Antimicrobial Susceptibility Profiles of Commensal Escherichia coli Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023
Abstract
1. Introduction
2. Results
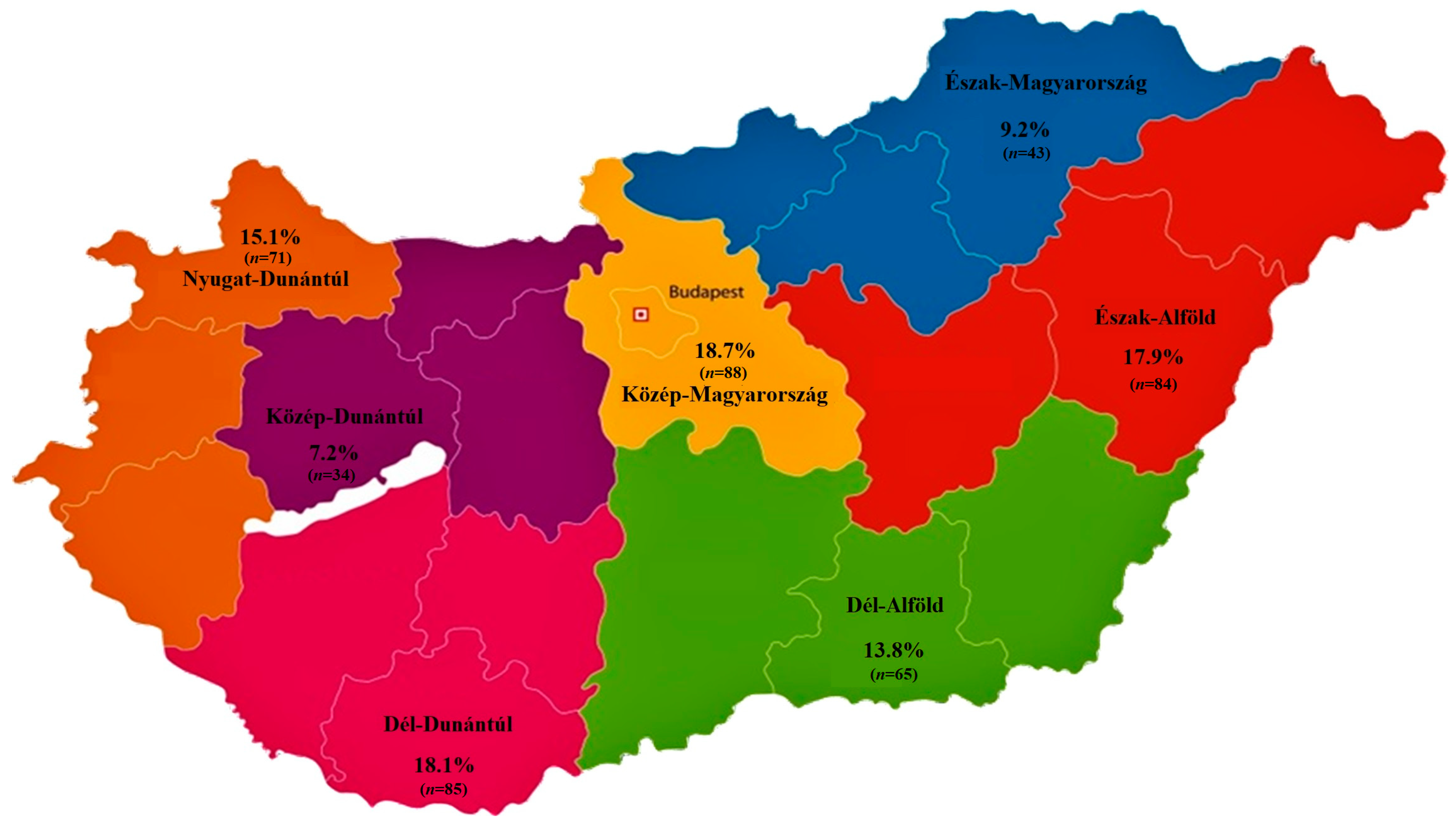
2.1. Regional Distribution and Origin of Received Samples
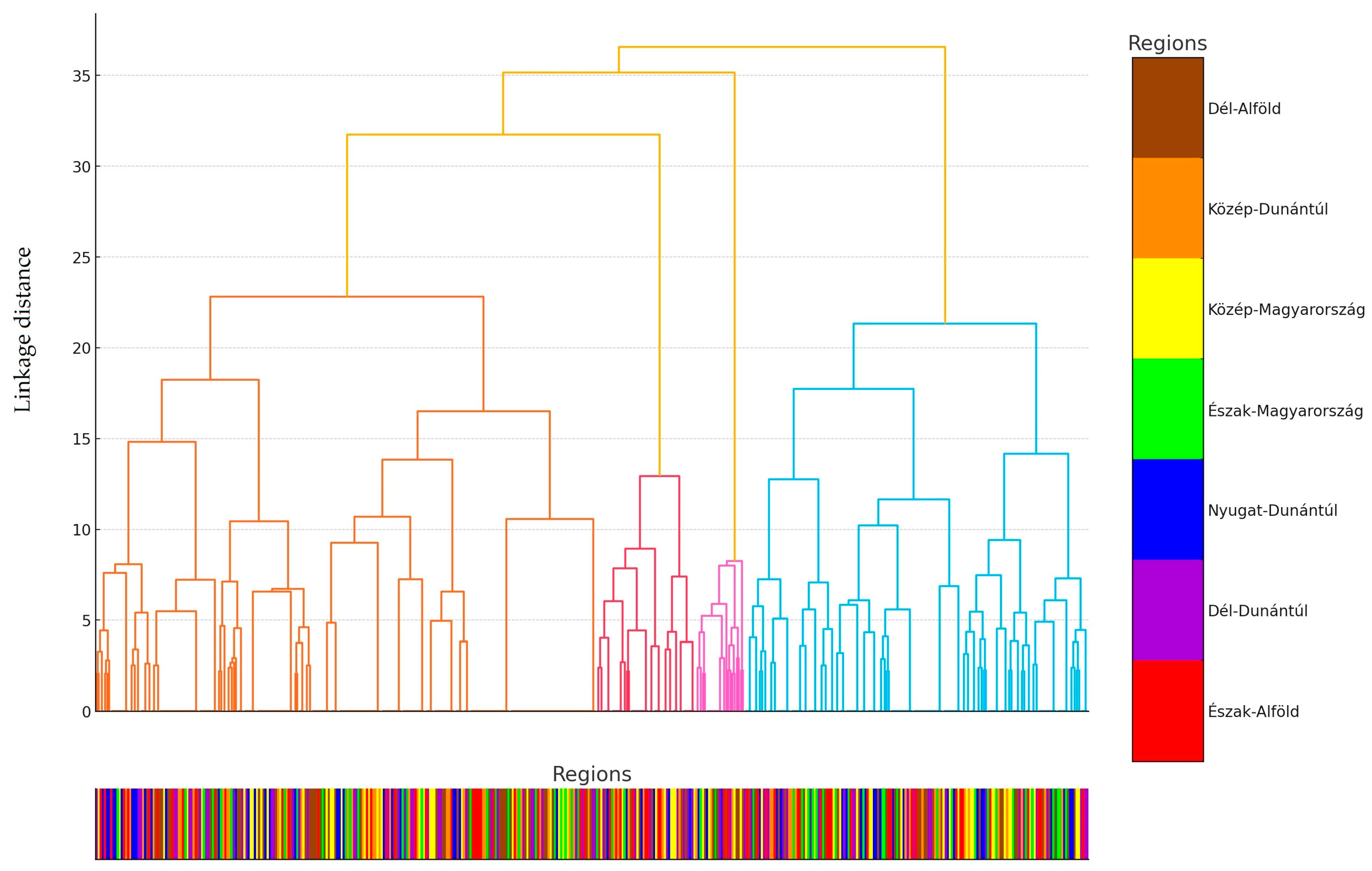
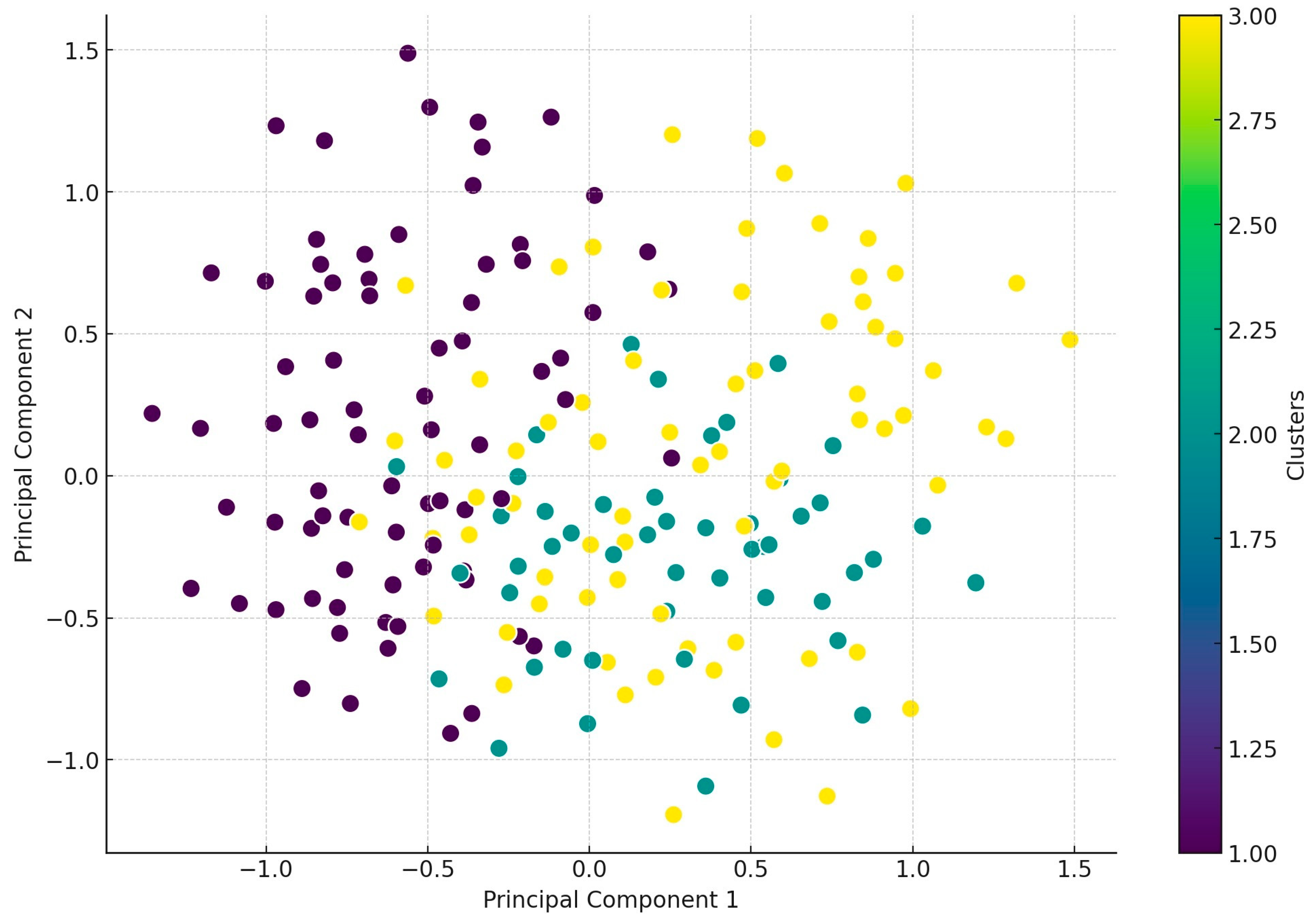
2.2. Antimicrobial Susceptibility Testing
3. Discussion
4. Materials and Methods
4.1. The Origin of Strains and Human Data
4.2. Minimum Inhibitory Concentration (MIC) Determination
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, J. Global Turkey Meat Market: Key Findings and Insights. Available online: https://www.thepoultrysite.com/news/2018/05/global-turkey-meat-market-key-findings-and-insights (accessed on 25 June 2023).
- Cook, A.; Reid-Smith, R.; Irwin, R.; McEwen, S.A.; Valdivieso-Garcia, A.; Ribble, C. Antimicrobial Resistance in Campylobacter, Salmonella, and Escherichia Coli Isolated from Retail Turkey Meat from Southern Ontario, Canada. J. Food Prot. 2009, 72, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Checkley, S.; Avery, B.; Chalmers, G.; Bohaychuk, V.; Gensler, G.; Reid-Smith, R.; Boerlin, P. Phenotypic and Genetic Characterization of Antimicrobial Resistance in Salmonella Serovars Isolated from Retail Meats in Alberta, Canada. Food Microbiol. 2012, 32, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Carson, C.; Léger, D. Antimicrobial Therapy of Selected Diseases in Turkeys, Laying Hens, and Minor Poultry Species in Canada. Can. Vet. J. 2013, 54, 1041–1052. [Google Scholar] [PubMed]
- Dec, M.; Nowaczek, A.; Stępień-Pyśniak, D.; Wawrzykowski, J.; Urban-Chmiel, R. Identification and Antibiotic Susceptibility of Lactobacilli Isolated from Turkeys. BMC Microbiol. 2018, 18, 168. [Google Scholar] [CrossRef]
- Franceschini, G.; Bottino, M.; Millet, I.; Martello, E.; Zaltron, F.; Favretto, A.R.; Vonesch, N.; Tomao, P.; Mannelli, A. Assessment of the Exposure of Turkey Farmers to Antimicrobial Resistance Associated with Working Practices. Vet. Sci. 2019, 6, 13. [Google Scholar] [CrossRef]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis–Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Mayrhofer, S.; Paulsen, P.; Smulders, F.J.M.; Hilbert, F. Antimicrobial Resistance in Commensal Escherichia Coli Isolated from Muscle Foods as Related to the Veterinary Use of Antimicrobial Agents in Food-Producing Animals in Austria. Microb. Drug Resist. 2006, 12, 278–283. [Google Scholar] [CrossRef]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils–Animal Health Aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Lopez, P.; Steenberg, B.; Lava, P.-H.; Chiarella, F.; Khaldoune, N. The Poultry Meat Sector Contributes to the EU Economy, Especially in Rural Areas. Available online: https://avec-poultry.eu/resources/annual-reports/2022-annual-report/ (accessed on 10 March 2025).
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Walker, B.; Barrett, S.; Polasky, S.; Galaz, V.; Folke, C.; Engström, G.; Ackerman, F.; Arrow, K.; Carpenter, S.; Chopra, K.; et al. Environment. Looming Global-Scale Failures and Missing Institutions. Science 2009, 325, 1345–1346. [Google Scholar] [CrossRef] [PubMed]
- Frimodt-Møller, N. Microbial Threat--The Copenhagen Recommendations Initiative of the EU. J. Vet. Med. B Infect. Dis. Vet. Public. Health 2004, 51, 400–402. [Google Scholar] [CrossRef]
- Hesp, A.; Veldman, K.; van der Goot, J.; Mevius, D.; van Schaik, G. Monitoring Antimicrobial Resistance Trends in Commensal Escherichia Coli from Livestock, the Netherlands, 1998 to 2016. Eurosurveillance 2019, 24, 1800438. [Google Scholar] [CrossRef]
- de Greeff, S.C.; Schoffelen, A.F.; Verduin, C.M. NethMap 2021. Consumption of Antimicrobial Agents and Antimicrobial Resistance among Medically Important Bacteria in the Netherlands in 2020/MARAN 2021. Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2020; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Bilthoven, The Netherlands, 2021. [Google Scholar]
- Mevius, D.; Heederik, D. Reduction of Antibiotic Use in Animals “Let’s Go Dutch”. J. Verbr. Lebensm. 2014, 9, 177–181. [Google Scholar] [CrossRef]
- Sibel, K.; Fatma, E.A.; Aziz, U.Ö.; Murat, Y.; Cansu, Ö.G.; Efsun, M.C. Determination of Subtypes, Serogroups, And Serotypes, Virulence, and/ or Toxigenic Properties of Escherichia Coli Isolated From Cattle, Sheep, and Goat Feces by Multiplex PCR. Kafkas Univ. Vet. Fak. Derg. 2024, 30, 155–160. [Google Scholar] [CrossRef]
- Lin, H.; Chen, W.; Zhou, R.; Yang, J.; Wu, Y.; Zheng, J.; Fei, S.; Wu, G.; Sun, Z.; Li, J.; et al. Characteristics of the Plasmid-Mediated Colistin-Resistance Gene Mcr-1 in Escherichia Coli Isolated from a Veterinary Hospital in Shanghai. Front. Microbiol. 2022, 13, 1002827. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, X.; Xue, Y.; Li, X.; Zhu, X.; Xue, Y. Drug Resistance and Genetic Relatedness of Escherichia Coli from Mink in Northeast China. Pak. Vet. J. 2023, 43, 824–827. [Google Scholar] [CrossRef]
- Ewers, C.; Janßen, T.; Wieler, L.H. Avian pathogenic Escherichia coli (APEC). Berl. Und Munch. Tierarztl. Wochenschr. 2003, 116, 381–395. [Google Scholar]
- Ewers, C.; Li, G.; Wilking, H.; Kießling, S.; Alt, K.; Antáo, E.-M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian Pathogenic, Uropathogenic, and Newborn Meningitis-Causing Escherichia Coli: How Closely Related Are They? Int. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef]
- Ewers, C.; Antão, E.-M.; Diehl, I.; Philipp, H.-C.; Wieler, L.H. Intestine and Environment of the Chicken as Reservoirs for Extraintestinal Pathogenic Escherichia Coli Strains with Zoonotic Potential. Appl. Environ. Microbiol. 2009, 75, 184–192. [Google Scholar] [CrossRef]
- Mei, X.; Zhonhong, L.; Ping, Z.; Weiqiang, L. Genomic Characteristics of ETT2 Gene Clusters in Avian Pathogenic Escherichia Coli Identified by Whole-Genome Sequencing. Pak. Vet. J. 2024, 44, 833–839. [Google Scholar] [CrossRef]
- Guabiraba, R.; Schouler, C. Avian Colibacillosis: Still Many Black Holes. FEMS Microbiol. Lett. 2015, 362, fnv118. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-H.; Lee, J.-H.; Park, S.-H.; Song, M.-O.; Park, S.-H.; Jung, H.-W.; Park, G.-Y.; Choi, S.-M.; Kim, M.-S.; Chae, Y.-Z.; et al. Antimicrobial Resistance Profiles among Escherichia Coli Strains Isolated from Commercial and Cooked Foods. Int. J. Food Microbiol. 2012, 159, 263–266. [Google Scholar] [CrossRef]
- Asikur, R.; Shahidur, R.C.; Hemayet, H.; Fahmy, G.E.; Layla, A.A.; Ruhena, B.; Mirza Synthia, S.; Rashedunnabi, A.; Mukter, H.; Rafiqul, I. Identification of Virulence Genes and Multidrug Resistance in Shiga-Toxin Producing Escherichia Coli (STEC) from Migratory and Captive Wild Birds. Pak. Vet. J. 2024, 44, 833–839. [Google Scholar] [CrossRef]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and Simple Determination of the Escherichia Coli Phylogenetic Group. Appl. Env. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.J.; Hansen, N.I.; Zaterka-Baxter, K.; Higgins, R.D.; Stoll, B.J. Emergence of Antibiotic Resistance-Associated Clones Among Escherichia Coli Recovered From Newborns With Early-Onset Sepsis and Meningitis in the United States, 2008-2009. J Pediatr. Infect Dis. Soc. 2016, 5, 269–276. [Google Scholar] [CrossRef]
- Ratshilingano, M.T.; du Plessis, E.M.; Duvenage, S.; Korsten, L. Characterization of Multidrug-Resistant Escherichia Coli Isolated from Two Commercial Lettuce and Spinach Supply Chains. J. Food Prot. 2022, 85, 122–132. [Google Scholar] [CrossRef]
- Olesen, B.; Scheutz, F.; Menard, M.; Skov, M.N.; Kolmos, H.J.; Kuskowski, M.A.; Johnson, J.R. Three-Decade Epidemiological Analysis of Escherichia Coli O15:K52:H1. J. Clin. Microbiol. 2009, 47, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Shibata, N.; Yamane, K.; Wachino, J.; Ito, K.; Arakawa, Y. Change in the Prevalence of Extended-Spectrum-Beta-Lactamase-Producing Escherichia Coli in Japan by Clonal Spread. J. Antimicrob. Chemother. 2009, 63, 72–79. [Google Scholar] [CrossRef]
- Ciccozzi, M.; Giufre’, M.; Accogli, M.; Lo Presti, A.; Graziani, C.; Cella, E.; Cerquetti, M. Phylogenetic Analysis of Multidrug-Resistant Escherichia Coli Clones Isolated from Humans and Poultry. New Microbiol. 2013, 36, 385–394. [Google Scholar]
- Moulin-Schouleur, M.; Répérant, M.; Laurent, S.; Brée, A.; Mignon-Grasteau, S.; Germon, P.; Rasschaert, D.; Schouler, C. Extraintestinal Pathogenic Escherichia Coli Strains of Avian and Human Origin: Link between Phylogenetic Relationships and Common Virulence Patterns. J. Clin. Microbiol. 2007, 45, 3366–3376. [Google Scholar] [CrossRef] [PubMed]
- Sacher-Pirklbauer, A.; Klein-Jöbstl, D.; Sofka, D.; Blanc-Potard, A.-B.; Hilbert, F. Phylogenetic Groups and Antimicrobial Resistance Genes in Escherichia Coli from Different Meat Species. Antibiotics 2021, 10, 1543. [Google Scholar] [CrossRef]
- Wasyl, D.; Hoszowski, A.; Zając, M.; Szulowski, K. Antimicrobial Resistance in Commensal Escherichia Coli Isolated from Animals at Slaughter. Front. Microbiol. 2013, 4, 221. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. European Centre for Disease Prevention and Control The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2016. EFSA J. 2018, 16, e05182. [Google Scholar] [CrossRef]
- Jócsák, G.; Schilling-Tóth, B.; Bartha, T.; Tóth, I.; Ondrašovičová, S.; Kiss, D.S. Metal Nanoparticles-Immersion in the „tiny” World of Medicine. Magy. Állatorvosok Lapja 2025, 147, 115–127. [Google Scholar] [CrossRef]
- Such, N.; Molnár, A.; Pál, L.; Farkas, V.; Menyhárt, L.; Husvéth, F.; Dublecz, K. The Effect of Pre- and Probiotic Treatment on the Gumboro-Titer Values of Broilers. Magy. Állatorvosok Lapja 2021, 143, 119–127. [Google Scholar]
- Hetényi, N.; Bersényi, A.; Hullár, I. Physiological Effects of Medium-Chain Fatty Acids and Triglycerides, and Their Potential Use in Poultry and Swine Nutrition: A Literature Review. Magy. Állatorvosok Lapja 2024, 146, 651–659. [Google Scholar] [CrossRef]
- Jerzsele, Á.; Somogyi, Z.; Szalai, M.; Kovács, D. Effects of Fermented Wheat Germ Extract on Artificial Salmonella Typhimurium Infection in Broiler Chickens. Magy. Állatorvosok Lapja 2020, 142, 77–85. [Google Scholar]
- Pomothy, J.M.; Barna, R.F.; Gere, E. The Effects of the Rosmarinic Acid in Livestock Animals: Literature Review. Magy. Állatorvosok Lapja 2020, 142, 567–576. [Google Scholar]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Kovács, D.; Palkovicsné Pézsa, N.; Farkas, O.; Jerzsele, Á. Usage of Antibiotic Alternatives in Pig Farming: Literature Review. Magy. Állatorvosok Lapja 2021, 143, 281–282. [Google Scholar]
- Essősy, M.; Fodor, I.; Ihnáth, Z.; Karancsi, Z.; Kovács, D.; Szalai, K.V.; Szentmiklósi, D.; Jerzsele, Á. The Possibilities of Antibiotic-Free Broiler-Hen Fattening, with Special Reference to the Use of Pre- and Probiotics. Magy. Állatorvosok Lapja 2020, 142, 397–407. [Google Scholar]
- Mag, P.; Németh, K.; Somogyi, Z.; Jerzsele, Á. Antibacterial therapy based on pharmacokinetic/ pharmacodynamic models in small animal medicine-1. Literature review. Magy. Állatorvosok Lapja 2023, 145, 419–438. [Google Scholar] [CrossRef]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. EUCAST: Växjö, Sweden, 2023. [Google Scholar]
- Giske, C.G.; Turnidge, J.; Cantón, R.; Kahlmeter, G. EUCAST Steering Committee Update from the European Committee on Antimicrobial Susceptibility Testing (EUCAST). J. Clin. Microbiol. 2022, 60, e0027621. [Google Scholar] [CrossRef]
- Shrestha, R.D.; Agunos, A.; Gow, S.P.; Deckert, A.E.; Varga, C. Associations between Antimicrobial Resistance in Fecal Escherichia Coli Isolates and Antimicrobial Use in Canadian Turkey Flocks. Front. Microbiol. 2022, 13, 954123. [Google Scholar] [CrossRef]
- Suwono, B.; Eckmanns, T.; Kaspar, H.; Merle, R.; Zacher, B.; Kollas, C.; Weiser, A.A.; Noll, I.; Feig, M.; Tenhagen, B.-A. Cluster Analysis of Resistance Combinations in Escherichia Coli from Different Human and Animal Populations in Germany 2014–2017. PLoS ONE 2021, 16, e0244413. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Gow, S.P.; Léger, D.F.; Carson, C.A.; Deckert, A.E.; Bosman, A.L.; Loest, D.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial Use and Antimicrobial Resistance Indicators-Integration of Farm-Level Surveillance Data From Broiler Chickens and Turkeys in British Columbia, Canada. Front. Vet. Sci. 2019, 6, 131. [Google Scholar] [CrossRef]
- Boulianne, M.; Arsenault, J.; Daignault, D.; Archambault, M.; Letellier, A.; Dutil, L. Drug Use and Antimicrobial Resistance among Escherichia Coli and Enterococcus Spp. Isolates from Chicken and Turkey Flocks Slaughtered in Quebec, Canada. Can. J. Vet. Res. 2016, 80, 49–59. [Google Scholar]
- Grobbel, M.; Hammerl, J.A.; Alt, K.; Irrgang, A.; Kaesbohrer, A.; Tenhagen, B.-A. Comparison of Antimicrobial Resistances in Escherichia Coli from Conventionally and Organic Farmed Poultry from Germany. Antibiotics 2022, 11, 1282. [Google Scholar] [CrossRef]
- Kerek, Á.; Szabó, Á.; Dobra, P.F.; Bárdos, K.; Ózsvári, L.; Fehérvári, P.; Bata, Z.; Molnár-Nagy, V.; Jerzsele, Á. Determining the In Vivo Efficacy of Plant-Based and Probiotic-Based Antibiotic Alternatives against Mixed Infection with Salmonella Enterica and Escherichia Coli in Domestic Chickens. Vet. Sci. 2023, 10, 706. [Google Scholar] [CrossRef]
- Rhasid, S.; F Alsayeqh, A.; Akhtar, T.; Abbas, R.Z.; Ashraf, R. Probiotics: Alternative to Antibiotics in Poultry Production. Int. J. Vet. Sci. 2023, 12, 45–53. [Google Scholar] [CrossRef]
- Nobili, G.; La Bella, G.; Basanisi, M.G.; Damato, A.M.; Coppola, R.; Migliorelli, R.; Rondinone, V.; Leekitcharoenphon, P.; Bortolaia, V.; La Salandra, G. Occurrence and Characterisation of Colistin-Resistant Escherichia Coli in Raw Meat in Southern Italy in 2018–2020. Microorganisms 2022, 10, 1805. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Varona, O.; Kaspar, H.; Grobbel, M.; Tenhagen, B.-A. Phenotypical Antimicrobial Resistance Data of Clinical and Non-Clinical Escherichia Coli from Poultry in Germany between 2014 and 2017. PLoS ONE 2020, 15, e0243772. [Google Scholar] [CrossRef]
- Hu, J.; Yang, J.; Chen, W.; Liu, Z.; Zhao, Q.; Yang, H.; Sun, Z.; Chen, X.; Li, J. Prevalence and Characteristics of Mcr-1-Producing Escherichia Coli in Three Kinds of Poultry in Changsha, China. Front. Microbiol. 2022, 13, 840520. [Google Scholar] [CrossRef] [PubMed]
- Gambi, L.; Rossini, R.; Menandro, M.L.; Franzo, G.; Valentini, F.; Tosi, G.; D’Incau, M.; Fiorentini, L. Virulence Factors and Antimicrobial Resistance Profile of Escherichia Coli Isolated from Laying Hens in Italy. Animals 2022, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, A.; Giovanardi, D.; Dotto, G.; Grilli, G.; Montesissa, C.; Boldrin, C.; Salata, C.; Giacomelli, M. Antimicrobial Resistance and Class 1 and 2 Integrons in Escherichia Coli from Meat Turkeys in Northern Italy. Avian Pathol. 2014, 43, 396–405. [Google Scholar] [CrossRef]
- Moawad, A.A.; Hotzel, H.; Hafez, H.M.; Ramadan, H.; Tomaso, H.; Braun, S.D.; Ehricht, R.; Diezel, C.; Gary, D.; Engelmann, I.; et al. Occurrence, Phenotypic and Molecular Characteristics of Extended-Spectrum Beta-Lactamase-Producing Escherichia Coli in Healthy Turkeys in Northern Egypt. Antibiotics 2022, 11, 1075. [Google Scholar] [CrossRef]
- Barnácz, F.; Kerek, Á.; Csirmaz, B.; Román, I.L.; Gál, C.; Horváth, Á.; Hajduk, E.; Szabó, Á.; Jerzsele, Á.; Kovács, L. The Status of Antimicrobial Resistance in Domestic Poultry with Different Breeding Purposes in Hungary between 2022-2023. Magy. Állatorvosok Lapja 2024, 146, 339–356. [Google Scholar] [CrossRef]
- Carmona-Cartaya, Y.; Hidalgo-Benito, M.; Borges-Mateus, L.M.; Pereda-Novales, N.; González-Molina, M.K.; Quiñones-Pérez, D. Community-Acquired Uropathogenic Escherichia Coli, Antimicrobial Susceptibility, and Extended-Spectrum Beta-Lactamase Detection. MEDICC Rev. 2022, 24, 20–25. [Google Scholar] [CrossRef]
- Jańczak, D.; Górecki, P.; Stryjek, R.; Zasada, A. Multidrug Resistance of Escherichia Coli Isolated from the Urinary Bladder of Dogs and Cats with Suspected Urinary Tract Infections. Ann. Agric. Env. Med. 2024, 31, 178–184. [Google Scholar] [CrossRef]
- Brown, S.A. Fluoroquinolones in Animal Health. J. Vet. Pharmacol. Ther. 1996, 19, 1–14. [Google Scholar] [CrossRef]
- Lam, J.C.; Lang, R.; Stokes, W. How I Manage Bacterial Prostatitis. Clin. Microbiol. Infect. 2023, 29, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kovács, L.; Klaucke, C.R.; Farkas, M.; Bakony, M.; Jurkovich, V.; Könyves, L. The Correlation between On-Farm Biosecurity and Animal Welfare Indices in Large-Scale Turkey Production. Poult. Sci. 2025, 104, 104598. [Google Scholar] [CrossRef]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity Situation of Large-Scale Poultry Farms in Hungary According to the Databases of National Food Chain Safety Office Centre for Disease Control and Biosecurity Audit System of Poultry Product Board of Hungary in the Period of 2021–2022. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Aliczki, K. A Magyarországi Pulyka Vertikum egy Évtizedes Fejlődési Pályájának Értékelése=Development of the Hungarian Turkey Sector in the Last Decade; Agrárgazdasági Kutató Intézet: Budapest, Hungary, 2014. [Google Scholar]
- CLSI Standards M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Lima-Filho, J.V.; Martins, L.V.; Nascimento, D.C.d.O.; Ventura, R.F.; Batista, J.E.C.; Silva, A.F.B.; Ralph, M.T.; Vaz, R.V.; Rabello, C.B.-V.; Silva, I. de M.M. da; et al. Zoonotic Potential of Multidrug-Resistant Extraintestinal Pathogenic Escherichia Coli Obtained from Healthy Poultry Carcasses in Salvador, Brazil. Braz. J. Infect. Dis. 2013, 17, 54–61. [Google Scholar] [CrossRef]
- Hesp, A.; van Schaik, G.; Wiegel, J.; Heuvelink, A.; Mevius, D.; Veldman, K. Antimicrobial Resistance Monitoring in Commensal and Clinical Escherichia Coli from Broiler Chickens: Differences and Similarities. Prev. Vet. Med. 2022, 204, 105663. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-Test? On Assumptions for Hypothesis Tests and Multiple Interpretations of Decision Rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Sibson, R. SLINK: An Optimally Efficient Algorithm for the Single-Link Cluster Method. Comput. J. 1973, 16, 30–34. [Google Scholar] [CrossRef]



| Antibiotics | Respiratory–Cloaca | Meat–Breeding | 3 Young–4 Adult | 5 Small–6 Medium |
|---|---|---|---|---|
| p-Values | ||||
| Ceftriaxone | 1.0000 | 0.0015 * | 0.0015 * | 0.7282 |
| Imipenem | 0.7206 | 0.0023 * | 0.0023 * | 0.0347 * |
| Doxycycline | 0.1669 | 0.6840 | 0.6840 | 0.1207 |
| 1 Potentiated sulfonamide | 1.0000 | 0.3791 | 0.3791 | 0.2935 |
| 2 Amoxicillin–clavulanic acid | 0.5670 | 1.0000 | 1.0000 | 1.0000 |
| Florfenicol | 1.0000 | 0.0001 * | 0.0001 * | 0.0002 * |
| Enrofloxacin | 1.0000 | 0.3556 | 0.3556 | 0.0002 * |
| Amoxicillin | 0.3418 | 0.1059 | 0.1059 | 0.8632 |
| Neomycin | 0.9131 | 0.1853 | 0.1853 | 0.8703 |
| Spectinomycin | 0.8368 | <0.0001 * | <0.0001 * | 0.4525 |
| Colistin | 0.2777 | <0.0001 * | <0.0001 * | 0.0006 * |
| Antibiotic | 1 BP * | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | MIC50 | MIC90 | 2 ECOFF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/mL | µg/mL | ||||||||||||||||||||||||
| Enrofloxacin | 1 2 | 6 | 0 | 1 | 5 | 24 | 24 | 22 | 13 | 13 | 22 | 45 | 35 | 14 | 38 | 55 | 73 | 32 | 22 | 13 | 9 | 4 | 8 | 128 | 0.125 |
| 1.3% | 0.0% | 0.2% | 1.1% | 5.1% | 5.1% | 4.7% | 2.8% | 2.8% | 4.7% | 9.6% | 7.4% | 3.0% | 8.1% | 11.7% | 15.5% | 6.8% | 4.7% | 2.8% | 1.9% | 0.9% | |||||
| Colistin | 2 | 2 | 0 | 0 | 0 | 11 | 17 | 36 | 45 | 103 | 83 | 33 | 10 | 12 | 6 | 4 | 3 | 1 | 1 | 5 | 21 | 77 | 0.5 | 1024 | 2 |
| 0.4% | 0.0% | 0.0% | 0.0% | 2.3% | 3.6% | 7.7% | 9.6% | 21.9% | 17.7% | 7.0% | 2.1% | 2.6% | 1.3% | 0.9% | 0.6% | 0.2% | 0.2% | 1.1% | 4.5% | 16.4% | |||||
| Ceftriaxone | 1 4 | 1 | 3 | 22 | 76 | 135 | 62 | 12 | 12 | 25 | 9 | 10 | 13 | 8 | 4 | 3 | 10 | 19 | 14 | 32 | 0.063 | 256 | 0.125 | ||
| 0.2% | 0.6% | 4.7% | 16.2% | 28.7% | 13.2% | 2.6% | 2.6% | 5.3% | 1.9% | 2.1% | 2.8% | 1.7% | 0.9% | 0.6% | 2.1% | 4.0% | 3.0% | 6.8% | |||||||
| Imipenem | 1 4 | 2 | 5 | 5 | 27 | 77 | 97 | 110 | 62 | 37 | 30 | 13 | 5 | 0.25 | 4 | 0.5 | |||||||||
| 0.4% | 1.1% | 1.1% | 5.7% | 16.4% | 20.6% | 23.4% | 13.2% | 7.9% | 6.4% | 2.8% | 1.1% | ||||||||||||||
| 3 Potentiated sulphonamide | 1 4 | 1 | 5 | 11 | 59 | 67 | 40 | 22 | 21 | 15 | 7 | 20 | 42 | 160 | 4 | 64 | 0.5 | ||||||||
| 0.2% | 1.1% | 2.3% | 12.6% | 14.3% | 8.5% | 4.7% | 4.5% | 3.2% | 1.5% | 4.3% | 8.9% | 34.0% | |||||||||||||
| Doxycycline | 1 16 | 4 | 1 | 0 | 29 | 60 | 32 | 47 | 73 | 101 | 97 | 20 | 2 | 3 | 1 | 16 | 64 | 8 | |||||||
| 0.9% | 0.2% | 0.0% | 6.2% | 12.8% | 6.8% | 10.0% | 15.5% | 21.5% | 20.6% | 4.3% | 0.4% | 0.6% | 0.2% | ||||||||||||
| Florfenicol | 1 16 | 2 | 9 | 43 | 84 | 126 | 56 | 28 | 16 | 62 | 38 | 6 | 16 | 256 | 16 | ||||||||||
| 0.4% | 1.9% | 9.1% | 17.9% | 26.8% | 11.9% | 6.0% | 3.4% | 13.2% | 8.1% | 1.3% | |||||||||||||||
| Neomycin | 32 | 8 | 56 | 66 | 13 | 22 | 37 | 129 | 59 | 26 | 37 | 17 | 64 | 512 | 8 | ||||||||||
| 1.7% | 11.9% | 14.0% | 2.8% | 4.7% | 7.9% | 27.4% | 12.6% | 5.5% | 7.9% | 3.6% | |||||||||||||||
| Amoxicillin | 1 32 | 1 | 2 | 4 | 4 | 17 | 6 | 22 | 73 | 41 | 11 | 16 | 11 | 29 | 29 | 52 | 152 | 128 | 1024 | 8 | |||||
| 0.2% | 0.4% | 0.9% | 0.9% | 3.6% | 1.3% | 4.7% | 15.5% | 8.7% | 2.3% | 3.4% | 2.3% | 6.2% | 6.2% | 11.1% | 32.3% | ||||||||||
| 4 Amoxicillin–clavulanic acid | 32 | 4 | 13 | 6 | 15 | 28 | 71 | 138 | 108 | 50 | 23 | 12 | 2 | 8 | 32 | 8 | |||||||||
| 0.9% | 2.8% | 1.3% | 3.2% | 6.0% | 15.1% | 29.4% | 23.0% | 10.6% | 4.9% | 2.6% | 0.4% | ||||||||||||||
| Spectinomycin | 128 | 1 | 8 | 66 | 64 | 110 | 84 | 68 | 33 | 36 | 64 | 512 | 64 | ||||||||||||
| 0.2% | 1.7% | 14.0% | 13.6% | 23.4% | 17.9% | 14.5% | 7.0% | 7.7% | |||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerzsele, Á.; Kerek, Á.; Barnácz, F.; Csirmaz, B.; Szabó, Á.; Kovács, L. Antimicrobial Susceptibility Profiles of Commensal Escherichia coli Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics 2025, 14, 305. https://doi.org/10.3390/antibiotics14030305
Jerzsele Á, Kerek Á, Barnácz F, Csirmaz B, Szabó Á, Kovács L. Antimicrobial Susceptibility Profiles of Commensal Escherichia coli Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics. 2025; 14(3):305. https://doi.org/10.3390/antibiotics14030305
Chicago/Turabian StyleJerzsele, Ákos, Ádám Kerek, Franciska Barnácz, Bence Csirmaz, Ábel Szabó, and László Kovács. 2025. "Antimicrobial Susceptibility Profiles of Commensal Escherichia coli Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023" Antibiotics 14, no. 3: 305. https://doi.org/10.3390/antibiotics14030305
APA StyleJerzsele, Á., Kerek, Á., Barnácz, F., Csirmaz, B., Szabó, Á., & Kovács, L. (2025). Antimicrobial Susceptibility Profiles of Commensal Escherichia coli Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics, 14(3), 305. https://doi.org/10.3390/antibiotics14030305







