Staphylococci Species. Pathogens 2022, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, F.; Alvarez-Ordóñez, A.; Oliveira, M.; Ferreira, S.; Lovisolo, S.; Vono, C.; Cannizzo, F.T.; Chiesa, F.; Civera, T.; Di Ciccio, P. Effect of Neutral Electrolyzed Water on Biofilm Formed by Meat-Related Listeria Monocytogenes: Intraspecies Variability and Influence of the Growth Surface Material. Int. J. Food Microbiol. 2025, 431, 111064. [Google Scholar] [CrossRef]
- Silva, A.S.; Duarte, E.A.A.; Oliveira, T.A.S.D.; Evangelista-Barreto, N.S. Identification of Listeria Monocytogenes in Cattle Meat Using Biochemical Methods and Amplification of the Hemolysin Gene. An. Acad. Bras. Ciencias 2020, 92, e20180557. [Google Scholar] [CrossRef]
- Doijad, S.P.; Barbuddhe, S.B.; Garg, S.; Poharkar, K.V.; Kalorey, D.R.; Kurkure, N.V.; Rawool, D.B.; Chakraborty, T. Biofilm-Forming Abilities of Listeria Monocytogenes Serotypes Isolated from Different Sources. PLoS ONE 2015, 10, e0137046. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 15.0; European Committee on Antimicrobial Susceptibility Testing (EUCAST): Växjö, Sweden, 2025. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Esteves, A.; Panera-Martínez, S.; Capita, R.; Alonso-Calleja, C. Quantification of Total and Viable Cells and Determination of Serogroups and Antibiotic Resistance Patterns of Listeria Monocytogenes in Chicken Meat from the North-Western Iberian Peninsula. Antibiotics 2022, 11, 1828. [Google Scholar] [CrossRef]
- Henriques, A.R.; Melo Cristino, J.; Fraqueza, M.J. Genetic Characterization of Listeria Monocytogenes Isolates from Industrial and Retail Ready-to-Eat Meat-Based Foods and Their Relationship with Clinical Strains from Human Listeriosis in Portugal. J. Food Prot. 2017, 80, 551–560. [Google Scholar] [CrossRef]
- Hodžić, S.; Hukić, M. Presence and Serological Characteristics of Listeria Monocytogenes in Meat and Meat Products. HealthMED 2012, 6, 2593–2599. [Google Scholar]
- Cavalcanti, A.A.C.; Limeira, C.H.; de Siqueira, I.N.; de Lima, A.C.; de Medeiros, F.J.P.; de Souza, J.G.; Medeiros, N.G.d.A.; Filho, A.A.d.O.; de Melo, M.A. The Prevalence of Listeria Monocytogenes in Meat Products in Brazil: A Systematic Literature Review and Meta-Analysis. Res. Vet. Sci. 2022, 145, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Boukili, M.; Rhazi Filali, F.; Lafkih, N.; Bouymajane, A.; Sefiani, M.; Moumni, M. Prevalence, Characterization and Antimicrobial Resistance of Listeria Monocytogenes Isolated from Beef Meat in Meknes City, Morocco. Germs 2020, 10, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Duma, M.N.; Ciupescu, L.M.; Dan, S.D.; Crisan-Reget, O.L.; Tabaran, A. Virulence and Antimicrobial Resistance of Listeria Monocytogenes Isolated from Ready-to-Eat Food Products in Romania. Microorganisms 2024, 12, 954. [Google Scholar] [CrossRef]
- Miranda, J.M.; Vázquez, B.I.; Fente, C.A.; Calo-Mata, P.; Cepeda, A.; Franco, C.M. Comparison of Antimicrobial Resistance in Escherichia Coli, Staphylococcus Aureus, and Listeria Monocytogenes Strains Isolated from Organic and Conventional Poultry Meat. J. Food Prot. 2008, 71, 2537–2542. [Google Scholar] [CrossRef]
- Kawacka, I.; Pietrzak, B.; Schmidt, M.; Olejnik-Schmidt, A. Listeria Monocytogenes Isolates from Meat Products and Processing Environment in Poland Are Sensitive to Commonly Used Antibiotics, with Rare Cases of Reduced Sensitivity to Ciprofloxacin. Life 2023, 13, 821. [Google Scholar] [CrossRef]
- Tayeb, B.A.; Mohamed-Sharif, Y.H.; Choli, F.R.; Haji, S.S.; Ibrahim, M.M.; Haji, S.K.; Rasheed, M.J.; Mustafa, N.A. Antimicrobial Susceptibility Profile of Listeria Monocytogenes Isolated from Meat Products: A Systematic Review and Meta-Analysis. Foodborne Pathog. Dis. 2023, 20, 315–333. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of Antibiotic Residues in Animal Food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Gargano, V.; Sciortino, S.; Gambino, D.; Costa, A.; Agozzino, V.; Reale, S.; Alduina, R.; Vicari, D. Antibiotic Susceptibility Profile and Tetracycline Resistance Genes Detection in Salmonella Spp. Strains Isolated from Animals and Food. Antibiotics 2021, 10, 809. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Gomes, J.P.; Coelho, A.; Batista, R.; Saraiva, C.; Esteves, A.; Martins, Â.; Contente, D.; Diaz-Formoso, L.; et al. Listeria Monocytogenes from Food Products and Food Associated Environments: Antimicrobial Resistance, Genetic Clustering and Biofilm Insights. Antibiotics 2024, 13, 447. [Google Scholar] [CrossRef]
- Piechota, M.; Kot, B.; Frankowska-Maciejewska, A.; Gruzewska, A.; Woźniak-Kosek, A. Biofilm Formation by Methicillin-Resistant and Methicillin-Sensitive Staphylococcus Aureus Strains from Hospitalized Patients in Poland. Biomed Res. Int. 2018, 2018, 4657396. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, G.; Dilhari, A.; Gayani, B.; Kottegoda, N.; Samaranayake, L.; Weerasekera, M. Influence of Laboratory Culture Media on in Vitro Growth, Adhesion, and Biofilm Formation of Pseudomonas Aeruginosa and Staphylococcus Aureus. Med. Princ. Pract. 2019, 28, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, C.; Soteyome, T.; Zhao, X.; Xia, J.; Xu, W.; Mao, Y.; Peng, R.; Chen, J.; Xu, Z.; et al. Inhibitory Effects of Two Types of Food Additives on Biofilm Formation by Foodborne Pathogens. Microbiologyopen 2019, 8, e00853. [Google Scholar] [CrossRef]
- Vargová, M.; Zigo, F.; Výrostková, J.; Farkašová, Z.; Rehan, I.F. Biofilm-Producing Ability of Staphylococcus Aureus Obtained from Surfaces and Milk of Mastitic Cows. Vet. Sci. 2023, 10, 386. [Google Scholar] [CrossRef]
- Di Ciccio, P.; Rubiola, S.; Panebianco, F.; Lomonaco, S.; Allard, M.; Bianchi, D.M.; Civera, T.; Chiesa, F. Biofilm Formation and Genomic Features of Listeria Monocytogenes Strains Isolated from Meat and Dairy Industries Located in Piedmont (Italy). Int. J. Food Microbiol. 2022, 378, 109784. [Google Scholar] [CrossRef]
- EN ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria spp. ISO: Geneva, Switzerland, 2017.
- Silva, V.; Hermenegildo, S.; Ferreira, C.; Manaia, C.M.; Capita, R.; Alonso-Calleja, C.; Carvalho, I.; Pereira, J.E.; Maltez, L.; Capelo, J.L.; et al. Genetic Characterization of Methicillin-Resistant Staphylococcus Aureus Isolates from Human Bloodstream Infections: Detection of MLSB Resistance. Antibiotics 2020, 9, 375. [Google Scholar] [CrossRef]
- Oniciuc, E.A.; Cerca, N.; Nicolau, A.I. Compositional Analysis of Biofilms Formed by Staphylococcus Aureus Isolated from Food Sources. Front. Microbiol. 2016, 7, 390. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of Multiple Methods for Quantification of Microbial Biofilms Grown in Microtiter Plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef]
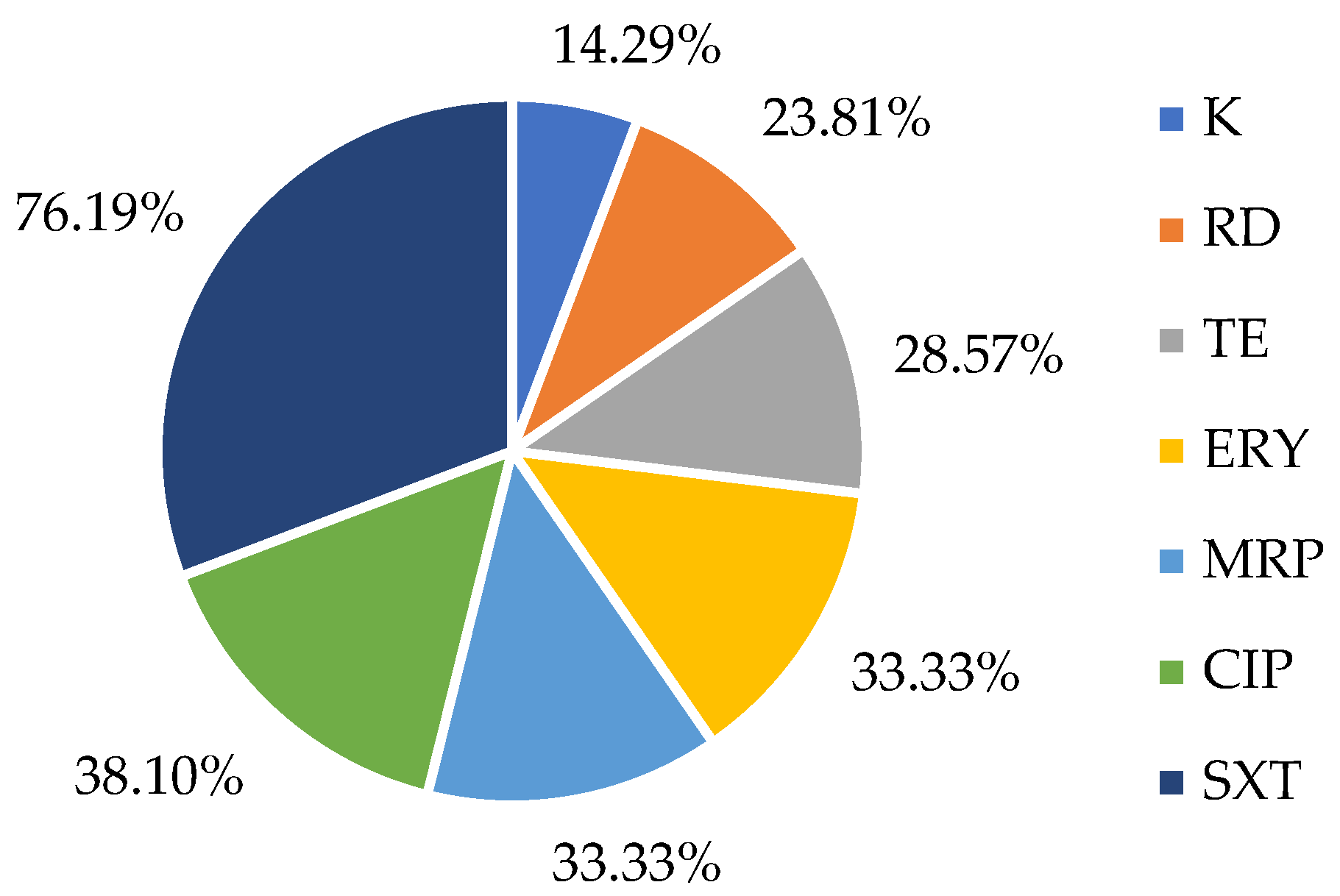

| Antimicrobial Resistance | ||||
|---|---|---|---|---|
| Isolate Code | Phenotype | Genotype | Virulence Gene Detected | Source |
| JP01 | - | - | hlyA | Alheira |
| JP02 | SXT | - | hlyA | Alheira |
| JP03 | TE | - | hlyA | Meat skewer |
| JP04 | TE | - | hlyA | Meat skewer |
| JP05 | RD-CIP-K-ERY-MRP-SXT | aadA, tetK | hlyA | Minced meat (bovine) |
| JP06 | RD-CIP-K-ERY-MRP-SXT | aadA, tetK | hlyA | Minced meat (bovine) |
| JP07 | RD-CIP-ERY-TE-MRP-SXT | ermC, tetL | hlyA | Minced meat (poultry) |
| JP08 | RD-CIP-ERY-TE-MRP-SXT | blaOXA-48, tetK, tetL | hlyA | Minced meat (poultry) |
| JP09 | CIP-ERY-MRP-SXT | blaOXA-48, tetK, tetL | hlyA | Minced meat (poultry) |
| JP10 | RD-CIP-K-ERY-TE-MRP-SXT | aadA, blaOXA-48 | hlyA | Minced meat (poultry) |
| JP11 | - | - | hlyA | Breaded meat |
| JP12 | SXT | - | hlyA | Fresh sausage (swine) |
| JP13 | CIP-SXT | - | hlyA | Fresh sausage (swine) |
| JP14 | - | - | hlyA | Minced meat (bovine) |
| JP15 | TE-SXT | - | hlyA | Meat skewer |
| JP16 | SXT | - | hlyA | Meat skewer |
| JP17 | SXT | - | hlyA | Meat skewer |
| JP18 | MRP-SXT | - | hlyA | Minced meat (poultry) |
| JP19 | CIP-SXT | - | hlyA | Minced meat (poultry) |
| JP20 | ERY-SXT | msr(A/B) | hlyA | Alheira |
| JP21 | SXT | - | hlyA | Alheira |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, J.; Silva, V.; Poeta, P.; Saraiva, C. Antimicrobial Resistance Profile and Biofilm Formation of Listeria monocytogenes Isolated from Meat. Antibiotics 2025, 14, 454. https://doi.org/10.3390/antibiotics14050454
Paiva J, Silva V, Poeta P, Saraiva C. Antimicrobial Resistance Profile and Biofilm Formation of Listeria monocytogenes Isolated from Meat. Antibiotics. 2025; 14(5):454. https://doi.org/10.3390/antibiotics14050454
Chicago/Turabian StylePaiva, Joana, Vanessa Silva, Patrícia Poeta, and Cristina Saraiva. 2025. "Antimicrobial Resistance Profile and Biofilm Formation of Listeria monocytogenes Isolated from Meat" Antibiotics 14, no. 5: 454. https://doi.org/10.3390/antibiotics14050454
APA StylePaiva, J., Silva, V., Poeta, P., & Saraiva, C. (2025). Antimicrobial Resistance Profile and Biofilm Formation of Listeria monocytogenes Isolated from Meat. Antibiotics, 14(5), 454. https://doi.org/10.3390/antibiotics14050454









