Novel Antibacterial Approaches and Therapeutic Strategies
Abstract
1. Introduction
2. Factors Contributing to the Increase in Bacterial Infections
3. Search for Novel Targets and Therapies
4. Challenges in Developing Antibiotics Targeting Gram-Negative Outer Membranes
4.1. Structural Differences Between the Cell Wall (CW) of Gram-Positive and Gram-Negative Bacteria
4.2. Efflux Pumps and β-Lactamases
4.3. Strategies Based on Disrupting the OM
- The LolCDE Complex—The ATP-binding cassette (ABC) transporter LolCDE extracts lipoproteins from the inner membrane and is critical for their subsequent delivery to the OM. Recent structural studies have shed light on the transport mechanism of lipoproteins by LolCDE, involving significant rearrangements in the transmembrane helices causing large-scale “extrusion” movement of lipoprotein from the membrane [41]. Muñoz et al. [3] recently designed a novel class of selective and potential Gram-negative pathogenic bacteria active antibiotics called lolamicin [3]. For the drug design, they targeted the essential LolCDE complex, which mediates the transport of OM-specific lipoproteins to the periplasm via the cytoplasmic membrane [44]. Lolamicin displayed activity against an MDR-isolates collection of 130 strains via efficacy against multiple murine pneumonia and septicemia models, as well as protection against C. difficile gut infection in a mouse model of the microbiome [5]. They suggested that lolamicin is active against Gram-negative pathogenic bacteria but inactive toward commensals (both Gram-positive and -negative) based on low-sequence homology between the two classes of organisms. Thus, lolamicin seems to be at least a potential candidate for testing and possible application against human infections.
- Chaperones: LolA and LolB are periplasmic chaperone proteins that transfer lipoproteins across the periplasm and insert them into OM. If either LolA or LolB is disrupted, lipoproteins become mislocalized and defects in OM assembly arise [45].
- Inhibition of Lipoprotein Diacylglyceryl Transferase (Lgt): It catalyzes the first step in lipoprotein biogenesis, where it acylates lipoproteins in the inner membrane. Lgt inhibitors like G2824 mess up OM permeability to make bacteria more susceptible to serum killing and antibiotics. Importantly, these inhibitors show activity against strains resistant to other lipoprotein pathway inhibitors [46].
4.4. These Complexities Pose Their Own Challenges in Clinical Development
5. Emerging Antibiotics and Strategies
5.1. Derivatives of Tetracycline (Eravacycline)
5.2. Cefiderocol
5.3. Aminoglycoside Derivatives (Plazomicin)
5.4. Delafloxacin
5.5. New Glycopeptides (Carbomycin)
5.6. Nanomaterials and Biomedicine
5.7. Antibiotic Potentiators
5.8. Bacteriophage Therapy
6. Post-Genomic Era Antibacterial Drug Discovery
6.1. Histidine Kinases in Two-Component (TCS) and Quorum Sensing Systems
6.2. Lipid Biosynthesis Pathways
6.3. Protein Synthesis Inhibition (Hybrid Antimicrobial Peptides)
6.4. The Bacterial Cell Wall and Membrane as Targets
| Target | Description | Mechanism of Action | Key Examples/Notes |
|---|---|---|---|
| Histidine Kinases (HKs) | Part of Two-Component Systems (TCS) involved in bacterial stress response and antibiotic resistance | Inhibition of HKs disrupts bacterial signaling pathways, reducing pathogenicity and virulence. | |
| Quorum Sensing Systems | Bacterial communication system regulating virulence and biofilm formation. | Inhibiting quorum sensing reduces pathogenicity and biofilm formation. |
|
| Lipid Biosynthesis Pathways | Essential for bacterial membrane integrity in Gram-negative bacteria | Inhibition of lipid A biosynthesis weakens bacterial outer membrane, increasing susceptibility to antibiotics. | |
| Protein Synthesis Inhibition | Critical for bacterial survival and growth. | Inhibition of ribosome function disrupts protein synthesis. | |
| Protein Kinase Inhibitors | Enzymes involved in bacterial growth and virulence, especially in M. tuberculosis. | Inhibition of serine/threonine kinases disrupts bacterial signaling and growth. |
|
| Peptidoglycan and Membrane Targets | Peptidoglycan layer is essential for bacterial cell wall integrity. | Disruption of peptidoglycan weakens the bacterial cell wall, leading to bacterial death. |
|
7. What Is New in Bacterial Genome Sequencing
7.1. Predicting Antibiotic Resistance Using Whole-Genome Sequencing
- Antimicrobial Resistance Prediction: The use of WGS data to predict resistance phenotypes (particularly in Gram-negative bacteria) is currently being explored. Machine learning models developed on WGS data can predict resistance to multiple antibiotics with a high degree of accuracy, enabling real-time clinical decisions and potentially swift treatment tailored to the group’s type and required level of therapy [85]. This could provide in silico antibiograms, thereby assisting the correlative management of resistant infections.
- Genomic Databases for Resistance Genes: Novel tools such as BacAnt and ARTS are available for genome annotation regarding antimicrobial resistance genes (ARGs) and mobile genetic elements (MGEs), which are crucial for monitoring the transmission of resistance genes. Such bioinformatics platforms automate the detection of ARGs and MGEs, aiding epidemiological studies and resistance monitoring [86].
7.2. Discovery of New Antibiotics Through Genome Mining
- Triggering of Silent Gene Clusters: Many genes responsible for producing antibiotics remain silent during normal laboratory conditions. With improvements in genome mining approaches such as CRISPR/gene-editing technologies, these silent clusters have been induced for expression, producing, e.g., lignin or lactobacilli [17].
- Drug Discovery with Comparative Genomics: Over the years, based on comparative genomic approaches, highly conserved genes across bacterial species have been essential as novel drug targets. Despite the inherent redundancy in secondary metabolite gene clusters, it has been possible to connect several novel chemical structures with antibacterial activity to their respective gene clusters for rational antibiotic design [87].
7.3. A Novel Approach for Antibiotic Resistance Surveillance—Metagenomics
- Environmental Resistome Studies: Most ARGs are present in environmental bacteria long before they can be recovered from clinical pathogens. Methods such as metagenomics and metatranscriptomics could further increase the detection of unknown ARGs in bacteria that may not be culturable [88].
- Predictive Surveillance for Emerging Resistance—High-throughput genomic sequencing integrated with functional genomics enables the observation of resistance genes across space and time. Such predictive surveillance is necessary to detect and mitigate emerging resistance threats early enough [89].
7.4. Third-Generation Sequencing and Producing Complete Genomes
8. Applying Genomic Advances to Improve Public Health
8.1. Monitoring Antimicrobial Resistance Using Genomics
- AMR Surveillance platforms in clinical settings: Several bioinformatics platforms have been developed, such as ResFinder, CARD, and MEGARes, to catalog ARGs and their dissemination through clinical and environmental samples. These enable real-time tracking of resistance mechanisms and have become an integral aspect of global surveillance [92]. In vitro activity against P. aeruginosa and Citrobacter spp. demonstrated the potential of nanopore sequencing for monitoring plasmid dissemination and resistance gene development during hospital outbreaks, which allows rapid action to stop the spread of resistant strains [93].
- Metagenomics applied to environmental samples and animal habitats: Metagenomic approaches for environmental and animal habitats are increasingly being used to identify reservoirs of ARGs that could transfer to human pathogens. Thus, understanding the environmental transmission routes/pathways of antibiotic resistance is essential, and surveillance programs that incorporate environmental sampling are needed to identify potential community sources [94]. Genomics is also being utilized for the detection of emerging pathogens and their antibiotic resistance profiles in populations. Projecting genomic diversity onto genotype-based diagnostics provides a more streamlined pathogen surveillance strategy than random sampling and can improve the identification of new resistance variants in pathogens like Neisseria gonorrhoeae [95].
8.2. Improving Detection and Response to Outbreaks
- Outbreak Investigations and Responses: WGS has been important in multiple outbreak investigations, allowing strain differentiation between outbreak and sporadic isolates. This ability provides better identification of transmission chains, such as WGS-based tracking of Staphylococcus pseudintermedius infections in companion animals and their connections to pathogens in humans [96].
- Real-Time Genomic Tracking: With portable sequencing technologies like nanopore sequencing, bacterial infections can be tracked in real time from a genomic perspective. These technologies are also advantageous in resource-limited settings and public health emergencies when genomic data can be used critically to guide interventions [93].
8.3. The Advent of Personalized Medicine and Precision Public Health
8.4. One Health Surveillance
9. AI for Antibiotic Discovery
9.1. Small-Molecule Antibiotics and Antimicrobial Peptides Discovery
9.2. Predicting Antibiotic Resistance
9.3. AI and Antibacterial Combination Treatments
9.4. Advantages and Challenges
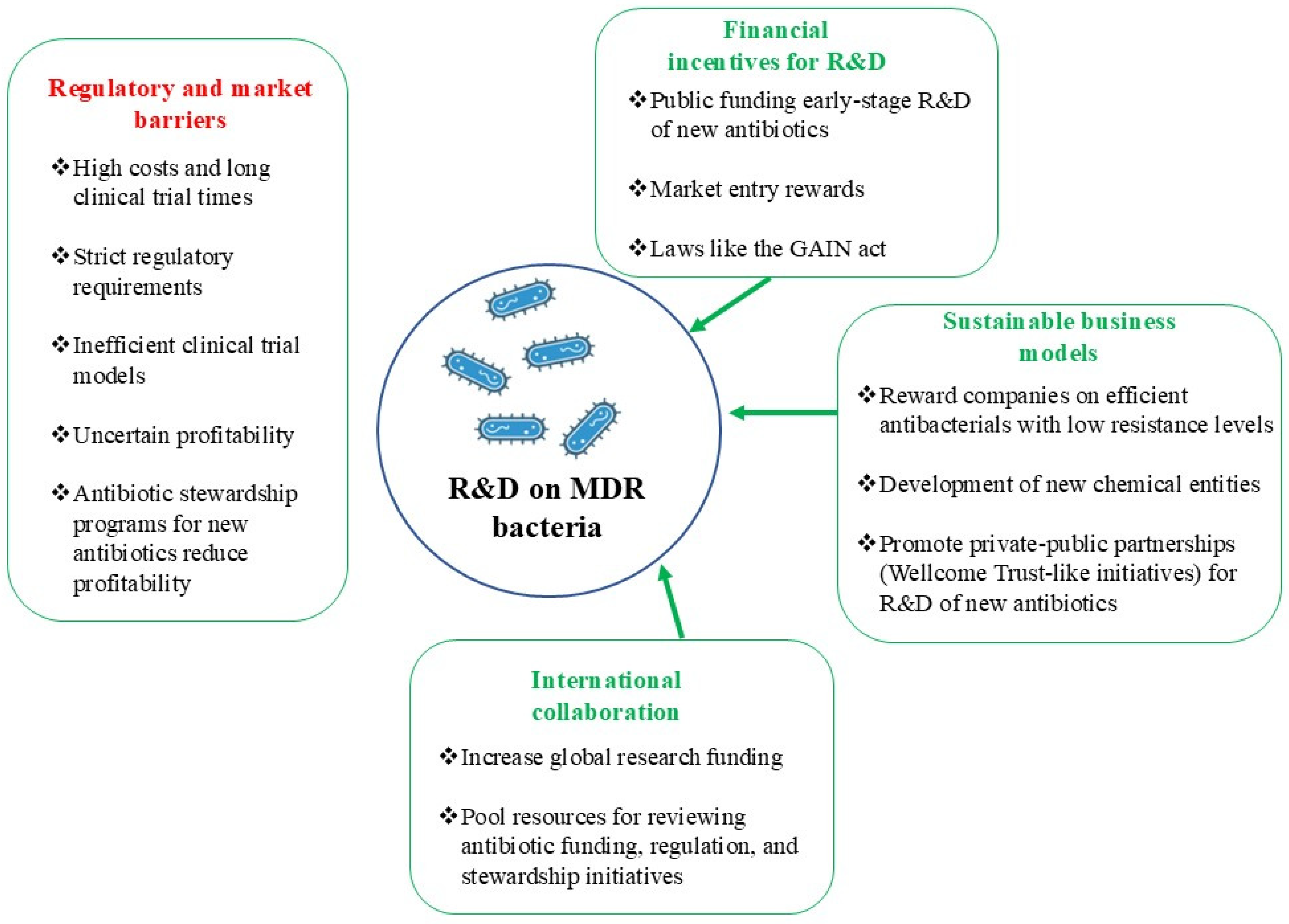
10. Decreased Industry Investment in Antibacterial Drug Development
10.1. Unprofitable Antibiotics
10.2. Challenges in Science and Technology
10.3. Obstacles in Regulation and Market
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hendriksen, R.S.; Munk, P.; Njage, P.; Van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 10, 112410. [Google Scholar] [CrossRef]
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1.
- Muñoz, K.A.; Ulrich, R.J.; Vasan, A.K.; Sinclair, M.; Wen, P.-C.; Holmes, J.R.; Lee, H.Y.; Hung, C.-C.; Fields, C.J.; Tajkhorshid, E.; et al. A Gram-negative –selective antibiotic that spares the gut microbiome. Nature 2024, 630, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.S.; Choi, J.; Dantas, G. Approaches for characterizing and tracking hospital-associated multidrug-resistant bacteria. Cell Mol. Life Sci. 2021, 78, 2585–2606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.; Park, J.; Kang, M.; Yang, J.; Park, W. Gain and loss of antibiotic resistant genes in multidrug resistant bacteria: One Health perspective. J. Microbiol. 2021, 59, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.R.; Griffith, M.P.; Sundermann, A.J.; Shutt, K.A.; Saul, M.I.; Mustapha, M.M.; Marsh, J.W.; Cooper, V.S.; Harrison, L.H.; Van Tyne, D. Systematic detection of horizontal gene transfer across genera among multidrug-resistant bacteria in a single hospital. Elife 2020, 9, e53886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McInnes, R.S.; McCallum, G.E.; Lamberte, L.E.; van Schaik, W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol. 2020, 53, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.; Traykovska, M.; Penchovsky, R. Targeting FMN, TPP, SAM-I, and glmS Riboswitches with Chimeric Antisense Oligonucleotides for Completely Rational Antibacterial Drug Development. Antibiotics 2023, 12, 1607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Binepal, G.; Mabanglo, M.F.; Goodreid, J.D.; Leung, E.; Barghash, M.M.; Wong, K.S.; Lin, F.; Cossette, M.; Bansagi, J.; Song, B.; et al. Development of Antibiotics That Dysregulate the Neisserial ClpP Protease. ACS Infect. Dis. 2020, 6, 3224–3236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Talat, A.; Bashir, Y.; Khan, A.U. Repurposing of antibiotics: Sense or non-sense. Front. Pharmacol. 2022, 13, 833005. [Google Scholar] [CrossRef]
- Rezzoagli, C.; Archetti, M.; Mignot, I.; Baumgartner, M.; Kümmerli, R. Combining antibiotics with antivirulence compounds can have synergistic effects and reverse selection for antibiotic resistance in Pseudomonas aeruginosa. PLoS Biol. 2020, 18, e3000805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Provenzani, A.; Hospodar, A.R.; Meyer, A.L.; Leonardi Vinci, D.; Hwang, E.Y.; Butrus, C.M.; Polidori, P. Multidrug-resistant gram-negative organisms: A review of recently approved antibiotics and novel pipeline agents. Int. J. Clin. Pharm. 2020, 42, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Hart, E.M.; Mitchell, A.M.; Konovalova, A.; Grabowicz, M.; Sheng, J.; Han, X.; Rodriguez-Rivera, F.P.; Schwaid, A.G.; Malinverni, J.C.; Balibar, C.J.; et al. A small-molecule inhibitor of BamA impervious to efflux and the outer membrane permeability barrier. Proc. Natl. Acad. Sci. USA 2019, 116, 21748–21757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, S.; Adamiak, J.W.; Bonifay, V.; Mehla, J.; Zgurskaya, H.I.; Tan, D.S. Defining new chemical space for drug penetration into Gram-negative bacteria. Nat. Chem. Biol. 2020, 16, 1293–1302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durand-Reville, T.F.; Miller, A.A.; O’Donnell, J.P.; Wu, X.; Sylvester, M.A.; Guler, S.; Iyer, R.; Shapiro, A.B.; Carter, N.M.; Velez-Vega, C.; et al. Rational design of a new antibiotic class for drug-resistant infections. Nature 2021, 597, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.; Zhang, S.J.; Wang, W.G.; Jiang, H. Strategies for Discovering New Antibiotics from Bacteria in the Post-Genomic Era. Curr. Microbiol. 2020, 77, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Alkatheri, A.H.; Yap, P.S.X.; Abushelaibi, A.; Lai, K.S.; Cheng, W.H.; Lim, S.H.E. Microbial genomics:Innovative targets and mechanisms. Antibiotics 2023, 12, 190. [Google Scholar] [CrossRef]
- Talbot, G.H.; Jezek, A.; Murray, B.E.; Jones, R.N.; Ebright, R.H.; Nau, G.J.; Rodvold, K.A.; Newland, J.G.; Boucher, H.W.; Infectious Diseases Society of America. The Infectious Diseases Society of America’s 10 × ’20 Initiative (10 New Systemic Antibacterial Agents US Food and Drug Administration Approved by 2020): Is 20 × ’20 a Possibility? Clin. Infect. Dis. 2019, 69, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, L.; Gersbach, H. A Refunding Scheme to Incentivize Narrow-Spectrum Antibiotic Development. Bull. Math. Biol. 2022, 84, 59. [Google Scholar] [CrossRef]
- Ding, M.; Ye, Z.; Liu, L.; Wang, W.; Chen, Q.; Zhang, F.; Wang, Y.; Sjöling, Å.; Martín-Rodríguez, A.J.; Hu, R.; et al. Subinhibitory antibiotic concentrations promote the horizontal transfer of plasmid-borne resistance genes from Klebsiellae pneumoniae to Escherichia coli. Front. Microbiol. 2022, 13, 1017092. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kunhikannan, S.; Thomas, C.J.; Franks, A.E.; Mahadevaiah, S.; Kumar, S.; Petrovski, S. Environmental hotspots for antibiotic resistance genes. Microbiologyopen 2021, 10, e1197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic resistance genes in bacteria: Occurrence, spread, and control. J. Basic Microbiol. 2021, 61, 1049–1070. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Y.; Jin, M.; Yuan, Z.; Bond, P.; Guo, J. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020, 169, 115229. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Buckner, M.M.C. Non-antibiotic compounds associated with humans and the environment can promote horizontal transfer of antimicrobial resistance genes. Crit. Rev. Microbiol. 2023, 50, 993–1010. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A. Nanoantibiotics: A Novel Rational Approach to Antibiotic Resistant Infections. Curr. Drug Metab. 2019, 20, 720–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Greenberg, D.E.; Pifer, R.; Jiang, S.; Xiao, G.; Shelburne, S.A.; Koh, A.; Xie, Y.; Zhan, X. VAMPr: VAriant Mapping and Prediction of antibiotic resistance via explainable features and machine learning. PLoS Comput. Biol. 2020, 16, e1007511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di, Y.P.; Lin, Q.; Chen, C.; Montelaro, R.C.; Doi, Y.; Deslouches, B. Enhanced therapeutic index of an antimicrobial peptide in mice by increasing safety and activity against multidrug-resistant bacteria. Sci. Adv. 2020, 6, eaay6817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehman, K.M.; Grabowicz, M. Countering Gram-Negative Antibiotic Resistance: Recent Progress in Disrupting the Outer Membrane with Novel Therapeutics. Antibiotics 2019, 8, 163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venter, H.; Mowla, R.; Ohene-Agyei, T.; Ma, S. RND-type drug efflux pumps from Gram-negative bacteria: Molecular mechanism and inhibition. Front. Microbiol. 2015, 6, 377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumari, R.; Saraogi, I. Navigating Antibiotic Resistance in Gram-Negative Bacteria: Current Challenges and Emerging Therapeutic Strategies. Chemphyschem 2025, e202401057. [Google Scholar] [CrossRef] [PubMed]
- Vasan, A.K.; Haloi, N.; Ulrich, R.J.; Metcalf, M.E.; Wen, P.C.; Metcalf, W.W.; Hergenrother, P.J.; Shukla, D.; Tajkhorshid, E. Role of internal loop dynamics in antibiotic permeability of outer membrane porins. Proc. Natl. Acad. Sci. USA 2022, 119, e2117009119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, B.; Trout, R.E.L.; Chu, G.H.; McGarry, D.; Jackson, R.W.; Hamrick, J.C.; Daigle, D.M.; Cusick, S.M.; Pozzi, C.; De Luca, F.; et al. Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-β-lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. J. Med. Chem. 2020, 63, 2789–2801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herald, F.; Burgos, R.M. Clinical Evaluation of Meropenem-Vaborbactam Combination for the Treatment of Urinary Tract Infection: Evidence to Date. Infect. Drug Resist. 2023, 16, 555–568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hillyer, T.; Shin, W.S. Meropenem/Vaborbactam-A Mechanistic Review for Insight into Future Development of Combinational Therapies. Antibiotics 2024, 13, 472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, U.; Lee, C.R. Antimicrobial Agents That Inhibit the Outer Membrane Assembly Machines of Gram-Negative Bacteria. J. Microbiol. Biotechnol. 2019, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Jakob, R.P.; Marzinek, J.K.; Green, R.; Imai, Y.; Bolla, J.R.; Agustoni, E.; Robinson, C.V.; Bond, P.J.; Lewis, K.; et al. The antibiotic darobactin mimics a β-strand to inhibit outer membrane insertase. Nature 2021, 593, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Kany, A.M.; Fries, F.; Seyfert, C.E.; Porten, C.; Deckarm, S.; Chacón Ortiz, M.; Dubarry, N.; Vaddi, S.; Große, M.; Bernecker, S.; et al. In Vivo Activity Profiling of Biosynthetic Darobactin D22 against Critical Gram-Negative Pathogens. ACS Infect. Dis. 2024, 10, 4337–4346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klobucar, K.; Côté, J.P.; French, S.; Borrillo, L.; Guo, A.B.Y.; Serrano-Wu, M.H.; Lee, K.K.; Hubbard, B.; Johnson, J.W.; Gaulin, J.L.; et al. Chemical Screen for Vancomycin Antagonism Uncovers Probes of the Gram-Negative Outer Membrane. ACS Chem. Biol. 2021, 16, 929–942. [Google Scholar] [CrossRef] [PubMed]
- French, S.; Farha, M.; Ellis, M.J.; Sameer, Z.; Côté, J.P.; Cotroneo, N.; Lister, T.; Rubio, A.; Brown, E.D. Potentiation of Antibiotics against Gram-Negative Bacteria by Polymyxin B Analogue SPR741 from Unique Perturbation of the Outer Membrane. ACS Infect. Dis. 2020, 6, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- MacNair, C.R.; Brown, E.D. Outer membrane disruption overcomes intrinsic, acquired, and spontaneous antibiotic resistance. mBio 2020, 11, e01615-20. [Google Scholar] [CrossRef]
- Romano, K.P.; Hung, D.T. Targeting LPS biosynthesis and transport in gram-negative bacteria in the era of multi-drug resistance. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, S.; Zhou, R.; Wan, L.; Feng, S.; Song, K.; Xu, C.; Li, Y.; Liao, M. Mechanism of LolCDE as a molecular extruder of bacterial triacylated lipoproteins. Nat. Commun. 2021, 12, 4687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grabowicz, M.; Silhavy, T.J. Redefining the essential trafficking pathway for outer membrane lipoproteins. Proc. Nat. Acad. Sci. USA 2017, 114, 4769–4774. [Google Scholar] [CrossRef]
- Grabowicz, M. Lipoproteins and Their Trafficking to the Outer Membrane. EcoSal Plus. 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Komura, R.; Sano, T.; Pantua, H.; Storek, K.M.; Inaba, H.; Ogawa, H.; Noland, C.L.; Peng, Y.; Gloor, S.L.; et al. Inhibition of Escherichia coli Lipoprotein Diacylglyceryl Transferase Is Insensitive to Resistance Caused by Deletion of Braun’s Lipoprotein. J. Bacteriol. 2021, 203, e0014921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- May, K.L.; Lehman, K.M.; Mitchell, A.M.; Grabowicz, M. A Stress Response Monitoring Lipoprotein Trafficking to the Outer Membrane. mBio 2019, 10, e00618-19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, B.; Roy Choudhury, M.; Yang, X.; Benoit, S.L.; Womack, E.; Van Mouwerik Lyles, K.; Acharya, A.; Kumar, A.; Yang, C.; Pavlova, A.; et al. Restoring and Enhancing the Potency of Existing Antibiotics against Drug-Resistant Gram-Negative Bacteria through the Development of Potent Small-Molecule Adjuvants. ACS Infect. Dis. 2022, 8, 1491–1508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Antibiotic adjuvants: An alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit. Rev. Microbiol. 2019, 45, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Snydman, D.R.; McDermott, L.A.; Jacobus, N.V.; Kerstein, K.; Grossman, T.H.; Sutcliffe, J.A. Evaluation of the In Vitro Activity of Eravacycline against a Broad Spectrum of Recent Clinical Anaerobic Isolates. Antimicrob. Agents Chemother. 2018, 62, e02206-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, X.; Liang, Q.; Dai, X.; Wu, S.; Duan, C.; Luo, Z.; Xie, X. In vitro activities of eravacycline against clinical bacterial isolates: A multicenter study in Guangdong, China. Front. Microbiol. 2024, 15, 1504013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.Y.; Srinivas, P.; Pogue, J.M. Cefiderocol: A Novel Agent for the Management of Multidrug-Resistant Gram-Negative Organisms. Infect. Dis. Ther. 2020, 9, 17–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69 (Suppl. 7), S538–S543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karakonstantis, S.; Rousaki, M.; Kritsotakis, E.I. Cefiderocol: Systematic Review of Mechanisms of Resistance, Heteroresistance and In Vivo Emergence of Resistance. Antibiotics 2022, 11, 723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karakonstantis, S.; Rousaki, M.; Vassilopoulou, L.; Kritsotakis, E.I. Global prevalence of cefiderocol non-susceptibility in Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2024, 30, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Endimiani, A.; Hujer, K.M.; Hujer, A.M.; Armstrong, E.S.; Choudhary, Y.; Aggen, J.B.; Bonomo, R.A. ACHN-490, a neoglycoside with potent in vitro activity against multidrug-resistant Klebsiella pneumoniae isolates. Antimicrob. Agents Chemother. 2009, 53, 4504–4507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Kashikar, A.; Bush, K. In vitro activity of plazomicin against β-lactamase-producing carbapenem-resistant Enterobacteriaceae (CRE). J. Antimicrob. Chemother. 2017, 72, 2792–2795. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Liang, B.; Zhang, G.; Wang, J.; Zhu, M.; Cai, Y. Efficacy and Safety of Plazomicin in the Treatment of Enterobacterales Infections: A Meta-analysis of Randomized Controlled Trials. Open Forum Infect. Dis. 2022, 9, ofac429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tulkens, P.M.; Van Bambeke, F.; Zinner, S.H. Profile of a Novel Anionic Fluoroquinolone-Delafloxacin. Clin. Infect. Dis. 2019, 68 (Suppl. 3), S213–S222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ribeiro, Á.C.D.S.; Santos, F.F.; Valiatti, T.B.; Lenzi, M.H.; Santos, I.N.M.; Neves, R.F.B.; Moses, I.B.; Meneses, J.P.; Di Sessa, R.G.G.; Salles, M.J.; et al. Comparative in vitro activity of Delafloxacin and other antimicrobials against isolates from patients with acute bacterial skin, skin-structure infection and osteomyelitis. Braz. J. Infect. Dis. 2024, 28, 103867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turban, A.; Guérin, F.; Dinh, A.; Cattoir, V. Updated Review on Clinically-Relevant Properties of Delafloxacin. Antibiotics 2023, 12, 1241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol. Med. 2020, 26, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Culp, E.J.; Waglechner, N.; Wang, W.; Fiebig-Comyn, A.A.; Hsu, Y.P.; Koteva, K.; Sychantha, D.; Coombes, B.K.; Van Nieuwenhze, M.S.; Brun, Y.V.; et al. Evolution-guided discovery of antibiotics that inhibit peptidoglycan remodelling. Nature 2020, 578, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shi, S.; Zhang, X.; Han, F.; Dong, H. Newest perspectives of glycopeptide antibiotics: Biosynthetic cascades, novel derivatives, and new appealing antimicrobial applications. World J. Microbiol. Biotechnol. 2023, 39, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, J.; Zou, P.; Cui, Y.; Quan, L.; Gao, C.; Li, Z.; Gong, W.; Yang, M. Recent Advances in Strategies to Combat Bacterial Drug Resistance: Antimicrobial Materials and Drug Delivery Systems. Pharmaceutics 2023, 15, 1188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How nanomaterials are transforming drug delivery, bio-imaging, and diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Das, B.; Mahajan, D.; Rakonjac, J. Editorial: Antibiotic potentiators against drug-resistant pathogens: Discovery, development and clinical applications. Front. Microbiol. 2023, 14, 1173906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, J.; Chen, X. Current Promising Strategies against Antibiotic-Resistant Bacterial Infections. Antibiotics 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szymczak, M.; Pankowski, J.A.; Kwiatek, A.; Grygorcewicz, B.; Karczewska-Golec, J.; Sadowska, K.; Golec, P. An effective antibiofilm strategy based on bacteriophages armed with silver nanoparticles. Sci. Rep. 2024, 14, 9088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fihn, C.A.; Carlson, E.E. Targeting a highly conserved domain in bacterial histidine kinases to generate inhibitors with broad spectrum activity. Curr. Opin. Microbiol. 2021, 61, 107–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Yu, C.; Wu, H.; Li, G.; Li, C.; Wu, H.; Hong, W.; Yang, X.; You, X. Recent Advances in histidine kinase-targeted antimicrobial agents. Front. Chem. 2022, 10, 866392. [Google Scholar] [CrossRef]
- Mori, M.; Sammartino, J.C.; Costantino, L.; Gelain, A.; Meneghetti, F.; Villa, S.; Chiarelli, L.R. An Overview on the Potential Antimycobacterial Agents Targeting Serine/Threonine Protein Kinases from Mycobacterium tuberculosis. Curr. Top. Med. Chem. 2019, 19, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-Sensing Regulation of Antimicrobial Resistance in Bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernandes, P.B.; Reed, P.; Monteiro, J.M.; Pinho, M.G. Revisiting the Role of VraTSR in Staphylococcus aureus Response to Cell Wall-Targeting Antibiotics. J. Bacteriol. 2022, 204, e0016222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, J.; Cochrane, C.S.; Najeeb, J.; Gooden, D.; Sciandra, C.; Fan, P.; Lemaitre, N.; Newns, K.; Nicholas, R.A.; Guan, Z.; et al. Preclinical safety and efficacy characterization of an LpxC inhibitor against Gram-negative pathogens. Sci. Transl. Med. 2023, 15, eadf5668. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, P.; Hong, J. Structure- and Ligand-Dynamics-Based Design of Novel Antibiotics Targeting Lipid A Enzymes LpxC and LpxH in Gram-Negative Bacteria. Acc. Chem. Res. 2021, 54, 1623–1634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, S.; Li, D.; Yan, M.; Feng, X.; Lv, G.; Wu, G.; Jin, Y.; Wang, Y.; Yang, Z. The Complex Structure of Protein AaLpxC from Aquifex aeolicus with ACHN-975 Molecule Suggests an Inhibitory Mechanism at Atomic-Level against Gram-Negative Bacteria. Molecules 2021, 26, 1451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Q.; Cebrián, R.; Montalbán-López, M.; Ren, H.; Wu, W.; Kuipers, O.P. Outer-membrane-acting peptides and lipid II-targeting antibiotics cooperatively kill Gram-negative pathogens. Commun. Biol. 2021, 4, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dewangan, R.P.; Verma, D.P.; Verma, N.K.; Gupta, A.; Pant, G.; Mitra, K.; Habib, S.; Ghosh, J.K. Spermine-Conjugated Short Proline-Rich Lipopeptides as Broad-Spectrum Intracellular Targeting Antibacterial Agents. J. Med. Chem. 2022, 65, 5433–5448. [Google Scholar] [CrossRef] [PubMed]
- Grishin, S.Y.; Domnin, P.A.; Kravchenko, S.V.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Makarova, M.A.; Kurpe, S.R.; et al. Is It Possible to Create Antimicrobial Peptides Based on the Amyloidogenic Sequence of Ribosomal S1 Protein of P. aeruginosa? Int. J. Mol. Sci. 2021, 22, 9776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Panfilov, A.V.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Glyakina, A.V.; et al. Multiple Antimicrobial Effects of Hybrid Peptides Synthesized Based on the Sequence of Ribosomal S1 Protein from Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Zakhareva, A.P.; Zakharova, A.A.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Poshvina, D.V.; et al. Optimizing Antimicrobial Peptide Design: Integration of Cell-Penetrating Peptides, Amyloidogenic Fragments, and Amino Acid Residue Modifications. Int. J. Mol. Sci. 2024, 25, 6030. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhydzetski, A.; Głowacka-Grzyb, Z.; Bukowski, M.; Żądło, T.; Bonar, E.; Władyka, B. Agents Targeting the Bacterial Cell Wall as Tools to Combat Gram-Positive Pathogens. Molecules 2024, 29, 4065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dias, C.; Rauter, A.P. Membrane-targeting antibiotics: Recent developments outside the peptide space. Future Med. Chem. 2019, 11, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Van Camp, P.J.; Haslam, D.B.; Porollo, A. Prediction of Antimicrobial Resistance in Gram-Negative Bacteria From Whole-Genome Sequencing Data. Front. Microbiol. 2020, 11, 1013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hua, X.; Liang, Q.; Deng, M.; He, J.; Wang, M.; Hong, W.; Wu, J.; Lu, B.; Leptihn, S.; Yu, Y.; et al. BacAnt: A Combination Annotation Server for Bacterial DNA Sequences to Identify Antibiotic Resistance Genes, Integrons, and Transposable Elements. Front. Microbiol. 2021, 12, 649969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skinnider, M.A.; Johnston, C.W.; Gunabalasingam, M.; Merwin, N.J.; Kieliszek, A.M.; MacLellan, R.J.; Li, H.; Ranieri, M.R.M.; Webster, A.L.H.; Cao, M.P.T.; et al. Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences. Nat. Commun. 2020, 11, 6058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asante, J.; Osei Sekyere, J. Understanding antimicrobial discovery and resistance from a metagenomic and metatranscriptomic perspective: Advances and applications. Environ. Microbiol. Rep. 2019, 11, 62–86. [Google Scholar] [CrossRef] [PubMed]
- Sukhum, K.V.; Diorio-Toth, L.; Dantas, G. Genomic and Metagenomic Approaches for Predictive Surveillance of Emerging Pathogens and Antibiotic Resistance. Clin. Pharmacol. Ther. 2019, 106, 512–524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- La, T.-M.; Kim, J.-H.; Kim, T.; Lee, H.-J.; Lee, Y.; Shin, H.; Song, Y.; Ahn, G.; Hur, W.; Lee, J.-B.; et al. The optimal standard protocols for whole-genome sequencing of antibiotic-resistant pathogenic bacteria using third-generation sequencing platforms. Mol. Cell. Toxicol. 2021, 17, 493–501. [Google Scholar] [CrossRef]
- Khaledi, A.; Weimann, A.; Schniederjans, M.; Asgari, E.; Kuo, T.H.; Oliver, A.; Cabot, G.; Kola, A.; Gastmeier, P.; Hogardt, M.; et al. Predicting antimicrobial resistance in Pseudomonas aeruginosa with machine learning-enabled molecular diagnostics. EMBO Mol. Med. 2020, 12, e10264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hendriksen, R.S.; Bortolaia, V.; Tate, H.; Tyson, G.H.; Aarestrup, F.M.; McDermott, P.F. Using Genomics to Track Global Antimicrobial Resistance. Front. Public Health 2019, 7, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peter, S.; Bosio, M.; Gross, C.; Bezdan, D.; Gutierrez, J.; Oberhettinger, P.; Liese, J.; Vogel, W.; Dörfel, D.; Berger, L.; et al. Tracking of Antibiotic Resistance Transfer and Rapid Plasmid Evolution in a Hospital Setting by Nanopore Sequencing. mSphere 2020, 5, e00525-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pillay, S.; Calderón-Franco, D.; Urhan, A.; Abeel, T. Metagenomic-based surveillance systems for antibiotic resistance in non-clinical settings. Front. Microbiol. 2022, 13, 1066995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hicks, A.L.; Kissler, S.M.; Mortimer, T.D.; Ma, K.C.; Taiaroa, G.; Ashcroft, M.; Williamson, D.A.; Lipsitch, M.; Grad, Y.H. Targeted surveillance strategies for efficient detection of novel antibiotic resistance variants. Elife 2020, 9, e56367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tyson, G.H.; Ceric, O.; Guag, J.; Nemser, S.; Borenstein, S.; Slavic, D.; Lippert, S.; McDowell, R.; Krishnamurthy, A.; Korosec, S.; et al. Genomics accurately predicts antimicrobial resistance in Staphylococcus pseudintermedius collected as part of Vet-LIRN resistance monitoring. Vet. Microbiol. 2021, 254, 109006. [Google Scholar] [CrossRef] [PubMed]
- Stracy, M.; Snitser, O.; Yelin, I.; Amer, Y.; Parizade, M.; Katz, R.; Rimler, G.; Wolf, T.; Herzel, E.; Koren, G.; et al. Minimizing treatment-induced emergence of antibiotic resistance in bacterial infections. Science 2022, 375, 889–894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach-Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceric, O.; Tyson, G.H.; Goodman, L.B.; Mitchell, P.K.; Zhang, Y.; Prarat, M.; Cui, J.; Peak, L.; Scaria, J.; Antony, L.; et al. Enhancing the one health initiative by using whole genome sequencing to monitor antimicrobial resistance of animal pathogens: Vet-LIRN collaborative project with veterinary diagnostic laboratories in United States and Canada. BMC Vet. Res. 2019, 15, 130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hill, R.; Stentiford, G.D.; Walker, D.I.; Baker-Austin, C.; Ward, G.; Maskrey, B.H.; van Aerle, R.; Verner-Jeffreys, D.; Peeler, E.; Bass, D. Realising a global One Health disease surveillance approach: Insights from wastewater and beyond. Nat. Commun. 2024, 15, 5324, Erratum in: Nat. Commun. 2024, 15, 6412. https://doi.org/10.1038/s41467-024-50682-6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Environment Agency and Cefas. Shellfish as Bioindicator for Coastal Antimicrobial Resistance-Summary. 2023, pp. 1–51. Available online: https://assets.publishing.service.gov.uk/media/6540c73546532b000d67f5bb/Shellfish_as_bioindicators_for_coastal_antimicrobial_resistance_-_report.pdf (accessed on 12 April 2025).
- Hamet, P.; Tremblay, J. Artificial intelligence in medicine. Metabolism 2017, 69, S36–S40. [Google Scholar] [CrossRef]
- Talat, A.; Khan, A.U. Artificial intelligence as a smart approach to develop antimicrobial drug molecules: A paradigm to combat drug-resistant infections. Drug Discov. Today 2023, 28, 103491. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef] [PubMed]
- Parvatikar, P.P.; Patil, S.; Khaparkhuntikar, K.; Patil, S.; Singh, P.K.; Singh, P.K.; Sahana Raghavendra, V.; Kulkarni, R.V.; Raghu, A.V. Artificial intelligence: Machine learning approach for screening large database and drug discovery. Antivir. Res. 2023, 220, 105740. [Google Scholar] [CrossRef]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A deep learning approach to antibiotic discovery. Cell 2020, 181, 475–483. [Google Scholar] [CrossRef]
- Lluka, T.; Stokes, J.M. Antibiotic discovery in the artificial intelligence era. Ann. N. Y. Acad. Sci. 2023, 1519, 74–93. [Google Scholar] [CrossRef] [PubMed]
- Cesaro, A.; Bagheri, M.; Torres, M.; Wan, F.; de la Fuente-Nunez, C. Deep learning tools to accelerate antibiotic discovery. Expert Opin. Drug Discov. 2023, 18, 1245–1257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macedo, B.; Ribeiro Vaz, I.; Taveira Gomes, T. MedGAN: Optimized generative adversarial network with graph convolutional networks for novel molecule design. Sci. Rep. 2024, 14, 1212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Puentes, P.; Henao, M.C.; Cifuentes, J.; Muñoz-Camargo, C.; Reyes, L.H.; Cruz, J.C.; Arbeláez, P. Rational Discovery of Antimicrobial Peptides by Means of Artificial Intelligence. Membranes 2022, 12, 708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Oort, C.M.; Ferrell, J.B.; Remington, J.M.; Wshah, S.; Li, J. AMPGAN v2: Machine Learning-Guided Design of Antimicrobial Peptides. J. Chem. Inf. Model. 2021, 61, 2198–2207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, M.; Zhang, Y.; Wang, M.; Ma, L.Z. dsAMP and dsAMPGAN: Deep Learning Networks for Antimicrobial Peptides Recognition and Generation. Antibiotics 2024, 13, 948. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, J.; Zhang, J.; Yu, Q.; Ji, J.; Li, J.; He, S.; Zhu, Z. TG-CDDPM: Text-guided antimicrobial peptides generation based on conditional denoising diffusion probabilistic model. Brief. Bioinform. 2024, 26, bbae644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos-Júnior, C.D.; Torres, M.D.; Duan, Y.; del Río, Á.R.; Schmidt, T.S.; Chong, H.; Fullam, A.; Kuhn, M.; Zhu, C.; Houseman, A.; et al. Discovery of antimicrobial peptides in the global microbiome with machine learning. Cell 2024, 187, 3761–3778.e16. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yan, Y.; Links, M.G.; Li, L.; Dillon, J.R.; Horsch, M.; Kusalik, A. Antimicrobial resistance genetic factor identification from whole-genome sequence data using deep feature selection. BMC Bioinform. 2019, 20 (Suppl. 15), 535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, A.L.H.; To, C.C.K.; Chan, R.C.K.; Wong, J.S.H.; Lui, G.C.Y.; Cheung, I.Y.Y.; Chow, V.C.Y.; Lai, C.K.C.; Ip, M.; Lai, R.W.M. Predicting antibiotic susceptibility in urinary tract infection with artificial intelligence-model performance in a multi-centre cohort. JAC Antimicrob. Resist. 2024, 6, dlae121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melo, M.C.R.; Maasch, J.R.M.A.; de la Fuente-Nunez, C. Accelerating antibiotic discovery through artificial intelligence. Commun. Biol. 2021, 4, 1050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madani, B.M. The Application of Artificial Intelligence in Antibiotic Discovery: An Overview of Current and Future Perspective. Int. J. Adv. Res. 2023, 11, 1326–1332. [Google Scholar] [CrossRef]
- Roche-Lima, A.; Rosado-Quiñones, A.M.; Feliu-Maldonado, R.A.; Figueroa-Gispert, M.D.M.; Díaz-Rivera, J.; Díaz-González, R.G.; Carrasquillo-Carrion, K.; Nieves, B.G.; Colón-Lorenzo, E.E.; Serrano, A.E. Antimalarial Drug Combination Predictions Using the Machine Learning Synergy Predictor (MLSyPred©) tool. Acta Parasitol. 2024, 69, 415–425. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mott, B.T.; Eastman, R.T.; Guha, R.; Sherlach, K.S.; Siriwardana, A.; Shinn, P.; McKnight, C.; Michael, S.; Lacerda-Queiroz, N.; Patel, P.R.; et al. High-throughput matrix screening identifies synergistic and antagonistic antimalarial drug combinations. Sci. Rep. 2015, 5, 13891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mason, D.J.; Eastman, R.T.; Lewis, R.P.I.; Stott, I.P.; Guha, R.; Bender, A. Using Machine Learning to Predict Synergistic Antimalarial Compound Combinations With Novel Structures. Front. Pharmacol. 2018, 9, 1096. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anahtar, M.N.; Yang, J.H.; Kanjilal, S. Applications of machine learning to the problem of antimicrobial resistance: An emerging model for translational research. J. Clin. Microbiol. 2021, 59, e01260-20. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Yan, S.; Zhen, B. A machine learning method for predicting molecular antimicrobial activity. Sci. Rep. 2025, 15, 6559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiecek, J.; Buckland-Merrett, G. A View from the Wellcome Trust Drug-Resistant Infections Priority Programme: Creating a Sustainable Research and Development Ecosystem to Meet the Global Need for Antibiotics. ACS Infect. Dis. 2020, 6, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Bergkessel, M.; Forte, B.; Gilbert, I.H. Small-Molecule Antibiotic Drug Development: Need and Challenges. ACS Infect. Dis. 2023, 9, 2062–2071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Church, N.A.; McKillip, J.L. Antibiotic resistance crisis: Challenges and imperatives. Biologia 2021, 76, 1535–1550. [Google Scholar] [CrossRef]
- Lanini, S.; Ioannidis, J.P.A.; Vairo, F.; Pletschette, M.; Portella, G.; Di Bari, V.; Mammone, A.; Pisapia, R.; Merler, S.; Nguhuni, B.; et al. Non-inferiority versus superiority trial design for new antibiotics in an era of high antimicrobial resistance: The case for post-marketing, adaptive randomised controlled trials. Lancet Infect. Dis. 2019, 19, e444–e451. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.M.; Lindahl, O.; Harbarth, S.; de Kraker, M.E.A.; Edwards, S.; Hollis, A. Industry incentives and antibiotic resistance: An introduction to the antibiotic susceptibility bonus. J. Antibiot. 2020, 73, 421–428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walesch, S.; Birkelbach, J.; Jézéquel, G.; Haeckl, F.P.J.; Hegemann, J.D.; Hesterkamp, T.; Hirsch, A.K.H.; Hammann, P.; Müller, R. Fighting antibiotic resistance-strategies and (pre)clinical developments to find new antibacterials. EMBO Rep. 2023, 24, e56033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutescu, I.A.; Hillier, S.A. Encouraging the Development of New Antibiotics: Are Financial Incentives the Right Way Forward? A Systematic Review and Case Study. Infect. Drug Resist. 2021, 14, 415–434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Antibiotic/Strategy | Class/Type | Target Pathogens | Mechanism of Action | Key Notes |
|---|---|---|---|---|
| Eravacycline | Tetracycline derivative | Gram-positive and Gram-negative bacteria, including A. baumannii, E. coli | Overcomes tetracycline resistance mechanisms like efflux pumps and ribosomal protection proteins | Broad-spectrum activity against MDR bacteria. Approved for complicated intra-abdominal infections. |
| Cefiderocol | Siderophore cephalosporin | Carbapenem-resistant Enterobacterales, P. aeruginosa | Uses bacterial iron transport system (siderophore mechanism) to penetrate Gram-negative bacteria and bypass resistance mechanisms. | Effective against Gram-negative bacteria. |
| Plazomicin | Aminoglycoside derivative | MDR Enterobacterales, including carbapenemase producers | Evades aminoglycoside-modifying enzymes | Approved for complicated UTIs. |
| Delafloxacin | Fluoroquinolone | Gram-positive and Gram-negative bacteria, including MRSA | Increased potency in acidic environments; effective for skin and soft tissue infections. | Effective in acidic environments found at infection sites. |
| Corbomycin | Glycopeptide | Gram-positive pathogens, including MRSA | Binds to bacterial cell wall, preventing autolysin activity, which differs from traditional glycopeptides like vancomycin. | Unique mechanism with low resistance development. |
| Nanomaterials | Nanotechnology-based strategy | Various resistant pathogens | Nanoparticles improve antibiotic delivery, increase bacterial internalization, and enhance biofilm penetration. | Enhances antibiotic effectiveness and reduces dosage requirements. |
| MD-124 | Antibiotic potentiator | Drug-resistant Gram-negative bacteria | Permeabilizes bacterial outer membrane, enhancing antibiotic efficacy (e.g., colistin). | Potentiators offer a cost-effective strategy to combat resistance. |
| Bacteriophage Therapy | Phage therapy | Antibiotic-resistant bacteria (P. aeruginosa in CF patients) | Use bacteriophages (viruses) to target and destroy specific bacterial pathogens. | Alternative to traditional antibiotics, with promising results in personalized medicine approaches. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niño-Vega, G.A.; Ortiz-Ramírez, J.A.; López-Romero, E. Novel Antibacterial Approaches and Therapeutic Strategies. Antibiotics 2025, 14, 404. https://doi.org/10.3390/antibiotics14040404
Niño-Vega GA, Ortiz-Ramírez JA, López-Romero E. Novel Antibacterial Approaches and Therapeutic Strategies. Antibiotics. 2025; 14(4):404. https://doi.org/10.3390/antibiotics14040404
Chicago/Turabian StyleNiño-Vega, Gustavo A., Jorge A. Ortiz-Ramírez, and Everardo López-Romero. 2025. "Novel Antibacterial Approaches and Therapeutic Strategies" Antibiotics 14, no. 4: 404. https://doi.org/10.3390/antibiotics14040404
APA StyleNiño-Vega, G. A., Ortiz-Ramírez, J. A., & López-Romero, E. (2025). Novel Antibacterial Approaches and Therapeutic Strategies. Antibiotics, 14(4), 404. https://doi.org/10.3390/antibiotics14040404








