Seasonal Change in Microbial Diversity: Bile Microbiota and Antibiotics Resistance in Patients with Bilio-Pancreatic Tumors: A Retrospective Monocentric Study (2010–2020)
Abstract
1. Introduction
2. Results
3. Discussion
Limitations
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sicily Region. Department of Health. Available online: https://www.regione.sicilia.it/istituzioni/regione/strutture-regionali/assessorato-salute/dipartimento-attivita-sanitarie-osservatorio-epidemiologico/epidemiologia-prevenzione/epidemiologia/salute/atlante (accessed on 14 January 2025).
- Kim, Y.A.; Kim, H.J.; Kang, M.J.; Han, S.; Park, H.M.; Park, S. Increased diagnosis of hepato-biliary-pancreatic cancer after cholecystectomy: A population-based study. Sci. Rep. 2025, 15, 411. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, P.; Serra, N.; Assunta Fasciana, T.M.; Giammanco, A.; Rea, T.; Napolitano, M.S.; Lucchesi, A.; Cascio, A.; Sergi, C.M. Microbial profile in bile from pancreatic and extra-pancreatic biliary tract cancer. PLoS ONE 2024, 19, e0294049. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, P.; Serra, N.; D’Arpa, F.; Agrusa, A.; Gulotta, G.; Fasciana, T.; Rodolico, V.; Giammanco, A.; Sergi, C. The microbiota of the bilio-pancreatic system: A cohort, STROBE-compliant study. Infect. Drug Resist. 2019, 12, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Serra, N.; Di Carlo, P.; D’arpa, F.; Battaglia, E.; Fasciana, T.; Gulotta, G.; Maida, C.M.; Rodolico, V.; Giammanco, A.; Sergi, C. Human bile microbiota: A retrospective study focusing on age and gender. J. Infect. Public Health 2021, 14, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, P.; Serra, N.; Gulotta, G.; Giammanco, A.; Colomba, C.; Melfa, G.; Fasciana, T.; Sergi, C. Bactibilia in diseases of the biliary tract and pancreatic gland in patients older than 80 years: A STROBE-retrospective cohort study in a teaching hospital in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Serra, N.; Di Carlo, P.; Gulotta, G.; d’Arpa, F.; Giammanco, A.; Colomba, C.; Melfa, G.; Fasciana, T.; Sergi, C. Bactibilia in women affected with diseases of the biliary tract and pancreas. A STROBE guidelines-adherent cross-sectional study in Southern Italy. J. Med. Microbiol. 2018, 67, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Di Carlo, P.; Gulotta, G.; D’Arpa, F. Biliary microbiota in pancreatic cancer. HPB 2019, 21, 1790. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Zureikat, A.H.; Novelli, P.M.; Lopez, C.D.; Kardosh, A.; Chung, K.Y.; Devane, A.M.; Hatoum, H.; Meredith, K.L.; Zervos, E.E.; et al. Sepsis and pancreatic cancer: Biliary stents a significant risk factor in patients undergoing chemotherapy. J. Clin. Oncol. 2021, 39 (Suppl. S3), 399. [Google Scholar] [CrossRef]
- Argiroff, W.A.; Carrell, A.A.; Klingeman, D.M.; Dove, N.C.; Muchero, W.; Veach, A.M.; Wahl, T.; Lebreux, S.J.; Webb, A.B.; Peyton, K.; et al. Seasonality and longer-term development generate temporal dynamics in the Populus microbiome. mSystems 2024, 9, e00886-23. [Google Scholar] [CrossRef]
- Man, W.H.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef]
- Gunawan, W.B.; Abadi, M.N.P.; Fadhillah, F.S.; Nurkolis, F.; Pramono, A. The interlink between climate changes, gut microbiota, and aging processes. Hum. Nutr. Metab. 2023, 32, 200193. [Google Scholar] [CrossRef]
- Malta, G.; Serra, N.; Spatola, G.F.; Maida, C.M.; Graziano, G.; Di Raimondo, D.; Fasciana, T.M.; Caputo, V.; Giammanco, A.; Capuano, A.; et al. The Impact of the Seasonal and Geographical Distribution of Tuberculosis in Sicily: A 6-Year Retrospective Study (2018–2023). J. Clin. Med. 2024, 13, 3546. [Google Scholar] [CrossRef]
- Smits, S.A.; Leach, J.; Sonnenburg, E.D.; Gonzalez, C.G.; Lichtman, J.S.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Elias, J.E.; et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017, 357, 802–806. [Google Scholar] [CrossRef]
- Ramsey, E.G.; Royer, J.; Bookstaver, P.B.; Justo, J.A.; Kohn, J.; Albrecht, H.; Al-Hasan, M.N. Seasonal variation in antimicrobial resistance rates of community-acquired Escherichia coli bloodstream isolates. Int. J. Antimicrob. Agents 2019, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, F.; Rasool, M.H.; Shafiq, M.; Aslam, B.; Khurshid, M. Emergence of Carbapenem-Resistant Uropathogenic Escherichia coli (ST405 and ST167) Strains Carrying blaCTX-M-15, blaNDM-5 and Diverse Virulence Factors in Hospitalized Patients. Pathogens 2024, 13, 964. [Google Scholar] [CrossRef]
- Roux, J.; Nekkab, N.; Astagneau, P.; Crépey, P. Time-series modelling for the quantification of seasonality and forecasting antibiotic-resistant episodes: Application to carbapenemase-producing Enterobacteriaceae episodes in France over 2010–20. J. Antimicrob. Chemother. 2020, 76, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Sicily Region. Department of Health. Available online: https://www.qualitasiciliassr.it/?q=rete-laboratori (accessed on 14 January 2025).
- Di Carlo, P.; Gulotta, G.; Casuccio, A.; Pantuso, G.; Raineri, M.; Farulla, C.A.; Bonventre, S.; Guadagnino, G.; Ingrassia, D.; Cocorullo, G.; et al. KPC-3 Klebsiella pneumoniaeST258 clone infection in postoperative abdominal surgery patients in an intensive care setting: Analysis of a case series of 30 patients. BMC Anesthesiol. 2013, 13, 13. [Google Scholar] [CrossRef]
- Di Carlo, P.; Serra, N.; Lo Sauro, S.; Carelli, V.M.; Giarratana, M.; Signorello, J.C.; Lucchesi, A.; Manta, G.; Napolitano, M.S.; Rea, T.; et al. Epidemiology and Pattern of Resistance of Gram-Negative Bacteria Isolated from Blood Samples in Hospitalized Patients: A Single Center Retrospective Analysis from Southern Italy. Antibiotics 2021, 10, 1402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maugeri, G.; Calvo, M.; Bongiorno, D.; Bivona, D.; Migliorisi, G.; Privitera, G.F.; Scalia, G.; Stefani, S. Sequencing Analysis of Invasive Carbapenem-Resistant Klebsiella pneumoniae Isolates Secondary to Gastrointestinal Colonization. Microorganisms 2025, 13, 89. [Google Scholar] [CrossRef]
- Politi, C.; Mobrici, M.; Parlongo, R.M.; Spoto, B.; Tripepi, G.; Pizzini, P.; Cutrupi, S.; Franco, D.; Tino, R.; Farruggio, G.; et al. Role of Gut Microbiota in Overweight Susceptibility in an Adult Population in Italy. Nutrients 2023, 15, 2834. [Google Scholar] [CrossRef]
- Sisti, D.; Pazienza, V.; Piccini, F.; Citterio, B.; Baffone, W.; Zeppa, S.D.; Biavasco, F.; Prospero, E.; De Luca, A.; Artico, M.; et al. A proposal for the reference intervals of the Italian microbiota “scaffold” in healthy adults. Sci. Rep. 2022, 12, 3952. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the Fecal Bile Acid Profile by Gut Microbiota in Cirrhosis. J. Hepatol. 2013, 58, 949. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of Gut Microbiota with Bile Acid Metabolism and its Influence on Disease States. Appl. Microbiol. Biotechnol. 2017, 101, 47. [Google Scholar] [CrossRef]
- Miura, F.; Okamoto, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Pitt, H.A.; Gomi, H.; Solomkin, J.S.; Schlossberg, D.; Han, H.; et al. Tokyo Guidelines 2018: Initial management of acute biliary infection and flowchart for acute cholangitis. J. Hepatobiliary Pancreat. Sci. 2018, 25, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Coppola, A.; La Vaccara, V.; Farolfi, T.; Fiore, M.; Cascone, C.; Ramella, S.; Spoto, S.; Ciccozzi, M.; Angeletti, S.; Coppola, R.; et al. Different Biliary Microbial Flora Influence Type of Complications after Pancreaticoduodenectomy: A Single Center Retrospective Analysis. J. Clin. Med. 2021, 10, 2180. [Google Scholar] [CrossRef]
- Papaefthymiou, A.; Landi, R.; Arvanitakis, M.; Tringali, A.; Gkolfakis, P. Endoscopic retrograde cholangiopancreatography: A comprehensive review as a single diagnostic tool. Best Pract. Res. Clin. Gastroenterol. 2025, 101976, in press, corrected proof. [Google Scholar] [CrossRef]
- Perencevich, E.N.; McGregor, J.C.; Shardell, M.; Furuno, J.P.; Harris, A.D.; Morris, J.G.; Fisman, D.N.; Johnson, J.A. Summer Peaks in the Incidences of Gram-Negative Bacterial Infection Among Hospitalized Patients. Infect. Control Hosp. Epidemiol. 2008, 29, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Richet, H. Seasonality in Gram-negative and healthcare-associated infections. Clin. Microbiol. Infect. 2012, 18, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.G.; Ruban, K.; Donders, F.; Reybrouck, R. Lab-Based Retrospective 10-Year Analysis Shows Seasonal Variation of Vaginal Candida Infection Rates in Belgium. J. Clin. Med. 2022, 11, 574. [Google Scholar] [CrossRef]
- Yalçın, B.; Tamer, E.; Toy, G.G.; Öztaş, P.; Hayran, M.; Allı, N. The prevalence of skin diseases in the elderly: Analysis of 4099 geriatric patients. Int. J. Dermatol. 2006, 45, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, Y.; Jeon, J.; Gwak, H.J.; Kim, M.; Kang, K.; Kim, Y.; Jeong, J.; Jung, Y.K.; Lee, K.G.; et al. Association of Microbial Dysbiosis with Gallbladder Diseases Identified by Bile Microbiome Profiling. J. Korean Med. Sci. 2021, 36, e189. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, M.; Tang, L.; Wang, B.; Huang, Y.; Xu, Y.; Li, Y. Epidemiological, clinical and microbiological characteristics of patients with biliary tract diseases with positive bile culture in a tertiary hospital. BMC Infect. Dis. 2024, 24, 1010. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Xie, C.; Zhao, Y.; Liao, J.; Li, S.; Zhang, Y.; Wang, D.; Hua, K.; Gu, Y.; Du, J.; et al. The gut microbiota-bile acid axis in cholestatic liver disease. Mol. Med. 2024, 30, 104. [Google Scholar] [CrossRef]
- Elvevi, A.; Laffusa, A.; Gallo, C.; Invernizzi, P.; Massironi, S. Any Role for Microbiota in Cholangiocarcinoma? A Comprehensive Review. Cells 2023, 12, 370. [Google Scholar] [CrossRef]
- Bock, L.; Aguilar-Bultet, L.; Egli, A.; Battegay, M.; Kronenberg, A.; Vogt, R.; Kaufmann, C.; Tschudin-Sutter, S. Air temperature and incidence of extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae. Environ. Res. 2022, 215, 114146. [Google Scholar] [CrossRef]
- Kaier, K.; Frank, U.; Conrad, A.; Meyer, E. Seasonal and ascending trends in the incidence of carriage of extended-spectrum ß-lactamase-producing Escherichia coli and Klebsiella species in 2 German hospitals. Infect. Control. Hosp. Epidemiol. 2010, 31, 1154–1159. [Google Scholar]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Chang. 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Schreiber, P.W.; Dunic, M.; Wolfensberger, A.; Clack, L.; Falk, C.; Sax, H.; Kuster, S.P. Seasonal differences in central line-associated bloodstream infection incidence rates in a Central European setting: Results from prospective surveillance. Am. J. Infect. Control 2019, 47, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Guyot, A.; Turton, J.; Garner, D. Outbreak of Stenotrophomonas maltophilia on an intensive care unit. J. Hosp. Infect. 2013, 85, 303–307. [Google Scholar] [CrossRef]
- Psoter, K.J.; De Roos, A.J.; Wakefield, J.; Mayer, J.; Rosenfeld, M. Season is associated with Pseudomonas aeruginosa acquisition in young children with cystic fibrosis. Clin. Microbiol. Infect. 2013, 19, E483–E489. [Google Scholar] [CrossRef] [PubMed]
- Cassone, M.; Mantey, J.; Gontjes, K.J.; Lansing, B.J.; Gibson, K.E.; Wang, J.; Mody, L. Seasonal Patterns in Incidence and Antimicrobial Resistance of Common Bacterial Pathogens in Nursing Home Patients and Their Rooms. Front. Public Health 2021, 9, 671428. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.F.; Silva, D.; Rodrigues, A.; Pina-Vaz, C. Colistin Update on Its Mechanism of Action and Resistance, Present and Future Challenges. Microorganisms 2020, 8, 1716. [Google Scholar] [CrossRef]
- Hachem, R.Y.; Chemaly, R.F.; Ahmar, C.A.; Jiang, Y.; Boktour, M.R.; Rjaili, G.A.; Bodey, G.P.; Raad, I.I. Colistin is effective in treatment of infections caused by multidrug-resistant Pseudomonas aeruginosa in cancer patients. Antimicrob. Agents Chemother. 2007, 51, 1905–1911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rout, B.P.; Behera, B.; Sahu, K.K.; Praharaj, I.; Otta, S. An overview of colistin resistance: A breach in last line defense. Med. J. Armed Forces India 2023, 79, 516. [Google Scholar] [CrossRef]
- Capuozzo, M.; Zovi, A.; Langella, R.; Ottaiano, A.; Cascella, M.; Scognamiglio, M.; Ferrara, F. Optimizing Antibiotic Use: Addressing Resistance Through Effective Strategies and Health Policies. Antibiotics 2024, 13, 1112. [Google Scholar] [CrossRef] [PubMed]
- Meschiari, M.; Lòpez-Lozano, J.-M.; Di Pilato, V.; Gimenez-Esparza, C.; Vecchi, E.; Bacca, E.; Orlando, G.; Franceschini, E.; Sarti, M.; Pecorari, M.; et al. A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob. Resist. Infect. Control 2021, 10, 123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dyson, P.J.; Banat, I.M.; Quinn, G.A. War and peace: Exploring microbial defence systems as a source of new antimicrobial therapies. Front. Pharmacol. 2025, 15, 1504901. [Google Scholar] [CrossRef]
- Sciattella, P.; Fornero, A.; Giordano, S.M.A.; De Angelis, C.G.; Cattel, F. The economic burden of post-endoscopic retrograde cholangiopancreatography (ERCP) procedure infections in Italy. Glob. Reg. Health Technol. Assess. 2024, 11, 258–264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, C.; Hong, Y.K. Pancreatic Cancer. In Passing the General Surgery Oral Board Exam; Neff, M., Beekley, A., Yoon-Flannery, K., Ratnasekera, A., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takagi, T.; Suzuki, T.; Shimizu, H.; Shibukawa, G.; Nakajima, Y.; Takeda, Y.; Noguchi, Y.; Kobayashi, R.; Imamura, H.; et al. A new preprocedural predictive risk model for post-endoscopic retrograde cholangiopancreatography pancreatitis: The SuPER model. eLife 2025, 13, RP101604. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Policlinic University Hospital “Paolo Giaccone” University of Palermo. Available online: https://intranet.policlinico.pa.it/pub/documenti/browse.do?dispatch=documentRead&documentId=1323c0545872ac910158731923c50019 (accessed on 2 February 2025).
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, K.; De Brito, C.B.; Byndloss, M.X. Metabolic tug-of-war: Microbial metabolism shapes colonization resistance against enteric pathogens. Cell Chem. Biol. 2025, 32, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Xiao, Y.; Weiss, S.T.; Chen, X.; Kelly, C.P.; Liu, Y. A computational method to dissect colonization resistance of the gut microbiota against pathogens. Cell Rep. Methods 2023, 3, 100576. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.M.; Beley, G.J.; Dable-Tupas, G.; Talampas-Abundo, M.D.; Añonuevo, J.J.J.; Sahai, S. Human microbiome and infectious diseases. In Human Microbiome Drug Targets; Elsevier: Amsterdam, The Netherlands, 2024; pp. 151–164. [Google Scholar] [CrossRef]
- Sevcikova, A.; Izoldova, N.; Stevurkova, V.; Kasperova, B.; Chovanec, M.; Ciernikova, S.; Mego, M. The Impact of the Microbiome on Resistance to Cancer Treatment with Chemotherapeutic Agents and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Parameters | Sample |
|---|---|
| Patients | 90 |
| Age at hospitalization | |
| Mean ± SD | 75.5 ± 10.1 |
| Median (IQR) | 76.0 (70.0, 83.0) |
| Time of hospitalization (days) | |
| Mean ± SD | 15.6 ± 22.2 |
| Median (IQR) | 8.0 (4.0, 17.0) |
| Gender | |
| Male | 58.9% (53) |
| Female | 41.1% (37) |
| Isolates * | |
| Gram − | 87.8% (79) |
| Gram + | 31.1% (28) |
| Candida spp. | 21.1% (19) |
| Tumor type | |
| Gallbladder carcinoma | 5.6% (5) |
| Cholangiocarcinoma | 28.9% (26) |
| Pancreatic cancer | 65.6% (59) |
| Comorbidity † | |
| Acute coronary syndrome (ACS) | 20.0% (18) |
| Autoimmune diseases (AD) | 8.9% (8) |
| Chronic heart failure (CHF) | 34.4% (31) |
| Chronic obstructive pulmonary disease (COPD) | 15.6% (14) |
| Chronic renal failure (CRF) | 20.0% (18) |
| Dementia (DM) | 15.6% (14) |
| Diabetes | 48.9% (44) |
| Hypertension | 57.8% (52) |
| Hemodialysis (HD) | 3.3% (3) |
| Liver cirrhosis (LC) | 32.2% (29) |
| Isolated Strains | Total | Autumn (Au) | Summer (Su) | Winter (Wi) | Spring (Sp) | Among Seasons p-Value |
|---|---|---|---|---|---|---|
| Total | 149 | 27.5% (41/149) | 30.9% (46/149) | 20.8% (31/149) | 20.8% (31/149) | |
| Gram − | 68.5% (102/149) | 28.4% (29/102) | 34.3% (35/102) | 21.6% (22/102) | 15.7% (16/102) | p = 0.0353 * (Cgf) Summer **, p = 0.0197 (Z) |
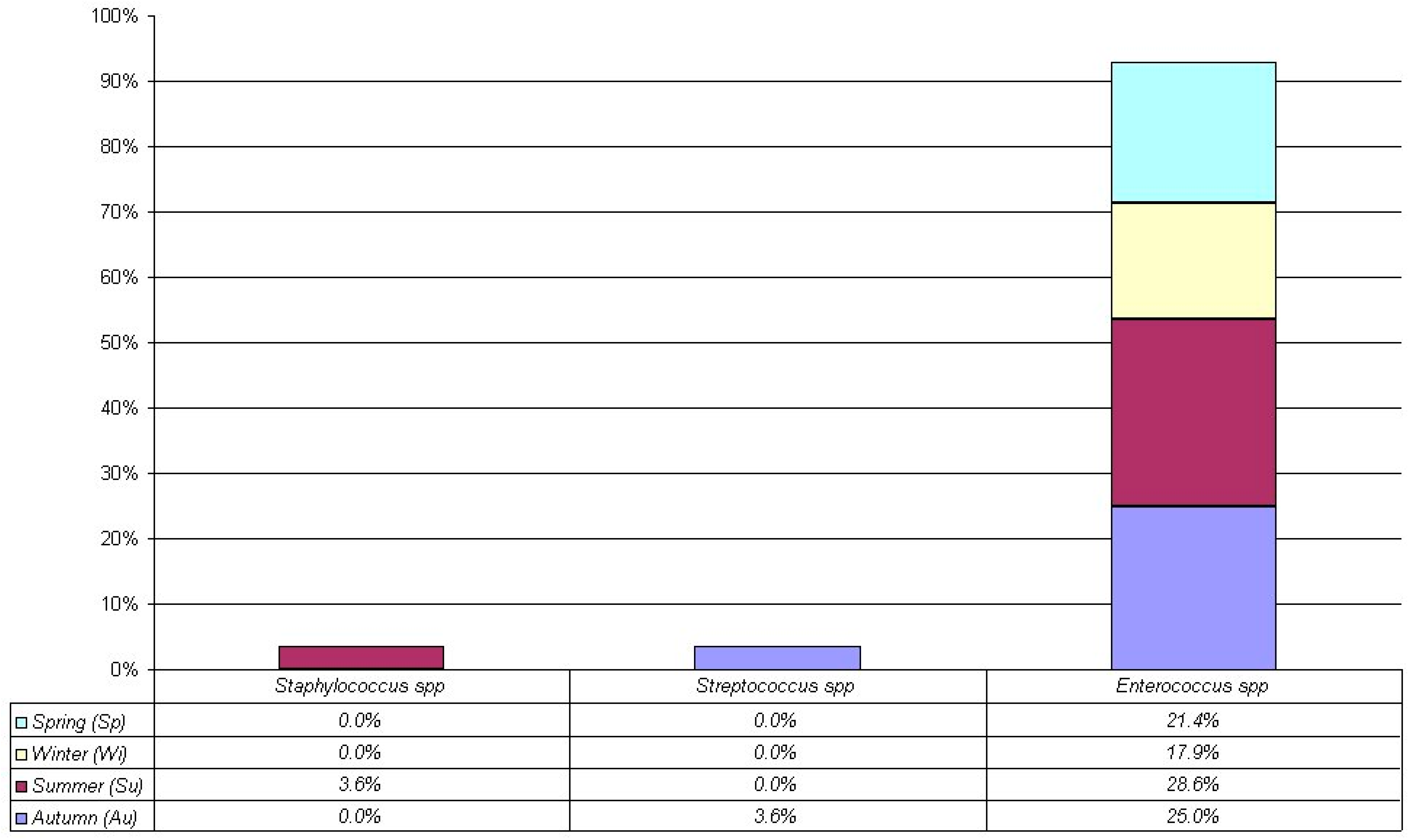
| Gram + | 18.8% (28/149) | 28.6% (8/28) | 32.1% (9/28) | 17.9% (5/28) | 21.4% (6/28) | p = 0.86 (Cgf) |
| Candida spp. | 12.8% (19/149) | 21.1% (4/19) | 10.5% (2/19) | 15.8% (3/19) | 52.6% (10/19) | p = 0.0429 * (Cgf) Spring **, p = 0.0003 (Z) |
| Isolates/Antibiotics | TOTAL Resistance | Autumn (Au) % (n) | Summer (Su) % (n) | Winter (Wi) % (n) | Spring (Sp) % (n) | Among Seasons p-Value (Test) |
|---|---|---|---|---|---|---|
| Gram − (102) | n = 102 | 28.4% (29/102) | 34.3% (35/102) | 21.6% (22/102) | 15.7% (16/102) | |
| (1) 3GC | 94.1% (96) | 100% (29) | 94.3% (33) | 90.9% (20) | 87.5% (14) | p = 0.0261 * (Cgf) Su **, p = 0.0226 Sp ***, p = 0.0264 |
| (2) Cefepime | 82.4% (84) | 93.1% (27) | 91.4% (32) | 72.7% (16) | 56.3% (9) | p = 0.0014 * (Cgf) Su **, p = 0.0021 |
| (3) Fluoroquinolones | 88.2% (90) | 96.6% (28) | 94.3% (33) | 86.4% (19) | 62.5% (10) | p = 0.0033 * (Cgf) Su **, p = 0.0052 Sp ***, p = 0.0053 |
| (4) Aminoglycosides | 71.6% (73) | 79.3% (23) | 74.3% (26) | 68.2% (15) | 56.3% (9) | p = 0.0204 * (Cgf) Su **, p = 0.0228 Sp ***, p = 0.02 |
| (5) Fosfomycin | 69.6% (71) | 75.9% (22) | 77.1% (27) | 63.6% (14) | 50.0% (8) | p = 0.0074 * (Cgf) Su **, p = 0.0050 Sp ***, p = 0.0134 |
| (6) Piperacillin- tazobactam | 78.4% (80) | 79.3% (23) | 91.4% (32) | 72.7% (16) | 56.3% (9) | p = 0.0023 * (Cgf) Su **, p = 0.0005 Sp ***, p = 0.0086 |
| (7) Carbapenem | 40.2% (41) | 37.9% (11) | 45.7% (16) | 31.8% (7) | 43.8% (7) | p = 0.15 (Cgf) |
| (8) Colistin | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | p = N/A |
| Analysis in the season p-value (test) | p < 0.001 * (Q) (1), (2), (3), (4), (5), (6) > (7) **, p < 0.05 (B) (1), (2), (3), (4), (5), (6) > (8) **, p < 0.05 (B) | p < 0.001 * (Q) (1), (2), (3), (4) and (6) > (7) **, p < 0.05 (B) (1), (2), (3), (4), (5), (6), (7) > (8)**, p < 0.05 (B) | p < 0.001 * (Q) (1), (2), (3) and (6) > (7) **, p < 0.05 (B) (1), (2), (3), (4), (5), (6), (7) > (8) **, p < 0.05 (B) | p < 0.001 * (Q) (1) > (7) **, p < 0.05 (B) (1), (2), (3), (4), (5), (6) > (8) **, p < 0.05 (B) | p < 0.001 * (Q) (1), (2), (3), (4), (5), (6) > (8) **, p < 0.05 (B) | |
| Gram + (28) | n = 28 | 28.6% (8/28) | 32.1% (9/28) | 17.9% (5/28) | 21.4% (6/28) | |
| (1) Oxacillin | 10.7% (3) | 25.0% (2) | 0.0% (0) | 0.0% (0) | 16.7% (1) | p = N/A |
| (2) Ampicillin | 39.3% (11) | 50.0% (4) | 22.2% (2) | 40.0% (2) | 50.0% (3) | p = 0.80 (Cgf) |
| (3) Vancomycin | 28.6% (8) | 25.0% (2) | 33.3% (3) | 40.0% (2) | 16.7% (1) | p = N/A |
| (4) Gentamicin | 32.1% (9) | 37.5% (3) | 22.2% (2) | 20.0% (1) | 50.0% (3) | p = N/A |
| Analysis in the season p-value (test) | p = 0.19 (Q) | N/A | N/A | N/A | N/A | |
| Candida spp. (19) | n = 19 | 21.1% (4/19) | 10.5% (2/19) | 15.8% (3/19) | 2.6% (10/19) | |
| (1) Fluconazole | 10.5% (2) | 50.0% (2) | 0.0% (0) | 0.0% (0) | 0.0% (0) | p = N/A |
| (2) Echinocandins | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | p = N/A |
| Analysis in the season p-value (test) | N/A | N/A | N/A | N/A | N/A |
| Isolates Bacteria | TOTAL Resistance | Autumn (Au) % (n) | Summer (Su) % (n) | Winter (Wi) % (n) | Spring (Sp) % (n) | Among Seasons p-Value (Test) |
|---|---|---|---|---|---|---|
| E. coli (n = 21) | n = 6 | n = 6 | n = 5 | n = 4 | ||
| (1) 3GC | 90.5% (19) | 100% (6) | 100% (6) | 80.0% (4) | 75.0% (3) | p = 0.70 (Cgf) |
| (2) Cefepime | 81.0% (17) | 100% (6) | 100% (6) | 60.0% (3) | 50.0% (2) | p = 0.39 (Cgf) |
| (3) Fluoroquinolones | 85.7% (18) | 100% (6) | 100% (6) | 80.0% (4) | 50.0% (2) | p = 0.49 (Cgf) |
| (4) Aminoglycosides | 61.9% (13) | 83.3% (5) | 50.0% (3) | 60.0% (3) | 50.0% (2) | p = 0.69 (Cgf) |
| (5) Fosfomycin | 57.1% (12) | 83.3% (5) | 50.0% (3) | 40.0% (2) | 50.0% (2) | p = 0.57 (Cgf) |
| (6) Piperacillin-tazobactam | 81.0% (17) | 100% (6) | 100% (6) | 60.0% (3) | 50.0% (2) | p = 0.39 (Cgf) |
| (7) Carbapenem | 19.0% (4) | 33.3% (2) | 16.7% (1) | 0.0% (0) | 25.0% (1) | p = N/A |
| (8) Colistin | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | p = N/A |
| Analysis in the season p-value (test) | p < 0.001 * (Q) (1), (2), (3), (6) > (7), (8), p < 0.05 * (B) (4), (5) > (8), p < 0.05 * (B) | p < 0.001 * (Q) (1), (2), (3), (4), (5), (6) > (8), p < 0.05 * (B) | p < 0.001 * (Q) (1), (2), (3), (6) > (8), p < 0.05 * (B) | N/E | N/E | |
| Klebsiella spp. (n = 12) | n = 2 | n = 6 | n = 3 | n = 1 | ||
| (1) 3GC | 91.7% (11) | 100% (2) | 83.3% (5) | 100% (3) | 100% (1) | p = 0.36 (Cgf) |
| (2) Cefepime | 83.3% (10) | 100% (2) | 83.3% (5) | 66.7% (2) | 100% (1) | p = 0.31 (Cgf) |
| (3) Fluoroquinolones | 91.7% (11) | 100% (2) | 83.3% (5) | 100% (3) | 100% (1) | p = 0.36 (Cgf) |
| (4) Aminoglycosides | 66.7% (8) | 50.0% (1) | 66.7% (4) | 66.7% (2) | 100% (1) | p = N/A |
| (5) Fosfomycin | 66.7% (8) | 50.0% (1) | 66.7% (4) | 66.7% (2) | 100% (1) | p = N/A |
| (6) Piperacillin-tazobactam | 75.0% (9) | 50.0% (1) | 83.3% (5) | 66.7% (2) | 100% (1) | p = N/A |
| (7) Carbapenem | 58.3% (7) | 50.0% (1) | 50.0% (3) | 66.7% (2) | 100% (1) | p = N/A |
| (8) Colistin | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | p = 1.0 |
| Analysis in the season p-value (test) | p < 0.001 * (Q) (1), (2), (3), (4), 5), (6), (7) > (8), p < 0.05 * (B) | p = 0.18 (Q) | p < 0.001 * (Q) (1), (2), (3), (6) > (8), p < 0.05 * (B) | p = 0.051 (Q) | N/E | |
| Pseudomonas spp. (n = 27) | n = 9 | n = 8 | n = 7 | n = 3 | ||
| (1) 3GC | 96.4% (26) | 100% (9) | 100% (8) | 85.7% (6) | 100% (3) | p = 0.36 (Cgf) |
| (2) Cefepime | 85.2% (23) | 100% (9) | 100% (8) | 71.4% (5) | 33.3% (1) | p = 0.081 (Cgf) |
| (3) Fluoroquinolones | 85.2% (23) | 100% (9) | 100% (8) | 71.4% (5) | 33.3% (1) | p = 0.081 (Cgf) |
| (4) Aminoglycosides | 81.5% (22) | 88.9% (8) | 100% (8) | 71.4% (5) | 33.3% (1) | p = 0.11 (Cgf) |
| (5) Fosfomycin | 81.5% (22) | 88.9% (8) | 100% (8) | 71.4% (5) | 33.3% (1) | p = 0.11 (Cgf) |
| (6) Piperacillin-tazobactam | 77.8% (21) | 77.8% (7) | 100% (8) | 71.4% (5) | 33.3% (1) | p = 0.14 (Cgf) |
| (7) Carbapenem | 33.3% (9) | 22.2% (2) | 50.0% (4) | 28.6% (2) | 33.3% (1) | p = N/A |
| (8) Colistin | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | p = N/A |
| Analysis in the season p-value (test) | p < 0.001 * (Q) (1), (2), (3), (4), (5), (6) > (7), (8), p < 0.05 * (B) (7) > (8), p < 0.05 * (B) | p < 0.001 * (Q) (1), (2), (3), (4), (6) > (7), (8), p < 0.05 * (B) (5) > (8), p < 0.05 * (B) | p < 0.001 * (Q) (1), (2), (3), (4), (5), (6) > (8), p < 0.05 * (B) | p = 0.002 * (Q) (1), (2), (3), (4), (5), (6) > (8), p < 0.05 * (B) | p = 0.072 (Q) | |
| S. maltophilia (n = 12) | n = 2 | n = 3 | n = 3 | n = 4 | ||
| (1) 3GC | 100% (12) | 100% (2) | 100% (3) | 100% (3) | 100% (4) | p = 0.88 (Cgf) |
| (2) Cefepime | 100% (12) | 100% (2) | 100% (3) | 100% (3) | 100% (4) | p = 0.88 (Cgf) |
| (3) Fluoroquinolones | 100% (12) | 100% (2) | 100% (3) | 100% (3) | 100% (4) | p = 0.88 (Cgf) |
| (4) Aminoglycosides | 100% (12) | 100% (2) | 100% (3) | 100% (3) | 100% (4) | p = 0.88 (Cgf) |
| (5) Fosfomycin | 100% (12) | 100% (2) | 100% (3) | 100% (3) | 100% (4) | p = 0.88 (Cgf) |
| (6) Piperacil-lin-tazobactam | 100% (12) | 100% (2) | 100% (3) | 100% (3) | 100% (4) | p = 0.88 (Cgf) |
| (7) Carbapenem | 75.0% (9) | 50.0% (1) | 100% (3) | 66.7% (2) | 75.0% (3) | p = N/A |
| (8) Colistin | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | 0.0% (0) | p = N/A |
| Analysis in the season p-value (test) | p < 0.001 * (Q) (1), (2), (3), (4), (5), (6), (7) > (8), p < 0.05 * (B) | p = 0.12 (Q) | p = 0.004 * (Q) (1), (2), (3), (4), (5), (6), (7) > (8), p < 0.05 * (B) | p = 0.016 * (Q) (1), (2), (3), (4), (5), (6), (7) > (8), p < 0.05 * (B) | p = 0.016 * (Q) (1), (2), (3), (4), (5), (6), (7) > (8), p < 0.05 * (B) | |
| Enterococcus spp. (n = 24) | n = 7 | n = 7 | n = 4 | n = 6 | ||
| (1) Oxacillin | 8.3% (2) | 14.3% (1) | 0.0% (0) | 0.0% (0) | 16.7% (1) | p = N/A |
| (2) Ampicillin | 45.8% (11) | 57.1% (4) | 28.6% (2) | 50.0% (2) | 50.0% (3) | p = 0.80 |
| (3) Vancomycin | 33.3% (8) | 28.6% (2) | 42.9% (3) | 50.0% (2) | 16.7% (1) | p = N/A |
| (4) Gentamicin | 37.5% (9) | 42.9% (3) | 28.6% (2) | 25.0% (1) | 50.0% (3) | p = N/A |
| Analysis in the season p-value (test) | p < 0.001 * (Q) (2) > (1), p < 0.05 * (B) | p = 0.34 (Q) | p = 0.10 (Q) | p = 0.19 (Q) | p = 0.45 (Q) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Carlo, P.; Serra, N.; Sergi, C.M.; Toia, F.; Battaglia, E.; Fasciana, T.M.A.; Rodolico, V.; Giammanco, A.; Salamone, G.; Cordova, A.; et al. Seasonal Change in Microbial Diversity: Bile Microbiota and Antibiotics Resistance in Patients with Bilio-Pancreatic Tumors: A Retrospective Monocentric Study (2010–2020). Antibiotics 2025, 14, 283. https://doi.org/10.3390/antibiotics14030283
Di Carlo P, Serra N, Sergi CM, Toia F, Battaglia E, Fasciana TMA, Rodolico V, Giammanco A, Salamone G, Cordova A, et al. Seasonal Change in Microbial Diversity: Bile Microbiota and Antibiotics Resistance in Patients with Bilio-Pancreatic Tumors: A Retrospective Monocentric Study (2010–2020). Antibiotics. 2025; 14(3):283. https://doi.org/10.3390/antibiotics14030283
Chicago/Turabian StyleDi Carlo, Paola, Nicola Serra, Consolato Maria Sergi, Francesca Toia, Emanuele Battaglia, Teresa Maria Assunta Fasciana, Vito Rodolico, Anna Giammanco, Giuseppe Salamone, Adriana Cordova, and et al. 2025. "Seasonal Change in Microbial Diversity: Bile Microbiota and Antibiotics Resistance in Patients with Bilio-Pancreatic Tumors: A Retrospective Monocentric Study (2010–2020)" Antibiotics 14, no. 3: 283. https://doi.org/10.3390/antibiotics14030283
APA StyleDi Carlo, P., Serra, N., Sergi, C. M., Toia, F., Battaglia, E., Fasciana, T. M. A., Rodolico, V., Giammanco, A., Salamone, G., Cordova, A., Capuano, A., Spatola, G. F., Malta, G., & Cascio, A. (2025). Seasonal Change in Microbial Diversity: Bile Microbiota and Antibiotics Resistance in Patients with Bilio-Pancreatic Tumors: A Retrospective Monocentric Study (2010–2020). Antibiotics, 14(3), 283. https://doi.org/10.3390/antibiotics14030283











