Virucidal Activity of Lemon Juice Against Feline Calicivirus, Surrogate of Norovirus
Abstract
1. Introduction
2. Results
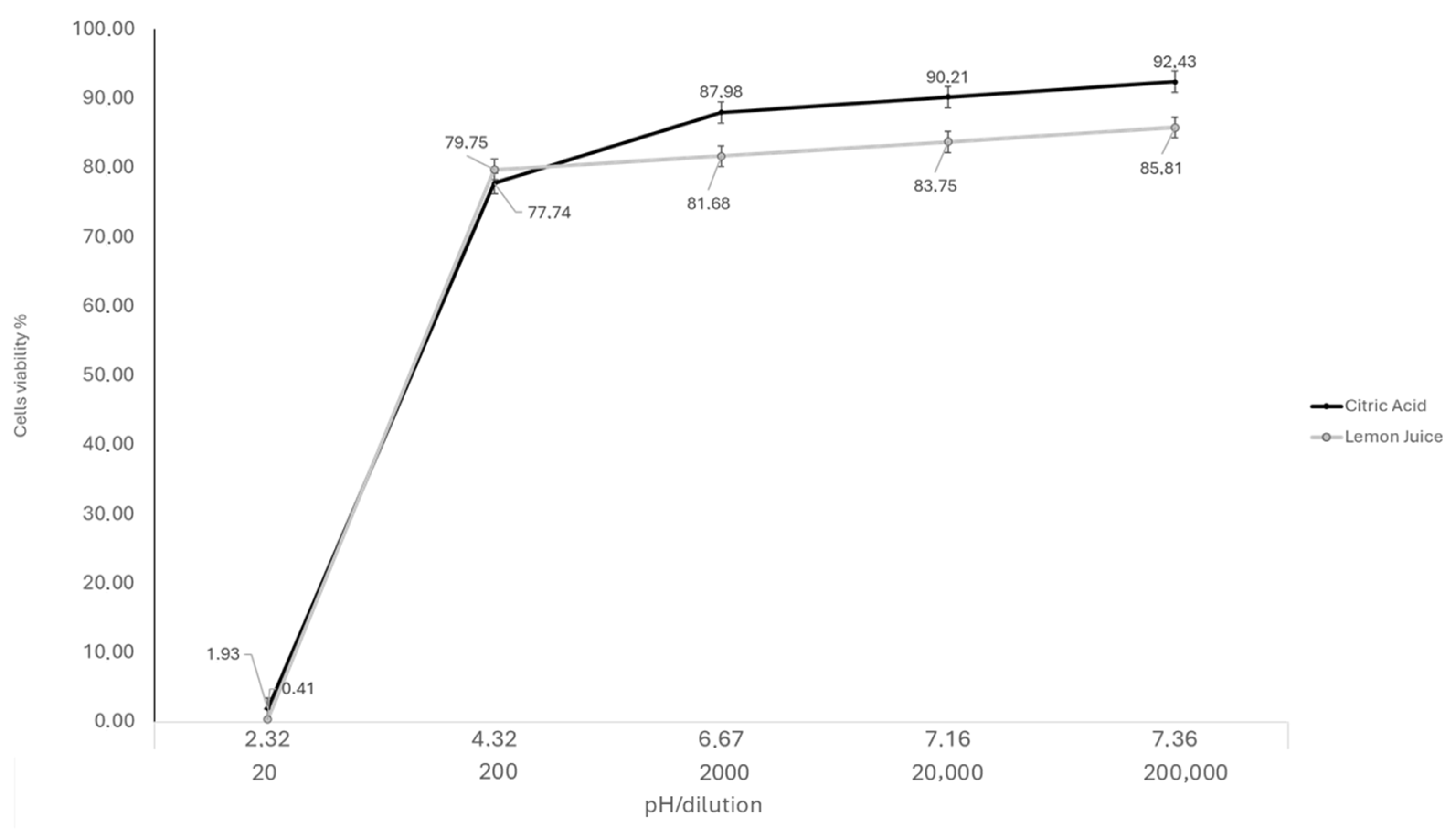
2.1. Cytotoxicity
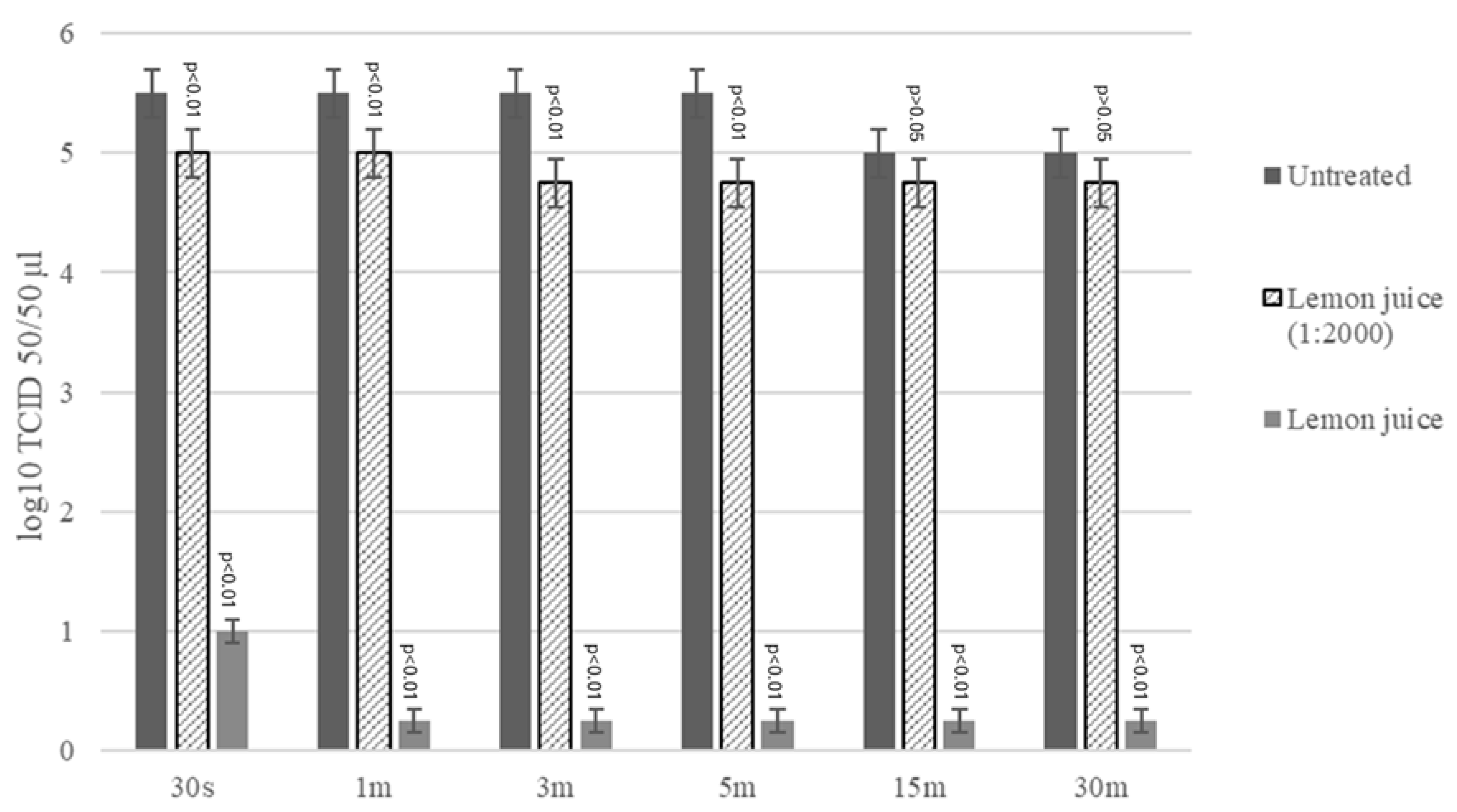
2.2. Virucidal Activity Assay LJ
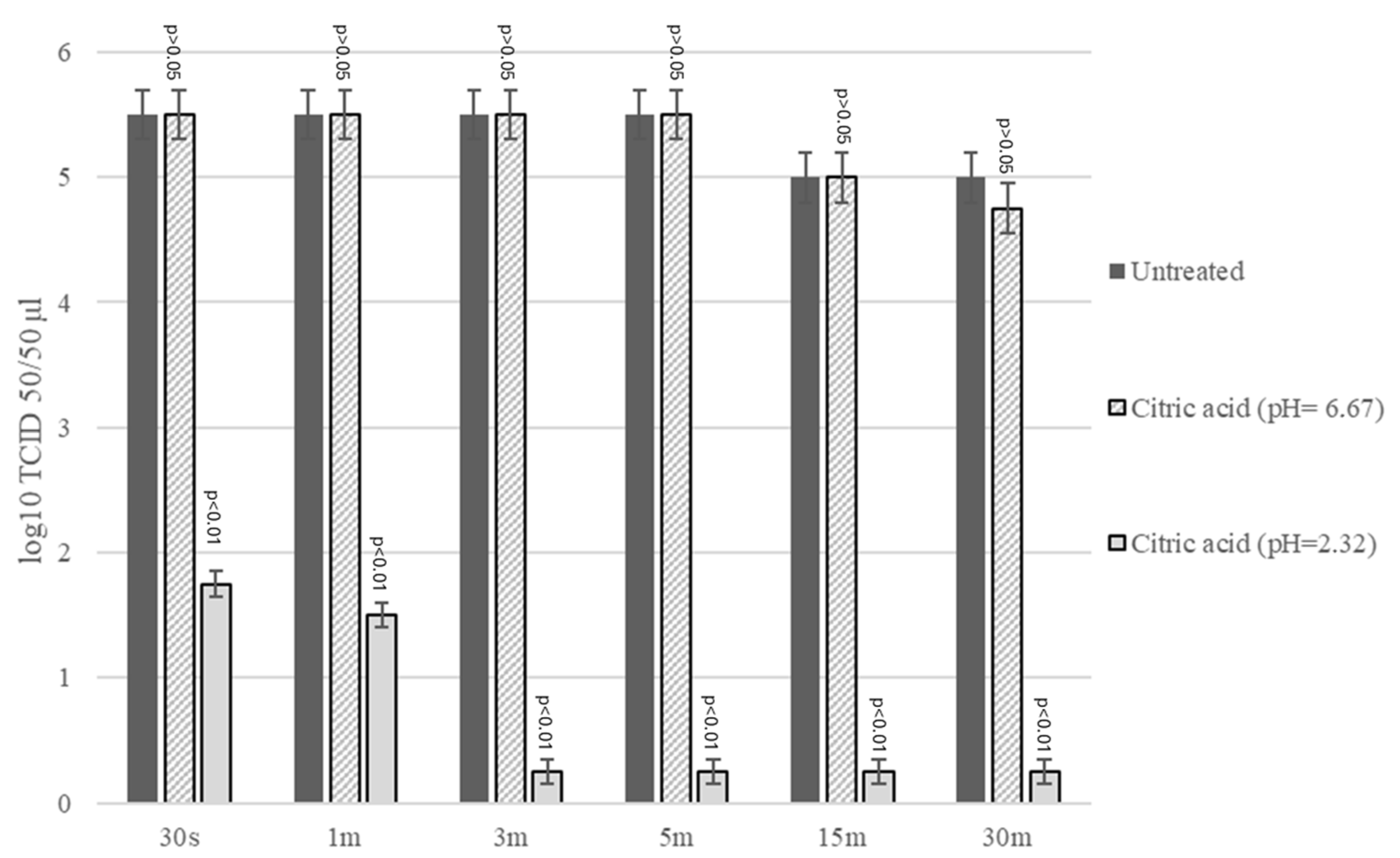
2.3. Virucidal Activity Assay CA
3. Discussion
4. Materials and Methods
4.1. Lemon Juice (LJ)
4.2. Citric Acid (CA)
4.3. Cells and Viruses
4.4. Cytotoxicity Assay
4.5. Virucidal Activity Assay LJ
4.6. Virucidal Activity Assay CA
4.7. Viral Titration
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Noroviruses—The State of the Art, Nearly Fifty Years after Their Initial Discovery. Viruses 2021, 13, 1541. [Google Scholar] [CrossRef] [PubMed]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Kniel, K.E. Comparing Human Norovirus Surrogates: Murine Norovirus and Tulane Virus. J. Food Prot. 2013, 76, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated Classification of Norovirus Genogroups and Genotypes. J. General. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef]
- Rachmadi, A.T.; Kitajima, M.; Watanabe, K.; Okabe, S.; Sano, D. Disinfection as a Selection Pressure on RNA Virus Evolution. Environ. Sci. Technol. 2018, 52, 2434–2435. [Google Scholar] [CrossRef]
- Ward, D. Handwashing Facilities in the Clinical Area: A Literature Review. Br. J. Nurs. 2000, 9, 82–86. [Google Scholar] [CrossRef]
- Winder, N.; Gohar, S.; Muthana, M. Norovirus: An Overview of Virology and Preventative Measures. Viruses 2022, 14, 2811. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.E.; Streby, A.; Moe, C.L. Vomiting as a Symptom and Transmission Risk in Norovirus Illness: Evidence from Human Challenge Studies. PLoS ONE 2016, 11, e0143759. [Google Scholar] [CrossRef]
- Chen, Y.; Lopman, B.A.; Hall, A.J.; Kambhampati, A.K.; Roberts, L.; Mason, J.; Vilen, K.; Salehi, E.; Fraser, A.; Adams, C. Factors Driving Norovirus Transmission in Long-Term Care Facilities: A Case-Level Analysis of 107 Outbreaks. Epidemics 2023, 42, 100671. [Google Scholar] [CrossRef]
- Kirby, A.E.; Shi, J.; Montes, J.; Lichtenstein, M.; Moe, C.L. Disease Course and Viral Shedding in Experimental Norwalk Virus and Snow Mountain Virus Infection. J. Med. Virol. 2014, 86, 2055–2064. [Google Scholar] [CrossRef]
- Cheng, H.-Y.; Hung, M.-N.; Chen, W.-C.; Lo, Y.-C.; Su, Y.-S.; Wei, H.-Y.; Chen, M.-Y.; Tuan, Y.-C.; Lin, H.-C.; Lin, H.-Y.; et al. Ice-Associated Norovirus Outbreak Predominantly Caused by GII.17 in Taiwan, 2015. BMC Public Health 2017, 17, 870. [Google Scholar] [CrossRef]
- Neetoo, H.; Juggoo, K.; Johaheer, H.; Ranghoo-Sanmukhiya, M.; Manoga, Z.; Gurib, N. A Study on the Occurrence of Human Enteric Viruses in Salad Vegetables and Seafood and Associated Health Risks for Consumers in Mauritius. Ital. J. Food Saf. 2023, 12, 11447. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, E.; Bertasi, B.; Galuppini, E.; Mangeri, L.; Meletti, F.; Tilola, M.; Carta, V.; Todeschi, S.; Losio, M.-N. Detection of Hepatitis A Virus and Norovirus in Different Food Categories: A 6-Year Survey in Italy. Food Environ. Virol. 2022, 14, 69–76. [Google Scholar] [CrossRef]
- Razafimahefa, R.M.; Ludwig-Begall, L.F.; Thiry, E. Cockles and Mussels, Alive, Alive, Oh—The Role of Bivalve Molluscs as Transmission Vehicles for Human Norovirus Infections. Transbound. Emerg. Dis. 2020, 67, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Lees, D. Viruses and Bivalve Shellfish. Int. J. Food Microbiol. 2000, 59, 81–116. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Engelbrektson, A.L.; Jiang, X.; Zhong, W.; Mandrell, R.E. Norovirus Recognizes Histo-Blood Group Antigens on Gastrointestinal Cells of Clams, Mussels, and Oysters: A Possible Mechanism of Bioaccumulation. J. Food Prot. 2007, 70, 2140–2147. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, M.; Zhao, F.; Su, L. Research Progress on Biological Accumulation, Detection and Inactivation Technologies of Norovirus in Oysters. Foods 2023, 12, 3891. [Google Scholar] [CrossRef]
- Serag, M.S.; Elfayoumy, R.A.; Mohesien, M.T. Essential Oils as Antimicrobial and Food Preservatives; IntechOpen: London, UK, 2022. [Google Scholar]
- Bailey, E.S.; Curcic, M.; Biros, J.; Erdogmuş, H.; Bac, N.; Sacco, A. Essential Oil Disinfectant Efficacy Against SARS-CoV-2 Microbial Surrogates. Front. Public. Health 2021, 9, 783832. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus Limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef]
- AL-ataby, I.A.; Talib, W.H. Daily Consumption of Lemon and Ginger Herbal Infusion Caused Tumor Regression and Activation of the Immune System in a Mouse Model of Breast Cancer. Front. Nutr. 2022, 9, 829101. [Google Scholar] [CrossRef]
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds of Citrus Limon for Food and Health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Rexrode, K.M.; Hu, F.; Albert, C.M.; Chae, C.U.; Rimm, E.B.; Stampfer, M.J.; Manson, J.E. Dietary Intakes of Flavonols and Flavones and Coronary Heart Disease in US Women. Am. J. Epidemiol. 2007, 165, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J. Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-Inflammatory Activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Suzuki, E.; Ohya, S.; Fukumoto, S.; Hiramitsu, M.; Sakaida, K.; Osawa, T.; Furuichi, Y. Lipid-Lowering Effect of Eriocitrin, the Main Flavonoid in Lemon Fruit, in Rats on a High-Fat and High-Cholesterol Diet. J. Food Sci. 2006, 71, S633–S637. [Google Scholar] [CrossRef]
- Del Río, J.A.; Fuster, M.D.; Gómez, P.; Porras, I.; García-Lidón, A.; Ortuño, A. Citrus Limon: A Source of Flavonoids of Pharmaceutical Interest. Food Chem. 2004, 84, 457–461. [Google Scholar] [CrossRef]
- Reddy, L.; Odhav, B.; Bhoola, K.D. Natural Products for Cancer Prevention: A Global Perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef]
- Vanamala, J.; Reddivari, L.; Yoo, K.S.; Pike, L.M.; Patil, B.S. Variation in the Content of Bioactive Flavonoids in Different Brands of Orange and Grapefruit Juices. J. Food Compos. Anal. 2006, 19, 157–166. [Google Scholar] [CrossRef]
- Galgano, M.; Capozza, P.; Pellegrini, F.; Cordisco, M.; Sposato, A.; Sblano, S.; Camero, M.; Lanave, G.; Fracchiolla, G.; Corrente, M.; et al. Antimicrobial Activity of Essential Oils Evaluated In Vitro against Escherichia coli and Staphylococcus aureus. Antibiotics 2022, 11, 979. [Google Scholar] [CrossRef]
- Galgano, M.; Mrenoshki, D.; Pellegrini, F.; Capozzi, L.; Cordisco, M.; Del Sambro, L.; Trotta, A.; Camero, M.; Tempesta, M.; Buonavoglia, D.; et al. Antibacterial and Biofilm Production Inhibition Activity of Thymus vulgaris L. Essential Oil against Salmonella spp. Isolates from Reptiles. Pathogens 2023, 12, 804. [Google Scholar] [CrossRef]
- Moura, V.S.; Olandin, L.D.; Mariano, B.S.; Rodrigues, J.; Devite, F.T.; Arantes, A.C.C.; Queiroga, C.L.; Sartoratto, A.; de Azevedo, F.A.; Bastianel, M. Antifungal Activity of Citrus Essential Oil in Controlling Sour Rot in Tahiti Acid Lime Fruits. Plants 2024, 13, 3075. [Google Scholar] [CrossRef]
- Allizond, V.; Cavallo, L.; Roana, J.; Mandras, N.; Cuffini, A.M.; Tullio, V.; Banche, G. In Vitro Antifungal Activity of Selected Essential Oils against Drug-Resistant Clinical Aspergillus spp. Strains. Molecules 2023, 28, 7259. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, F.; Camero, M.; Catella, C.; Fracchiolla, G.; Sblano, S.; Patruno, G.; Trombetta, C.M.; Galgano, M.; Pratelli, A.; Tempesta, M.; et al. Virucidal Activity of Lemon Essential Oil against Feline Calicivirus Used as Surrogate for Norovirus. Antibiotics 2023, 12, 322. [Google Scholar] [CrossRef]
- Khan, M.; Rauf, W.; Habib, F.-; Rahman, M.; Iqbal, S.; Shehzad, A.; Iqbal, M. Hesperidin Identified from Citrus Extracts Potently Inhibits HCV Genotype 3a NS3 Protease. BMC Complement. Med. Ther. 2022, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- Ewansiha, J.U. Evaluation of Antibacterial Potency of Citrus Limon (Lemon) Juice Against Some Pathogenic Organisms as Alternative Source of Chemotherapy. Eur. J. Biol. Biotechnol. 2020, 1, 1–8. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Song, J.; Liu, A.; Xiao, B.; Li, S.; Wen, Z.; Lu, Y.; Du, G. Research Progress of the Antiviral Bioactivities of Natural Flavonoids. Nat. Prod. Bioprospect. 2020, 10, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.; Isegawa, Y. Anti-Influenza Virus Activity of Citrullus Lanatus Var. Citroides as a Functional Food: A Review. Foods 2023, 12, 3866. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, Z.; Zhong, W.; Fu, F.; Li, G.; Guo, J.; Shan, Y. Preparation, Characterization, and Antioxidant Activity of Nanoemulsions Incorporating Lemon Essential Oil. Antioxidants 2022, 11, 650. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Thrupp, L.; Owens, J.; Cesario, T.; Shanbrom, E. Bactericidal Activity of Citrate against Gram-Positive Cocci. Lett. Appl. Microbiol. 2001, 33, 349–351. [Google Scholar] [CrossRef]
- Nagaoka, S.; Murata, S.; Kimura, K.; Mori, T.; Hojo, K. Antimicrobial Activity of Sodium Citrate against Streptococcus pneumoniae and Several Oral Bacteria. Lett. Appl. Microbiol. 2010, 51, 546–551. [Google Scholar] [CrossRef]
- Koromyslova, A.D.; White, P.A.; Hansman, G.S. Treatment of Norovirus Particles with Citrate. Virology 2015, 485, 199–204. [Google Scholar] [CrossRef]
- Cannon, J.L.; Papafragkou, E.; Park, G.W.; Osborne, J.; Jaykus, L.-A.; Vinjé, J. Surrogates for the Study of Norovirus Stability and Inactivation in the Environment: A Comparison of Murine Norovirus and Feline Calicivirus. J. Food Prot. 2006, 69, 2761–2765. [Google Scholar] [CrossRef]
- Boone, S.A.; Ijaz, M.K.; Bright, K.R.; Silva-Beltran, N.P.; Nims, R.W.; McKinney, J.; Gerba, C.P. Antiviral Natural Products, Their Mechanisms of Action and Potential Applications as Sanitizers and Disinfectants. Food Environ. Virol. 2023, 15, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Hall, A.J. Norovirus; Melhem, N.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-27208-1. [Google Scholar]
- Zhang, X.; Chen, C.; Du, Y.; Yan, D.; Jiang, D.; Liu, X.; Yang, M.; Ding, C.; Lan, L.; Hecht, R.; et al. Global Burden and Trends of Norovirus-Associated Diseases from 1990 to 2019: An Observational Trend Study. Front. Public Health 2022, 10, 905172. [Google Scholar] [CrossRef]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Walton, B. Sinclair The Organic Acids of Lemon Fruits. Botanical Gazette 1945, 107, 231–242. [Google Scholar]
- McLeod, M.; Belford, G.; Harlow, J.; Nasheri, N. Examining the Effect of Organic Acids on Inactivation of Hepatitis E Virus. J. Food Prot. 2022, 85, 1690–1695. [Google Scholar] [CrossRef]
- Hansman, G.S.; Shahzad-ul-Hussan, S.; McLellan, J.S.; Chuang, G.-Y.; Georgiev, I.; Shimoike, T.; Katayama, K.; Bewley, C.A.; Kwong, P.D. Structural Basis for Norovirus Inhibition and Fucose Mimicry by Citrate. J. Virol. 2012, 86, 284–292. [Google Scholar] [CrossRef]
- García, C.C.; Talarico, L.; Almeida, N.; Colombres, S.; Duschatzky, C.; Damonte, E.B. Virucidal Activity of Essential Oils from Aromatic Plants of San Luis, Argentina. Phytother. Res. 2003, 17, 1073–1075. [Google Scholar] [CrossRef]
- Li, Y.; Xue, L.; Gao, J.; Cai, W.; Zhang, Z.; Meng, L.; Miao, S.; Hong, X.; Xu, M.; Wu, Q.; et al. A Systematic Review and Meta-Analysis Indicates a Substantial Burden of Human Noroviruses in Shellfish Worldwide, with GII.4 and GII.2 Being the Predominant Genotypes. Food Microbiol. 2023, 109, 104140. [Google Scholar] [CrossRef]
- Chan, M.C.-W.; Cheung, S.K.C.; Mohammad, K.N.; Chan, J.C.M.; Estes, M.K.; Chan, P.K.S. Use of Human Intestinal Enteroids to Detect Human Norovirus Infectivity. Emerg. Infect. Dis. 2019, 25, 1730–1735. [Google Scholar] [CrossRef]
- Richards, G.P. Critical Review of Norovirus Surrogates in Food Safety Research: Rationale for Considering Volunteer Studies. Food Environ. Virol. 2012, 4, 6–13. [Google Scholar] [CrossRef]
- Di Martino, B.; Lanave, G.; Di Profio, F.; Melegari, I.; Marsilio, F.; Camero, M.; Catella, C.; Capozza, P.; Bányai, K.; Barrs, V.R.; et al. Identification of Feline Calicivirus in Cats with Enteritis. Transbound. Emerg. Dis. 2020, 67, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Ettayebi, K.; Salmen, W.; Imai, K.; Hagi, A.; Neill, F.H.; Atmar, R.L.; Prasad, B.V.V.; Estes, M.K. Antiviral Activity of Olanexidine-Containing Hand Rub against Human Noroviruses. mBio 2022, 13, e02848-21. [Google Scholar] [CrossRef]
- Terio, V.; Bottaro, M.; Pavoni, E.; Losio, M.N.; Serraino, A.; Giacometti, F.; Martella, V.; Mottola, A.; Di Pinto, A.; Tantillo, G. Occurrence of Hepatitis A and E and Norovirus GI and GII in Ready-to-Eat Vegetables in Italy. Int. J. Food Microbiol. 2017, 249, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Bostan, K.; Altan, E.; Muratoglu, K.; Turan, N.; Tan, D.; Helps, C.; Yilmaz, H. Investigations on the Frequency of Norovirus Contamination of Ready-to-Eat Food Items in Istanbul, Turkey, by Using Real-Time Reverse Transcription PCR. J. Food Prot. 2011, 74, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhao, F.; Tong, L.; Wang, S.; Zhou, D. Contamination, Bioaccumulation Mechanism, Detection, and Control of Human Norovirus in Bivalve Shellfish: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 8972–8985. [Google Scholar] [CrossRef]
- Menconi, V.; Lazzaro, E.; Bertola, M.; Guardone, L.; Mazzucato, M.; Prearo, M.; Bilska-Zajac, E.; Cortinovis, L.; Manfrin, A.; Arcangeli, G.; et al. The Occurrence of Freshwater Fish-Borne Zoonotic Helminths in Italy and Neighbouring Countries: A Systematic Review. Animals 2023, 13, 3793. [Google Scholar] [CrossRef]
- Merks, H.; Boone, R.; Janecko, N.; Viswanathan, M.; Dixon, B.R. Foodborne Protozoan Parasites in Fresh Mussels and Oysters Purchased at Retail in Canada. Int. J. Food Microbiol. 2023, 399, 110248. [Google Scholar] [CrossRef]
- Shamsi, S.; Barton, D.P. A Critical Review of Anisakidosis Cases Occurring Globally. Parasitol. Res. 2023, 122, 1733–1745. [Google Scholar] [CrossRef]
- Santos-Ferreira, N.; Mesquita, J.R.; Rivadulla, E.; Inácio, Â.S.; Martins da Costa, P.; Romalde, J.L.; Nascimento, M.S.J. Hepatitis E Virus Genotype 3 in Echinoderms: First Report of Sea Urchin (Paracentrotus lividus) Contamination. Food Microbiol. 2020, 89, 103415. [Google Scholar] [CrossRef]
- Onosi, O.; Upfold, N.S.; Jukes, M.D.; Luke, G.A.; Knox, C. The First Detection of Human Bocavirus Species 2 and 3 in Raw Sewage and Mussels in South Africa. Food Environ. Virol. 2020, 12, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Parisi, A.; Conversano, M.C.; Iannacci, A.; D’Emilio, F.; Mercurio, V.; Normanno, G. Food-Borne Bacteria Associated with Seafoods: A Brief Review. J. Food Qual. Hazards Control 2020, 7, 4–10. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Control of Hospital Waste. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3294–3309.e4. [Google Scholar]
- Chen, H.; Davidson, P.M.; Zhong, Q. Impacts of Sample Preparation Methods on Solubility and Antilisterial Characteristics of Essential Oil Components in Milk. Appl. Environ. Microbiol. 2014, 80, 907–916. [Google Scholar] [CrossRef]
- Nadi, A.; Shiravi, A.A.; Mohammadi, Z.; Aslani, A.; Zeinalian, M. Thymus Vulgaris, a Natural Pharmacy against COVID-19: A Molecular Review. J. Herb. Med. 2023, 38, 100635. [Google Scholar] [CrossRef] [PubMed]
- Camero, M.; Lanave, G.; Catella, C.; Capozza, P.; Gentile, A.; Fracchiolla, G.; Britti, D.; Martella, V.; Buonavoglia, C.; Tempesta, M. Virucidal Activity of Ginger Essential Oil against Caprine Alphaherpesvirus-1. Vet. Microbiol. 2019, 230, 150–155. [Google Scholar] [CrossRef]
- Lanave, G.; Pellegrini, F.; Triggiano, F.; De Giglio, O.; Lucente, M.S.; Diakoudi, G.; Catella, C.; Gentile, A.; Tardugno, R.; Fracchiolla, G.; et al. In Vitro Virucidal Activity of Different Essential Oils against Bovine Viral Diarrhea Virus Used as Surrogate of Human Hepatitis C Virus. Antibiotics 2024, 13, 514. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent ENDPOINTS12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
| Group of Compound | Metabolites |
|---|---|
| Flavonoids | flavonones: hesperidin, naringin flavones: apigenin, chrysoeriol, diosmetin, luteolin flavonols: isoramnethin, quercetin, rutoside dihydroxyflavonols: dihydroxyisoramnethin-7-O-rutinoside |
| Phenolic acids | Ferulic acid, synapic acid |
| Vitamins | vitamins: C (53 mg/L), A, B1, B2, B3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanave, G.; Pellegrini, F.; Catella, C.; Mateos, H.; Palazzo, G.; Gentile, A.; Diakoudi, G.; Burgio, M.; Tempesta, M.; Martella, V.; et al. Virucidal Activity of Lemon Juice Against Feline Calicivirus, Surrogate of Norovirus. Antibiotics 2025, 14, 273. https://doi.org/10.3390/antibiotics14030273
Lanave G, Pellegrini F, Catella C, Mateos H, Palazzo G, Gentile A, Diakoudi G, Burgio M, Tempesta M, Martella V, et al. Virucidal Activity of Lemon Juice Against Feline Calicivirus, Surrogate of Norovirus. Antibiotics. 2025; 14(3):273. https://doi.org/10.3390/antibiotics14030273
Chicago/Turabian StyleLanave, Gianvito, Francesco Pellegrini, Cristiana Catella, Helena Mateos, Gerardo Palazzo, Arturo Gentile, Georgia Diakoudi, Matteo Burgio, Maria Tempesta, Vito Martella, and et al. 2025. "Virucidal Activity of Lemon Juice Against Feline Calicivirus, Surrogate of Norovirus" Antibiotics 14, no. 3: 273. https://doi.org/10.3390/antibiotics14030273
APA StyleLanave, G., Pellegrini, F., Catella, C., Mateos, H., Palazzo, G., Gentile, A., Diakoudi, G., Burgio, M., Tempesta, M., Martella, V., & Camero, M. (2025). Virucidal Activity of Lemon Juice Against Feline Calicivirus, Surrogate of Norovirus. Antibiotics, 14(3), 273. https://doi.org/10.3390/antibiotics14030273











