A Regional Approach to Strengthening the Implementation of Sustainable Antimicrobial Stewardship Programs in Five Countries in East, Central, and Southern Africa
Abstract
1. Introduction
2. Results
2.1. Functionality of Drugs and Therapeutics Committees in Surveyed Hospitals
2.2. Leadership Commitment to Supporting AMS Programs in Surveyed Hospitals
2.3. Accountability and Responsibility in Surveyed Hospitals
2.4. AMS Actions in Surveyed Hospitals
2.5. Education and Training of Healthcare Workers in Surveyed Hospitals
2.6. Monitoring and Surveillance of Antimicrobial Use and AMR in Surveyed Hospitals
2.7. Reporting Feedback Within the Healthcare Facilities
3. Discussion
Study Limitations
4. Materials and Methods
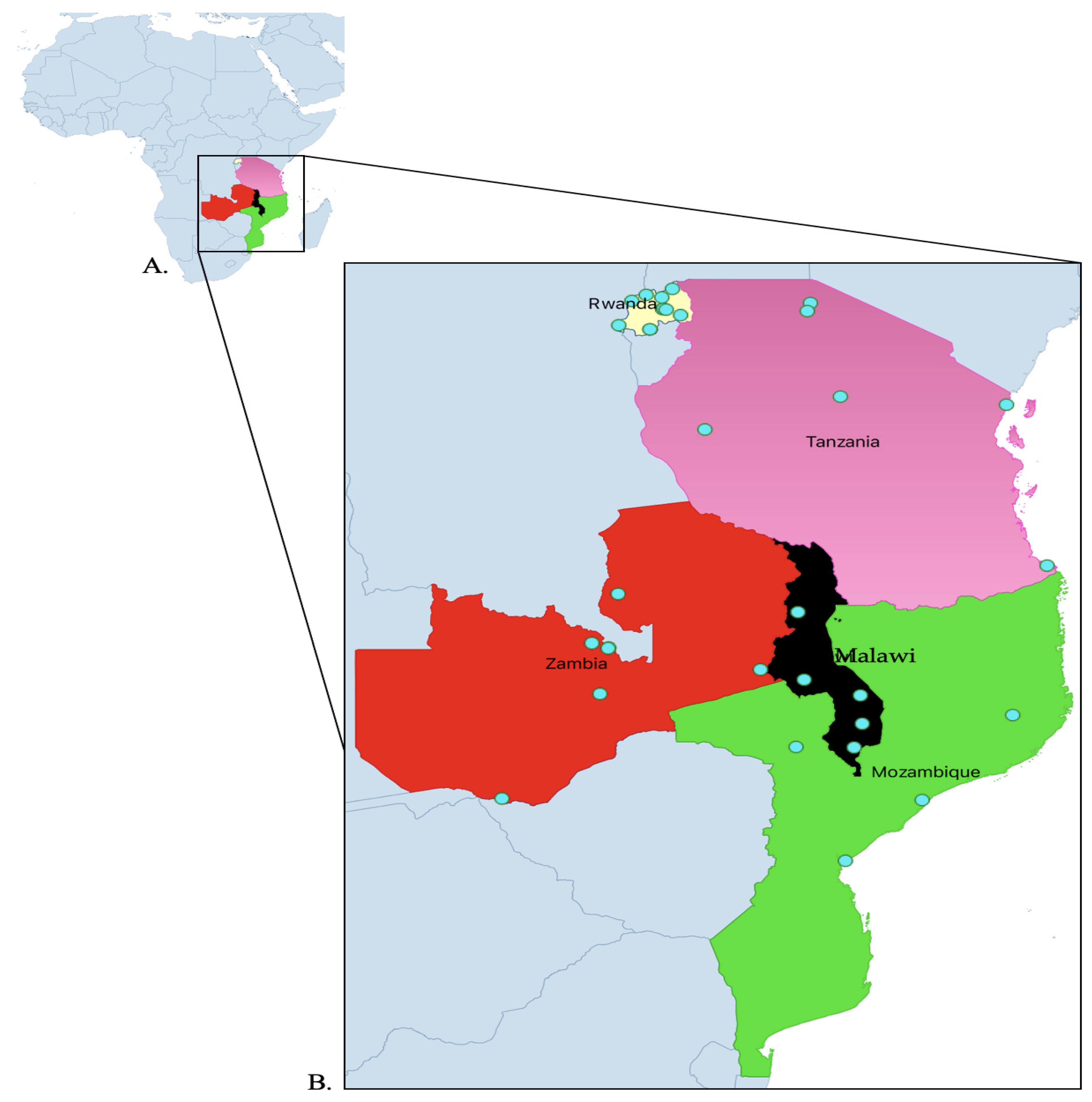
4.1. Study Design and Site Selection
4.2. The Approach
4.3. Data Collection Tool
4.4. Baseline Assessment
4.5. Implementation of the Project
4.6. Post-Intervention Data Collection (Endline Assessment)
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- E Clinical Medicine. Antimicrobial Resistance: A Top Ten Global Public Health Threat. EClinicalMedicine 2021, 41, 101221. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization Antimicrobial Resistance 2023; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Emil Vollset, S.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. In The Review on Antimicrobial Resistance; AMR Review: London, UK, 2016. [Google Scholar]
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Niba, L.L.; et al. Tackling Antimicrobial Resistance across Sub-Saharan Africa: Current Challenges and Implications for the Future. Expert. Opin. Drug Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- Sartorius, B.; Gray, A.P.; Davis Weaver, N.; Robles Aguilar, G.; Swetschinski, L.R.; Ikuta, K.S.; Mestrovic, T.; Chung, E.; Wool, E.E.; Han, C.; et al. The Burden of Bacterial Antimicrobial Resistance in the WHO African Region in 2019: A Cross-Country Systematic Analysis. Lancet Glob. Health 2024, 12, e201–e216. [Google Scholar] [CrossRef]
- Mudenda, S.; Chabalenge, B.; Daka, V.; Mfune, R.L.; Salachi, K.I.; Mohamed, S.; Mufwambi, W.; Kasanga, M.; Matafwali, S.K. Global Strategies to Combat Antimicrobial Resistance: A One Health Perspective. Pharmacol. Pharm. 2023, 14, 271–328. [Google Scholar] [CrossRef]
- Mendelson, M.; Morris, A.M.; Thursky, K.; Pulcini, C. How to Start an Antimicrobial Stewardship Programme in a Hospital. Clin. Microbiol. Infect. 2020, 26, 447–453. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What Is Antimicrobial Stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Chizimu, J.Y.; Mudenda, S.; Yamba, K.; Lukwesa, C.; Chanda, R.; Nakazwe, R.; Simunyola, B.; Shawa, M.; Kalungia, A.C.; Chanda, D.; et al. Antimicrobial Stewardship Situation Analysis in Selected Hospitals in Zambia: Findings and Implications from a National Survey. Front. Public Health 2024, 12, 1367703. [Google Scholar] [CrossRef] [PubMed]
- Chizimu, J.Y.; Mudenda, S.; Yamba, K.; Lukwesa, C.; Chanda, R.; Nakazwe, R.; Shawa, M.; Chambaro, H.; Kamboyi, H.K.; Kalungia, A.C.; et al. Antibiotic Use and Adherence to the WHO AWaRe Guidelines across 16 Hospitals in Zambia: A Point Prevalence Survey. JAC Antimicrob. Resist. 2024, 6, dlae170. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Shetty, N.; Patel, J.; Van Doorn, R.; Limmathurotsakul, D.; Feasey, N.A.; Okeke, I.N.; Peacock, S.J. Harnessing Alternative Sources of Antimicrobial Resistance Data to Support Surveillance in Low-Resource Settings. J. Antimicrob. Chemother. 2019, 74, 541–546. [Google Scholar] [CrossRef]
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, Animal and Environmental Contributors to Antibiotic Resistance in Low-Resource Settings: Integrating Behavioural, Epidemiological and One Health Approaches. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180332. [Google Scholar] [CrossRef]
- Nadimpalli, M.; Delarocque-Astagneau, E.; Love, D.C.; Price, L.B.; Huynh, B.T.; Collard, J.M.; Lay, K.S.; Borand, L.; Ndir, A.; Walsh, T.R.; et al. Combating Global Antibiotic Resistance: Emerging One Health Concerns in Lower-and Middle-Income Countries. Clin. Infect. Dis. 2018, 66, 963–969. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.; Dar, M.A.; Kaur, R.J.; Charan, J.; Iskandar, K.; Haque, M.; Murti, K.; Ravichandiran, V.; Dhingra, S. Menace of Antimicrobial Resistance in LMICs: Current Surveillance Practices and Control Measures to Tackle Hostility. J. Infect. Public Health 2022, 15, 172–181. [Google Scholar] [CrossRef]
- Gulumbe, B.H.; Danlami, M.B.; Abdulrahim, A. Closing the Antimicrobial Stewardship Gap-a Call for LMICs to Embrace the Global Antimicrobial Stewardship Accreditation Scheme. Antimicrob. Resist. Infect. Control 2024, 13, 19. [Google Scholar] [CrossRef]
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Hardcastle, T.C.; Haque, M.; Lugova, H.; Dhingra, S.; Sharma, P.; Islam, S.; et al. Surveillance of Antimicrobial Resistance in Low- and Middle-Income Countries: A Scattered Picture. Antimicrob. Resist. Infect. Control 2021, 10, 63. [Google Scholar] [CrossRef]
- Eden, T.; Burns, E.; Freccero, P.; Renner, L.; Paintsil, V.; Dolendo, M.; Scanlan, T.; Khaing, A.A.; Pina, M.; Islam, A.; et al. Are Essential Medicines Available, Reliable and Affordable in Low-Middle Income Countries? J. Cancer Policy 2019, 19, 100180. [Google Scholar] [CrossRef]
- Ozawa, S.; Shankar, R.; Leopold, C.; Orubu, S. Access to Medicines through Health Systems in Low-and Middle-Income Countries. Health Policy Plan 2019, 34, III1–III3. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and Socioeconomic Factors Contributing to Global Antimicrobial Resistance: A Univariate and Multivariable Analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial Resistance in Low- and Middle-Income Countries: Current Status and Future Directions. Expert. Rev. Anti Infect. Ther. 2022, 20, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling Antimicrobial Resistance in Low-Income and Middle-Income Countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef]
- Otaigbe, I.I.; Elikwu, C.J. Drivers of Inappropriate Antibiotic Use in Low- and Middle-Income Countries. JAC Antimicrob. Resist. 2023, 5, dlad062. [Google Scholar] [CrossRef]
- World Health Organization. Access to Essential Medicines, Vaccines and Health Technologies: Fact Sheet on Sustainable Development Goals (SDGs): Health Targets; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Huong, V.T.L.; Ngan, T.T.D.; Thao, H.P.; Tu, N.T.C.; Quan, T.A.; Nadjm, B.; Kesteman, T.; Van Kinh, N.; van Doorn, H.R. Improving Antimicrobial Use through Antimicrobial Stewardship in a Lower-Middle Income Setting: A Mixed-Methods Study in a Network of Acute-Care Hospitals in Viet Nam. J. Glob. Antimicrob. Resist. 2021, 27, 212–221. [Google Scholar] [CrossRef]
- World Health Organization. WHO Competency Framework for Health Workers’ Education and Training on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Kerr, F.; Sefah, I.A.; Essah, D.O.; Cockburn, A.; Afriyie, D.; Mahungu, J.; Mirfenderesky, M.; Ankrah, D.; Aggor, A.; Barrett, S.; et al. Practical Pharmacist-Led Interventions to Improve Antimicrobial Stewardship in Ghana, Tanzania, Uganda and Zambia. Pharmacy 2021, 9, 124. [Google Scholar] [CrossRef]
- Kpokiri, E.E.; Ladva, M.; Dodoo, C.C.; Orman, E.; Aku, T.A.; Mensah, A.; Jato, J.; Mfoafo, K.A.; Folitse, I.; Hutton-Nyameaye, A.; et al. Knowledge, Awareness and Practice with Antimicrobial Stewardship Programmes among Healthcare Providers in a Ghanaian Tertiary Hospital. Antibiotics 2022, 11, 6. [Google Scholar] [CrossRef]
- Mudenda, S.; Chabalenge, B.; Daka, V.; Jere, E.; Sefah, I.A.; Wesangula, E.; Yamba, K.; Nyamupachitu, J.; Mugenyi, N.; Mustafa, Z.U.; et al. Knowledge, Awareness and Practices of Healthcare Workers Regarding Antimicrobial Use, Resistance and Stewardship in Zambia: A Multi-Facility Cross-Sectional Study. JAC Antimicrob. Resist. 2024, 6, dlae076. [Google Scholar] [CrossRef]
- Amponsah, O.K.O.; Courtenay, A.; Kwame Ayisi-Boateng, N.; Abuelhana, A.; Opoku, D.A.; Kobina Blay, L.; Abruquah, N.A.; Osafo, A.B.; Danquah, C.B.; Tawiah, P.; et al. Assessing the Impact of Antimicrobial Stewardship Implementation at a District Hospital in Ghana Using a Health Partnership Model. JAC Antimicrob. Resist. 2023, 5, dlad084. [Google Scholar] [CrossRef]
- Knowles, R.; Chandler, C.; O’neill, S.; Sharland, M.; Mays, N. A Systematic Review of National Interventions and Policies to Optimize Antibiotic Use in Healthcare Settings in England. J. Antimicrob. Chemother. 2024, 79, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Ashiru-Oredope, D.; Garraghan, F.; Olaoye, O.; Krockow, E.M.; Matuluko, A.; Nambatya, W.; Babigumira, P.A.; Tuck, C.; Amofah, G.; Ankrah, D.; et al. Development and Implementation of an Antimicrobial Stewardship Checklist in Sub-Saharan Africa: A Co-Creation Consensus Approach. Healthcare 2022, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Zanichelli, V.; Sharland, M.; Cappello, B.; Moja, L.; Getahun, H.; Pessoa-Silva, C.; Sati, H.; van Weezenbeek, C.; Balkhy, H.; Simão, M.; et al. The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book and Prevention of Antimicrobial Resistance. Bull. World Health Organ. 2023, 101, 290–296. [Google Scholar] [CrossRef]
- Hsia, Y.; Lee, B.R.; Versporten, A.; Yang, Y.; Bielicki, J.; Jackson, C.; Newland, J.; Goossens, H.; Magrini, N.; Sharland, M. Use of the WHO Access, Watch, and Reserve Classification to Define Patterns of Hospital Antibiotic Use (AWaRe): An Analysis of Paediatric Survey Data from 56 Countries. Lancet Glob. Health 2019, 7, e861–e871. [Google Scholar] [CrossRef]
- Mudenda, S.; Daka, V.; Matafwali, S.K. World Health Organization AWaRe Framework for Antibiotic Stewardship: Where Are We Now and Where Do We Need to Go? An Expert Viewpoint. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e84. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries. A WHO Practical Toolkit; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Kakkar, A.K.; Shafiq, N.; Singh, G.; Ray, P.; Gautam, V.; Agarwal, R.; Muralidharan, J.; Arora, P. Antimicrobial Stewardship Programs in Resource-Constrained Environments: Understanding and Addressing the Need of the Systems. Front. Public Health 2020, 8, 140. [Google Scholar] [CrossRef]
- Veepanattu, P.; Singh, S.; Mendelson, M.; Nampoothiri, V.; Edathadatil, F.; Surendran, S.; Bonaconsa, C.; Mbamalu, O.; Ahuja, S.; Birgand, G.; et al. Building Resilient and Responsive Research Collaborations to Tackle Antimicrobial Resistance—Lessons Learnt from India, South Africa, and UK. Int. J. Infect. Dis. 2020, 100, 278–282. [Google Scholar] [CrossRef]
- Nassar, H.; Abu-Farha, R.; Barakat, M.; Alefishat, E. Antimicrobial Stewardship from Health Professionals’ Perspective: Awareness, Barriers, and Level of Implementation of the Program. Antibiotics 2022, 11, 99. [Google Scholar] [CrossRef]
- Joshi, M.P.; Chintu, C.; Mpundu, M.; Kibuule, D.; Hazemba, O.; Andualem, T.; Embrey, M.; Phulu, B.; Gerba, H. Multidisciplinary and Multisectoral Coalitions as Catalysts for Action against Antimicrobial Resistance: Implementation Experiences at National and Regional Levels. Glob. Public Health 2018, 13, 1781–1795. [Google Scholar] [CrossRef]
- World Health Organization. South African Antimicrobial Resistance National Strategy Framework: A One Health Approach 2018–2024; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Ministry of Health Kenya. Guidelines, Standards & Policies Portal; Ministry of Health Kenya: Nairobi, Kenya, 2019. [Google Scholar]
- World Health Organization. Vietnam: National Action Plan for Combating Drug Resistance; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Musoke, D.; Kitutu, F.E.; Mugisha, L.; Amir, S.; Brandish, C.; Ikhile, D.; Kajumbula, H.; Kizito, I.M.; Lubega, G.B.; Niyongabo, F.; et al. A One Health Approach to Strengthening Antimicrobial Stewardship in Wakiso District, Uganda. Antibiotics 2020, 9, 764. [Google Scholar] [CrossRef]
- Menezes, R.M.; Gonçalves, M.R.S.; Costa, M.M.d.M.; Krumennauer, E.C.; Carneiro, G.M.; Reuter, C.P.; Renner, J.D.P.; Carneiro, M. Antimicrobial Stewardship Programmes in Brazil: Introductory Analysis. Res. Soc. Dev. 2022, 11, e51011729444. [Google Scholar] [CrossRef]
- Government of the Republic of Zambia. Multi-Sectoral National Action Plan on Antimicrobial Resistance; Government of the Republic of Zambia: Lusaka, Zambia, 2017. [Google Scholar]
- World Health Organization. Kenya: National Action Plan on Prevention and Containment of Antimicrobial Resistance 2017–2022; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Chukwu, E.E.; Abuh, D.; Idigbe, I.E.; Osuolale, K.A.; Chuka-Ebene, V.; Awoderu, O.; Audu, R.A.; Ogunsola, F.T. Implementation of Antimicrobial Stewardship Programs: A Study of Prescribers’ Perspective of Facilitators and Barriers. PLoS ONE 2024, 19, e0297472. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, A.; Al Mutair, A.; Alhumaid, S.; Salih, S.; Alanazi, A.; Albarsan, H.; Abourayan, M.; Al Subaie, M. The Impact of Antimicrobial Stewardship Program Implementation at Four Tertiary Private Hospitals: Results of a Five-Years Pre-Post Analysis. Antimicrob. Resist. Infect. Control 2020, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Abdel Hadi, H.; Eltayeb, F.; Al Balushi, S.; Daghfal, J.; Ahmed, F.; Mateus, C. Evaluation of Hospital Antimicrobial Stewardship Programs: Implementation, Process, Impact, and Outcomes, Review of Systematic Reviews. Antibiotics 2024, 13, 253. [Google Scholar] [CrossRef]
- Jacobs, T.G.; Robertson, J.; Van Den Ham, H.A.; Iwamoto, K.; Bak Pedersen, H.; Mantel-Teeuwisse, A.K. Assessing the Impact of Law Enforcement to Reduce Over-the-Counter (OTC) Sales of Antibiotics in Low- And Middle-Income Countries; A Systematic Literature Review. BMC Health Serv. Res. 2019, 19, 536. [Google Scholar] [CrossRef]
- Cuevas, C.; Batura, N.; Wulandari, L.P.L.; Khan, M.; Wiseman, V. Improving Antibiotic Use through Behaviour Change: A Systematic Review of Interventions Evaluated in Low- And Middle-Income Countries. Health Policy Plan 2021, 36, 754–773. [Google Scholar] [CrossRef]
- Alabi, A.S.; Picka, S.W.; Sirleaf, R.; Ntirenganya, P.R.; Ayebare, A.; Correa, N.; Anyango, S.; Ekwen, G.; Agu, E.; Cook, R.; et al. Implementation of an Antimicrobial Stewardship Programme in Three Regional Hospitals in the South-East of Liberia: Lessons Learned. JAC Antimicrob. Resist. 2022, 4, dlac069. [Google Scholar] [CrossRef]
- Aiken, A.M.; Wanyoro, A.K.; Mwangi, J.; Juma, F.; Mugoya, I.K.; Scott, J.A.G. Changing Use of Surgical Antibiotic Prophylaxis in Thika Hospital, Kenya: A Quality Improvement Intervention with an Interrupted Time Series Design. PLoS ONE 2013, 8, e78942. [Google Scholar] [CrossRef]
- Brink, A.J.; Messina, A.P.; Feldman, C.; Richards, G.A.; Becker, P.J.; Goff, D.A.; Bauer, K.A.; Nathwani, D.; van den Bergh, D. Antimicrobial Stewardship across 47 South African Hospitals: An Implementation Study. Lancet Infect. Dis. 2016, 16, 1017–1025. [Google Scholar] [CrossRef]
- Abubakar, U.; Sulaiman, S.A.S.; Adesiyun, A.G. Impact of Pharmacist-Led Antibiotic Stewardship Interventions on Compliance with Surgical Antibiotic Prophylaxis in Obstetric and Gynecologic Surgeries in Nigeria. PLoS ONE 2019, 14, e0213395. [Google Scholar] [CrossRef]
- Kpokiri, E.E.; Taylor, D.G.; Smith, F.J. Development of Antimicrobial Stewardship Programmes in Low and Middle-Income Countries: A Mixed-Methods Study in Nigerian Hospitals. Antibiotics 2020, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Mzumara, G.W.; Mambiya, M.; Iroh Tam, P.Y. Protocols, Policies and Practices for Antimicrobial Stewardship in Hospitalized Patients in Least-Developed and Low-Income Countries: A Systematic Review. Antimicrob. Resist. Infect. Control 2023, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Shamas, N.; Stokle, E.; Ashiru-Oredope, D.; Wesangula, E. Challenges of Implementing Antimicrobial Stewardship Tools in Low to Middle Income Countries (LMICs). Infect. Prev. Pract. 2023, 5, 100315. [Google Scholar] [CrossRef] [PubMed]
- Sefah, I.A.; Chetty, S.; Yamoah, P.; Godman, B.; Bangalee, V. An Assessment of the Current Level of Implementation of the Core Elements of Antimicrobial Stewardship Programs in Public Hospitals in Ghana. Hosp. Pharm. 2024, 59, 367–377. [Google Scholar] [CrossRef]
- Aika, I.N.; Enato, E. Health Care Systems Administrators Perspectives on Antimicrobial Stewardship and Infection Prevention and Control Programs across Three Healthcare Levels: A Qualitative Study. Antimicrob. Resist. Infect. Control 2022, 11, 157. [Google Scholar] [CrossRef]
- Storr, J.; Twyman, A.; Zingg, W.; Damani, N.; Kilpatrick, C.; Reilly, J.; Price, L.; Egger, M.; Grayson, M.L.; Kelley, E.; et al. Core Components for Effective Infection Prevention and Control Programmes: New WHO Evidence-Based Recommendations. Antimicrob. Resist. Infect. Control 2017, 6, 6. [Google Scholar] [CrossRef]
- World Health Organization. Interim Practical Manual Supporting National Implementation of the WHO Guidelines on Core Components of Infection Prevention and Control Programmes; WHO: Geneva, Switzerland, 2017; pp. 1–77. [Google Scholar]
- World Health Organization. Improving Infection Prevention and Control at the Health Facility: An Interim Practical Manual; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Cheong, H.S.; Park, K.H.; Bin Kim, H.; Kim, S.W.; Kim, B.; Moon, C.; Lee, M.S.; Yoon, Y.K.; Jeong, S.J.; Kim, Y.C.; et al. Core Elements for Implementing Antimicrobial Stewardship Programs in Korean General Hospitals. Infect. Chemother. 2022, 54, 637–673. [Google Scholar] [CrossRef]
- Maki, G.; Smith, I.; Paulin, S.; Kaljee, L.; Kasambara, W.; Mlotha, J.; Chuki, P.; Rupali, P.; Singh, D.R.; Bajracharya, D.C.; et al. Feasibility Study of the World Health Organization Health Care Facility-Based Antimicrobial Stewardship Toolkit for Low-and Middle-Income Countries. Antibiotics 2020, 9, 556. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Yu, X.; Zhou, H.; Li, B.; Chen, G.; Ye, Z.; Wang, Y.; Cui, X.; Zheng, Y.; et al. Impact of Antimicrobial Stewardship Managed by Clinical Pharmacists on Antibiotic Use and Drug Resistance in a Chinese Hospital, 2010–2016: A Retrospective Observational Study. BMJ Open 2019, 9, e026072. [Google Scholar] [CrossRef]
- Siachalinga, L.; Mufwambi, W.; Lee, I.-H. Impact of Antimicrobial Stewardship Interventions to Improve Antibiotic Prescribing for Hospital Inpatients in Africa: A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2022, 129, 124–143. [Google Scholar] [CrossRef]
- Bantar, C.; Sartori, B.; Vesco, E.; Heft, C.; Saúl, M.; Salamone, F.; Oliva, M.E. A Hospitalwide Intervention Program to Optimize the Quality of Antibiotic Use: Impact on Prescribing Practice, Antibiotic Consumption, Cost Savings, and Bacterial Resistance. Clin. Infect. Dis. 2003, 37, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Nyoloka, N.; Richards, C.; Mpute, W.; Chadwala, H.M.; Kumwenda, H.S.; Mwangonde-Phiri, V.; Phiri, A.; Phillips, C.; Makanga, C. Pharmacist-Led Antimicrobial Stewardship Programme in Two Tertiary Hospitals in Malawi. Antibiotics 2024, 13, 480. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, J.; Cooper, L.; Afriyie, D.K.; Sefah, I.A.; Cockburn, A.; Kerr, F.; Cameron, E.; Goldthorpe, J.; Kurdi, A.; Andrew Seaton, R. Supporting Antimicrobial Stewardship in Ghana: Evaluation of the Impact of Training on Knowledge and Attitudes of Healthcare Professionals in Two Hospitals. JAC Antimicrob. Resist. 2020, 2, dlaa092. [Google Scholar] [CrossRef] [PubMed]
- Ashiru-Oredope, D.; Nabiryo, M.; Zengeni, L.; Kamere, N.; Makotose, A.; Olaoye, O.; Townsend, W.; Waddingham, B.; Matuluko, A.; Nambatya, W.; et al. Tackling Antimicrobial Resistance: Developing and Implementing Antimicrobial Stewardship Interventions in Four African Commonwealth Countries through a Health Partnership Model. J. Public Health Afr. 2023, 14, 2335. [Google Scholar] [CrossRef]
- Hassan, S.K.; Dahmash, E.Z.; Madi, T.; Tarawneh, O.; Jomhawi, T.; Alkhob, W.; Ghanem, R.; Halasa, Z. Four Years after the Implementation of Antimicrobial Stewardship Program in Jordan: Evaluation of Program’s Core Elements. Front. Public Health 2023, 11, 1078596. [Google Scholar] [CrossRef]
- Sabbatucci, M.; Ashiru-Oredope, D.; Barbier, L.; Bohin, E.; Bou-Antoun, S.; Brown, C.; Clarici, A.; Fuentes, C.; Goto, T.; Maraglino, F.; et al. Tracking Progress on Antimicrobial Resistance by the Quadripartite Country Self-Assessment Survey (TrACSS) in G7 Countries, 2017–2023: Opportunities and Gaps. Pharmacol. Res. 2024, 204, 107188. [Google Scholar] [CrossRef]
- Lazure, P.; Augustyniak, M.; Goff, D.A.; Villegas, M.V.; Apisarnthanarak, A.; Peloquin, S. Gaps and Barriers in the Implementation and Functioning of Antimicrobial Stewardship Programmes: Results from an Educational and Behavioural Mixed Methods Needs Assessment in France, the United States, Mexico and India. JAC Antimicrob. Resist. 2022, 4, dlac094. [Google Scholar] [CrossRef]
- Dodoo, C.C.; Odoi, H.; Mensah, A.; Asafo-Adjei, K.; Ampomah, R.; Obeng, L.; Jato, J.; Hutton-Nyameaye, A.; Aku, T.A.; Somuah, S.O.; et al. Development of a Local Antibiogram for a Teaching Hospital in Ghana. JAC Antimicrob. Resist. 2023, 5, dlad024. [Google Scholar] [CrossRef]
- Mathew, P.; Ranjalkar, J.; Chandy, S.J. Challenges in Implementing Antimicrobial Stewardship Programmes at Secondary Level Hospitals in India: An Exploratory Study. Front. Public Health 2020, 8, 493904. [Google Scholar] [CrossRef]
- East Central and Southern Africa-Health Community. Meeting Report Joint Regional Meeting on Antimicrobial Stewardship and Antimicrobial Surveillance in Eastern and Southern Africa Co-Sponsored by the East Central and Southern Africa-Health Community and Africa Centres for Disease; East Central and Southern Africa-Health Community: Arusha, Tanzania, 2023. [Google Scholar]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs. In Antibiotic Use; CDC: Atlanta, GA, USA, 2024; pp. 1–7. [Google Scholar]
- World Health Organization. WHO Policy Guidance on Integrated Antimicrobial Stewardship Activities; WHO: Geneva, Switzerland, 2021. [Google Scholar]








| Country | Hospital Level | Number of Hospitals |
|---|---|---|
| Malawi | Tertiary | 2 |
| Secondary | 3 | |
| Mozambique | Tertiary | 4 |
| Rwanda | Secondary | 7 |
| Tertiary | 4 | |
| Tanzania | Secondary | 6 |
| Zambia | Secondary | 2 |
| Tertiary | 4 | |
| Total | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesangula, E.; Chizimu, J.Y.; Mapunjo, S.; Mudenda, S.; Seni, J.; Mitambo, C.; Yamba, K.; Gashegu, M.; Nhantumbo, A.; Francis, E.; et al. A Regional Approach to Strengthening the Implementation of Sustainable Antimicrobial Stewardship Programs in Five Countries in East, Central, and Southern Africa. Antibiotics 2025, 14, 266. https://doi.org/10.3390/antibiotics14030266
Wesangula E, Chizimu JY, Mapunjo S, Mudenda S, Seni J, Mitambo C, Yamba K, Gashegu M, Nhantumbo A, Francis E, et al. A Regional Approach to Strengthening the Implementation of Sustainable Antimicrobial Stewardship Programs in Five Countries in East, Central, and Southern Africa. Antibiotics. 2025; 14(3):266. https://doi.org/10.3390/antibiotics14030266
Chicago/Turabian StyleWesangula, Evelyn, Joseph Yamweka Chizimu, Siana Mapunjo, Steward Mudenda, Jeremiah Seni, Collins Mitambo, Kaunda Yamba, Misbah Gashegu, Aquino Nhantumbo, Emiliana Francis, and et al. 2025. "A Regional Approach to Strengthening the Implementation of Sustainable Antimicrobial Stewardship Programs in Five Countries in East, Central, and Southern Africa" Antibiotics 14, no. 3: 266. https://doi.org/10.3390/antibiotics14030266
APA StyleWesangula, E., Chizimu, J. Y., Mapunjo, S., Mudenda, S., Seni, J., Mitambo, C., Yamba, K., Gashegu, M., Nhantumbo, A., Francis, E., Moremi, N., Athiany, H., & Matu, M. (2025). A Regional Approach to Strengthening the Implementation of Sustainable Antimicrobial Stewardship Programs in Five Countries in East, Central, and Southern Africa. Antibiotics, 14(3), 266. https://doi.org/10.3390/antibiotics14030266








