Predicting the Effect of Meropenem Against Klebsiella pneumoniae Using Minimum Inhibitory Concentrations Determined at High Inocula
Abstract
1. Introduction
2. Results
2.1. Meropenem MICs at Standard and High Inocula
2.2. Meropenem Pharmacodynamics with Carbapenemase-Non-Producing K. pneumoniae Strains
2.3. Meropenem Pharmacodynamics with Carbapenemase-Producing K. pneumoniae Strains
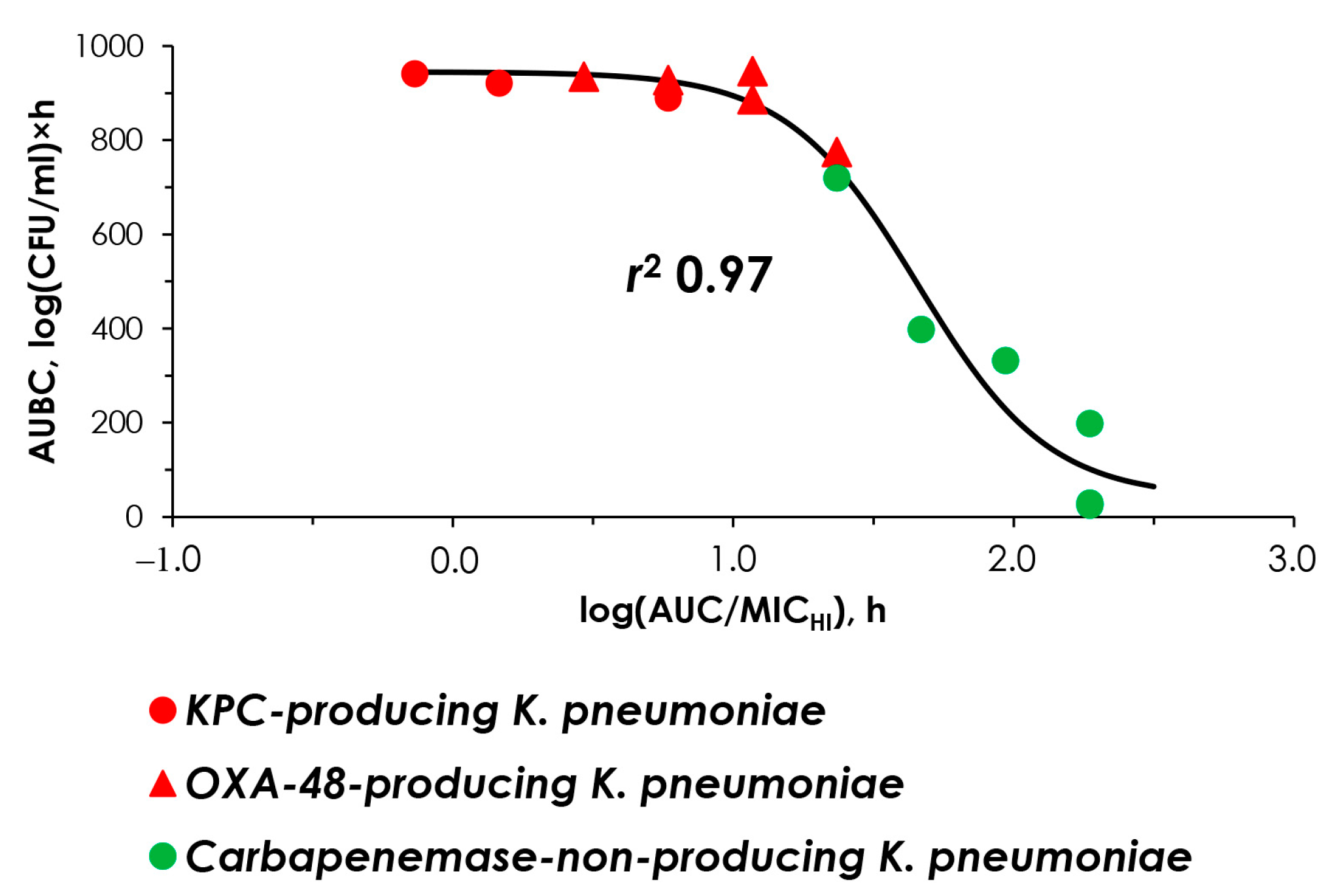
2.4. “AUC/MIC-Effect” and “AUC/MICHI-Effect” Relationships
2.5. Concordance Between the MIC and MICHI and Emergence of Resistance
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Agent and Bacterial Isolates
4.2. Susceptibility Testing
4.3. In Vitro Dynamic Model and Operational Procedure Used in the Pharmacodynamic Experiments
4.4. Antibiotic Dosing Regimens and Simulated Pharmacokinetic Profiles
4.5. Quantitation of the Antimicrobial Effect
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07; CLSI: Berwyn, PA, USA, 2019. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.0. 2023. Available online: http://www.eucast.org (accessed on 3 January 2025).
- Magréault, S.; Jauréguy, F.; Carbonnelle, E.; Zahar, J.R. When and How to Use MIC in Clinical Practice? Antibiotics 2022, 11, 1748. [Google Scholar] [CrossRef]
- Cantón, R.; Morosini, M.-I. Emergence and spread of antibiotic resistance following exposure to antibiotics. FEMS Microbiol. Rev. 2011, 35, 977–991. [Google Scholar] [CrossRef]
- Credito, K.; Kosowska-Shick, K.; Appelbaum, P.C. Mutant prevention concentrations of four carbapenems against gram-negative rods. Antimicrob. Agents Chemother. 2010, 54, 2692–2695. [Google Scholar] [CrossRef]
- Bao, X.L.; Tang, N.; Wang, Y.Z. Severe Klebsiella pneumoniae pneumonia complicated by acute intra-abdominal multiple arterial thrombosis and bacterial embolism: A case report. World J. Clin. Cases 2022, 10, 11101–11110. [Google Scholar] [CrossRef]
- Caron, F.; Alexandre, K.; Pestel-Caron, M.; Chassagne, P.; Grise, P.; Etienne, M. High bacterial titers in urine are predictive of abnormal postvoid residual urine in patients with urinary tract infection. Diagn. Microbiol. Infect. Dis. 2015, 83, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F. Native Infective Endocarditis: A State-of-the-Art-Review. Microorganisms 2024, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON527 (accessed on 3 January 2025).
- Lenhard, J.R.; Bulman, Z.P. Inoculum effect of β-lactam antibiotics. J. Antimicrob. Chemother. 2019, 74, 2825–2843. [Google Scholar] [CrossRef]
- Docobo-Pérez, F.; López-Cerero, L.; López-Rojas, R.; Egea, P.; Domínguez-Herrera, J.; Rodríguez-Baño, J.; Pascual, A.; Pachón, J. Inoculum effect on the efficacies of amoxicillin-clavulanate, piperacillin-tazobactam, and imipenem against extended-spectrum β-lactamase (ESBL)-producing and non-ESBL-producing Escherichia coli in an experimental murine sepsis model. Antimicrob. Agents Chemother. 2013, 57, 2109–2113. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Morinaga, Y.; Kaku, N.; Nakamura, S.; Uno, N.; Hasegawa, H.; Izumikawa, K.; Kohno, S.; Yanagihara, K. In vitro and in vivo activities of piperacillin-tazobactam and meropenem at different inoculum sizes of ESBL-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2014, 20, O831–O839. [Google Scholar] [CrossRef]
- Miller, W.R.; Seas, C.; Carvajal, L.P.; Diaz, L.; Echeverri, A.M.; Ferro, C.; Rios, R.; Porras, P.; Luna, C.; Gotuzzo, E.; et al. The cefazolin inoculum effect is associated with increased mortality in methicillin-susceptible Staphylococcus aureus bacteremia. Open Forum Infect. Dis. 2018, 5, ofy123. [Google Scholar] [CrossRef]
- Smelter, D.; McCrone, S.; Rose, W. Cefazolin inoculum effect predicts reduced susceptibility to other antibiotics and patient outcomes in MSSA endovascular infections. Open Forum Infect. Dis. 2020, 7 (Suppl. S1), S617. [Google Scholar] [CrossRef]
- Soriano, F.; Santamaría, M.; Ponte, C.; Castilla, C.; Fernández-Roblas, R. In vivo significance of the inoculum effect of antibiotics on Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 1988, 7, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Alieva, K.N.; Golikova, M.V.; Zinner, S.H. Role of volume and inoculum in MIC assessment: A study with meropenem and Klebsiella pneumoniae. J. Antimicrob. Chemother. 2024, 79, 2698–2707. [Google Scholar] [CrossRef]
- Ding, L.; Shen, S.; Chen, J.; Tian, Z.; Shi, Q.; Han, R.; Guo, Y.; Hu, F. Klebsiella pneumoniae carbapenemase variants: The new threat to global public health. Clin. Microbiol. Rev. 2023, 36, e0000823. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.S.; Thom, K.A.; Sharma, S.; Phillips, M.; Kristie Johnson, J.; Morgan, D.J. Emergence of Klebsiella pneumoniae carbapenemase-producing bacteria. South. Med. J. 2011, 104, 40–45. [Google Scholar] [CrossRef]
- Sadouki, Z.; McHugh, T.D.; Aarnoutse, R.; Ortiz Canseco, J.; Darlow, C.; Hope, W.; van Ingen, J.; Longshaw, C.; Manissero, D.; Mead, A.; et al. Application of the hollow fibre infection model (HFIM) in antimicrobial development: A systematic review and recommendations of reporting. J. Antimicrob. Chemother. 2021, 76, 2252–2259. [Google Scholar] [CrossRef]
- Hammond, R.J.H. Using Hollow Fiber to Model Treatment of Antimicrobial-Resistant Organisms. In Antibiotic Resistance Protocols. Methods in Molecular Biology; Gillespie, S.H., Ed.; Humana: New York, NY, USA, 2024; p. 2833. [Google Scholar] [CrossRef]
- Smith, K.P.; Kirby, J.E. The Inoculum Effect in the Era of Multidrug Resistance: Minor Differences in Inoculum Have Dramatic Effect on MIC Determination. Antimicrob. Agents Chemother. 2018, 62, e00433-18. [Google Scholar] [CrossRef] [PubMed]
- Sykes, R.B.; Matthew, M. The β-lactamases of gram-negative bacteria and their role in resistance to β-lactam antibiotics. J. Antimicrob. Chemother. 1976, 2, 115–157. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A.; Bhavnani, S.M.; Ambrose, P.G. The inoculum effect: Fact or artifact? Diagn. Microbiol. Infect. Dis. 2004, 50, 229–230. [Google Scholar] [CrossRef]
- Mizunaga, S.; Kamiyama, T.; Fukuda, Y.; Takahata, M.; Mitsuyama, J. Influence of inoculum size of Staphylococcus aureus and Pseudomonas aeruginosa on in vitro activities and in vivo efficacy of fluoroquinolones and carbapenems. J. Antimicrob. Chemother. 2005, 56, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Marum, D.; Manning, L.; Raby, E. Revisiting the inoculum effect for Streptococcus pyogenes with a hollow fibre infection model. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2137–2144. [Google Scholar] [CrossRef] [PubMed]
- Strukova, E.N.; Golikova, M.V.; Dovzhenko, S.A.; Kobrin, M.B.; Zinner, S.H. Pharmacodynamics of Doripenem Alone and in Combination with Relebactam in an In Vitro Hollow-Fiber Dynamic Model: Emergence of Resistance of Carbapenemase-Producing Klebsiella pneumoniae and the Inoculum Effect. Antibiotics 2023, 12, 1705. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Horinouchi, T.; Furusawa, C. Prediction of antibiotic resistance by gene expression profiles. Nat. Commun. 2014, 5, 5792. [Google Scholar] [CrossRef] [PubMed]
- Alieva, K.N.; Golikova, M.V.; Dovzhenko, S.A.; Kobrin, M.B.; Strukova, E.N.; Ageevets, V.A.; Avdeeva, A.A.; Sulian, O.S.; Sidorenko, S.V.; Zinner, S.H. Testing the mutant selection window hypothesis with meropenem: In vitro model study with OXA-48-producing Klebsiella pneumoniae. PLoS ONE 2023, 18, e0288660. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Zhao, X. Mutant selection window hypothesis updated. Clin. Infect. Dis. 2007, 44, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Croisier, D.; Etienne, M.; Bergoin, E.; Charles, P.E.; Lequeu, C.; Piroth, L.; Portier, H.; Chavanet, P. Mutant selection window in levofloxacin and moxifloxacin treatments of experimental pneumococcal pneumonia in a rabbit model of human therapy. Antimicrob. Agents Chemother. 2004, 48, 1699–1707. [Google Scholar] [CrossRef]
- Fujimura, S.; Nakano, Y.; Watanabe, A. A correlation between reduced susceptibilities to vancomycin and daptomycin among the MRSA isolates selected in mutant selection window of both vancomycin and daptomycin. J. Infect. Chemother. 2014, 20, 752–756. [Google Scholar] [CrossRef]
- Firsov, A.A.; Alieva, K.N.; Strukova, E.N.; Golikova, M.V.; Portnoy, Y.A.; Dovzhenko, S.A.; Kobrin, M.B.; Romanov, A.V.; Edelstein, M.V.; Zinner, S.H. Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to linezolid in an in vitro dynamic model. J. Antimicrob. Chemother. 2017, 72, 3100–3107. [Google Scholar] [CrossRef] [PubMed]
- Hleba, L.; Hlebová, M.; Kováčik, A.; Čuboň, J.; Medo, J. Carbapenemase Producing Klebsiella pneumoniae (KPC): What Is the Best MALDI-TOF MS Detection Method. Antibiotics 2021, 10, 1549. [Google Scholar] [CrossRef] [PubMed]
- Gülen, D.; Şafak, B.; Erdal, B.; Günaydın, B. Curcumin-meropenem synergy in carbapenem resistant Klebsiella pneumoniae curcumin-meropenem synergy. Iran. J. Microbiol. 2021, 13, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.; Sartor, A.; Bassetti, M.; Scarparo, C.; Pea, F.J. Is meropenem MIC increase against KPC-producing Klebsiella pneumoniae correlated with increased resistance rates against other antimicrobials with Gram-negative activity? Glob. Antimicrob. Resist. 2018, 14, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Fattouh, R.; Tijet, N.; McGeer, A.; Poutanen, S.M.; Melano, R.G.; Patel, S.N. What Is the Appropriate Meropenem MIC for Screening of Carbapenemase-Producing Enterobacteriaceae in Low-Prevalence Settings? Antimicrob. Agents Chemother. 2015, 60, 1556–1559. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, A.; Roorda, L.; Bosman, G.; Fluit, A.C.; Hermans, M.; Smits, P.H.; Ossewaarde, J.M. Multi-centre evaluation of real-time multiplex PCR for detection of carbapenemase genes OXA-48, VIM, IMP, NDM and KPC. BMC Infect. Dis. 2014, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- ISO 20776-1:2019; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents Against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. International Organization for Standardization: Geneva, Switzerland, 2019.
- MIC EUCAST. Available online: https://mic.eucast.org/ (accessed on 7 February 2025).
- Firsov, A.A.; Smirnova, M.V.; Strukova, E.N.; Vostrov, S.N.; Portnoy, Y.A.; Zinner, S.H. Enrichment of resistant Staphylococcus aureus at ciprofloxacin concentrations simulated within the mutant selection window: Bolus versus continuous infusion. Int. J. Antimicrob. Agents 2008, 32, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, E.; Gotfried, M.H.; Loutit, J.S.; Durso, S.; Griffith, D.C.; Dudley, M.N.; Rodvold, K.A. Meropenem-RPX7009 Concentrations in Plasma, Epithelial Lining Fluid, and Alveolar Macrophages of Healthy Adult Subjects. Antimicrob. Agents Chemother. 2015, 59, 7232–7239. [Google Scholar] [CrossRef]
- Firsov, A.A.; Lubenko, I.Y.; Smirnova, M.V.; Strukova, E.N.; Zinner, S.H. Enrichment of fluoroquinolone-resistant Staphylococcus aureus: Oscillating ciprofloxacin concentrations simulated at the upper and lower portions of the mutant selection window. Antimicrob. Agents Chemother. 2008, 52, 1924–1928. [Google Scholar] [CrossRef]
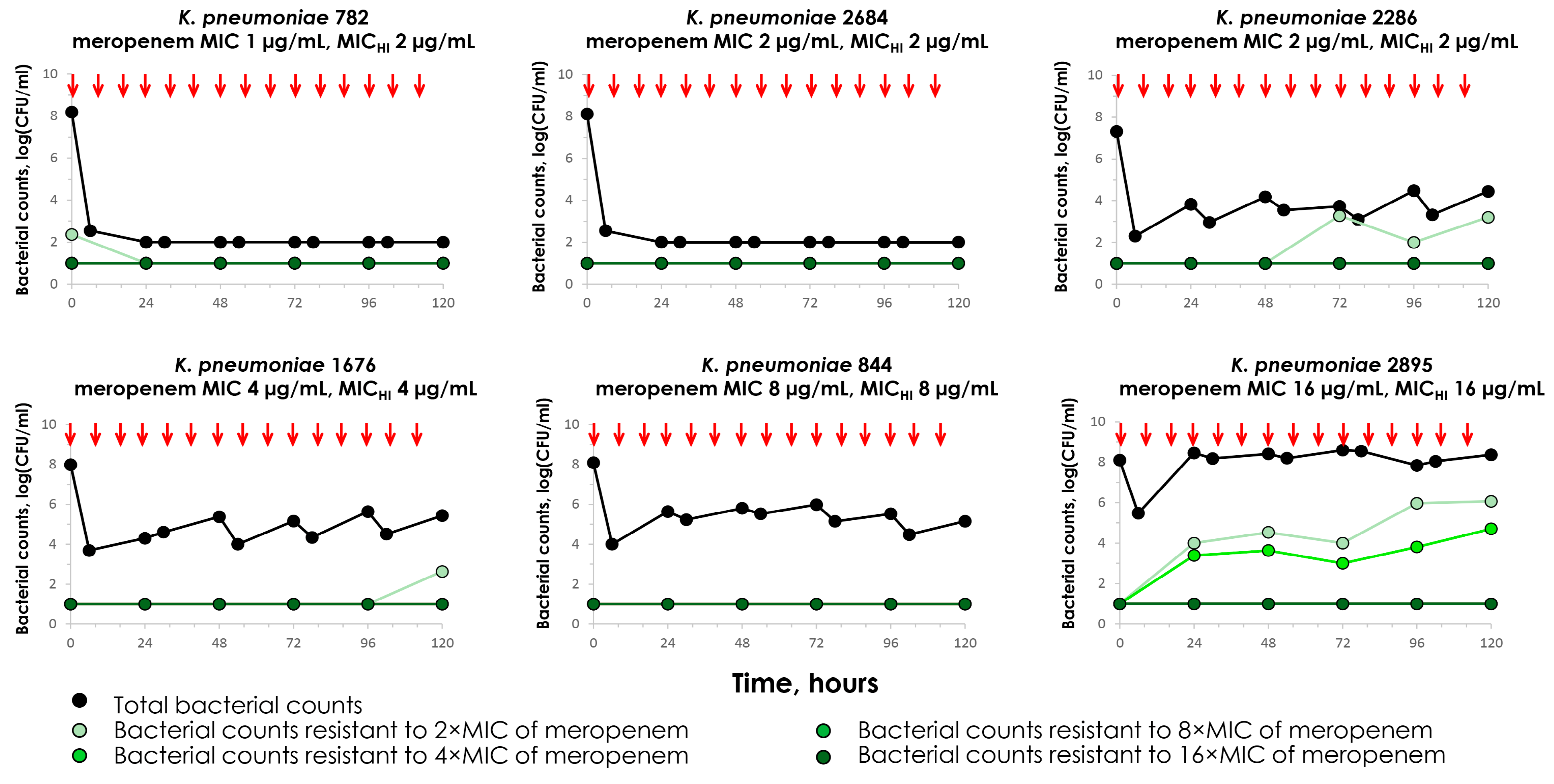
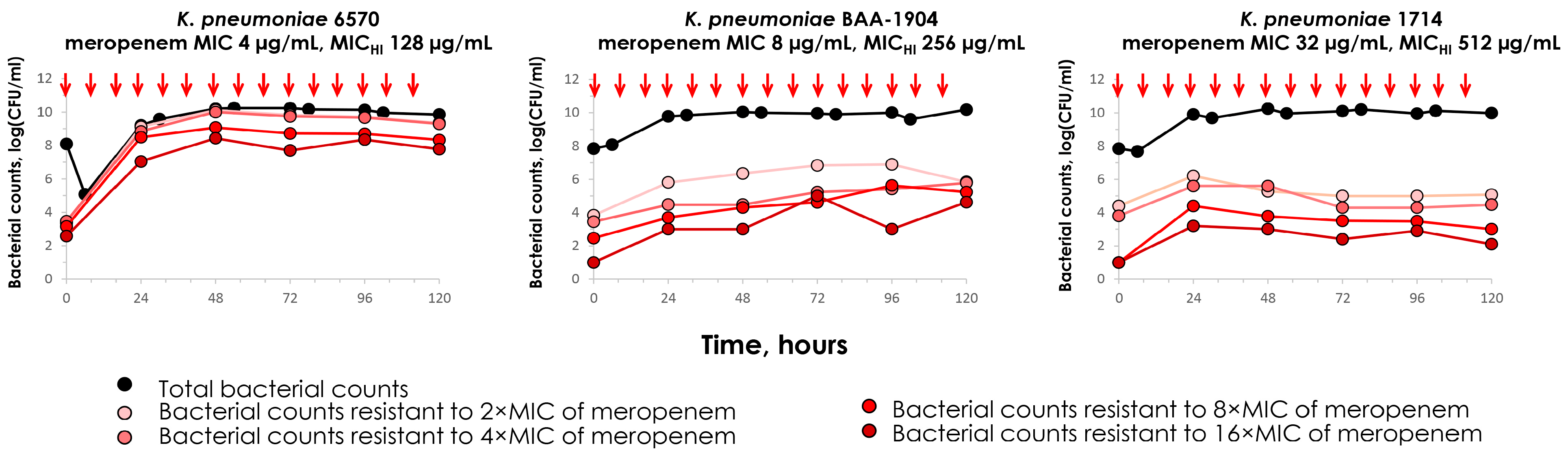
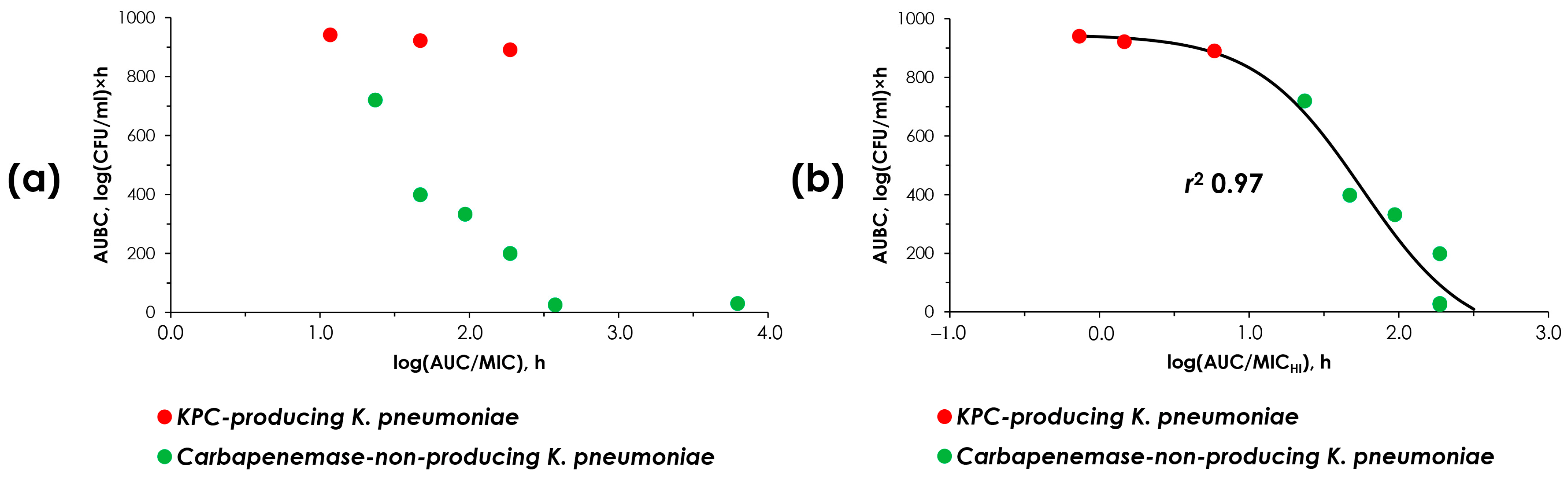
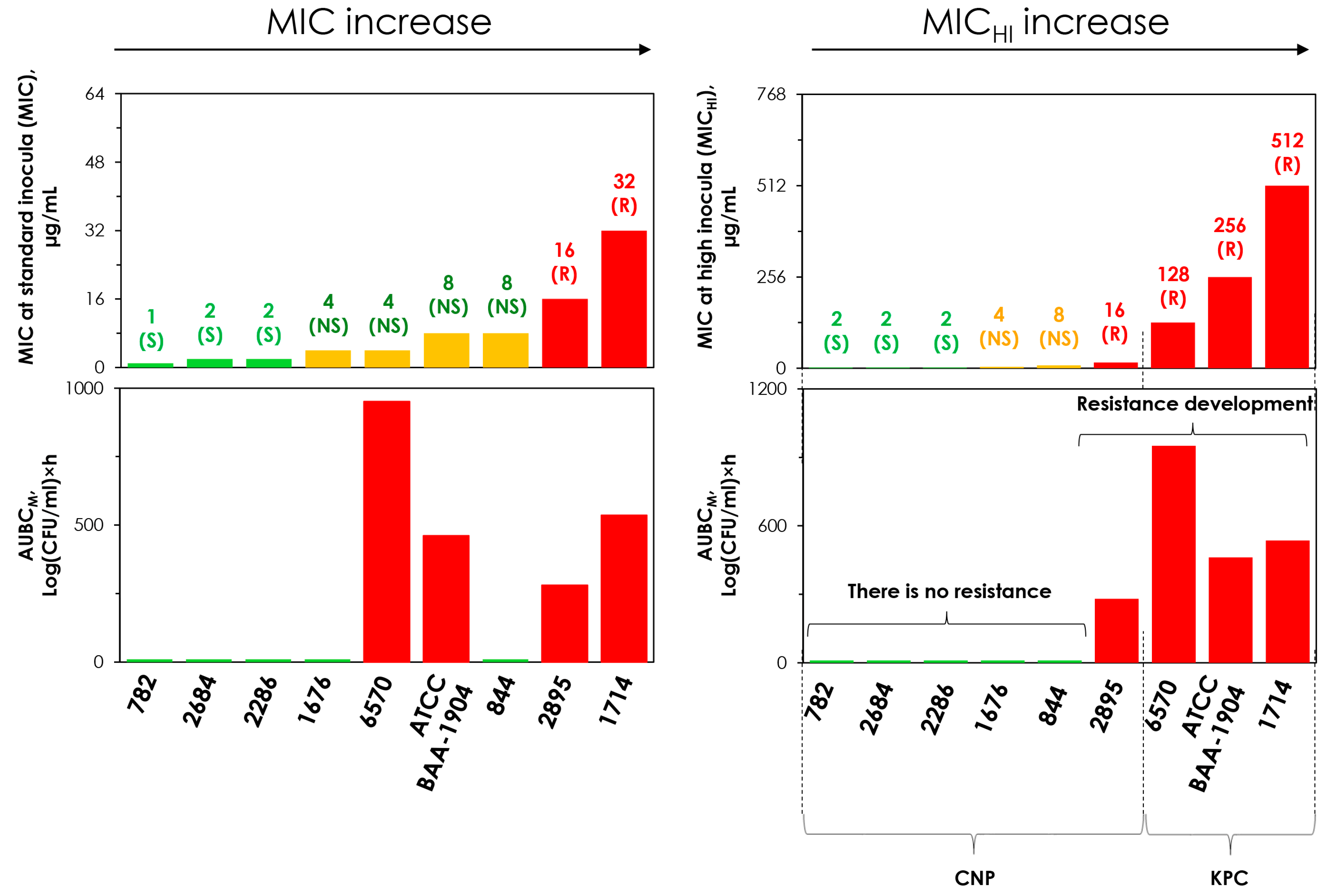
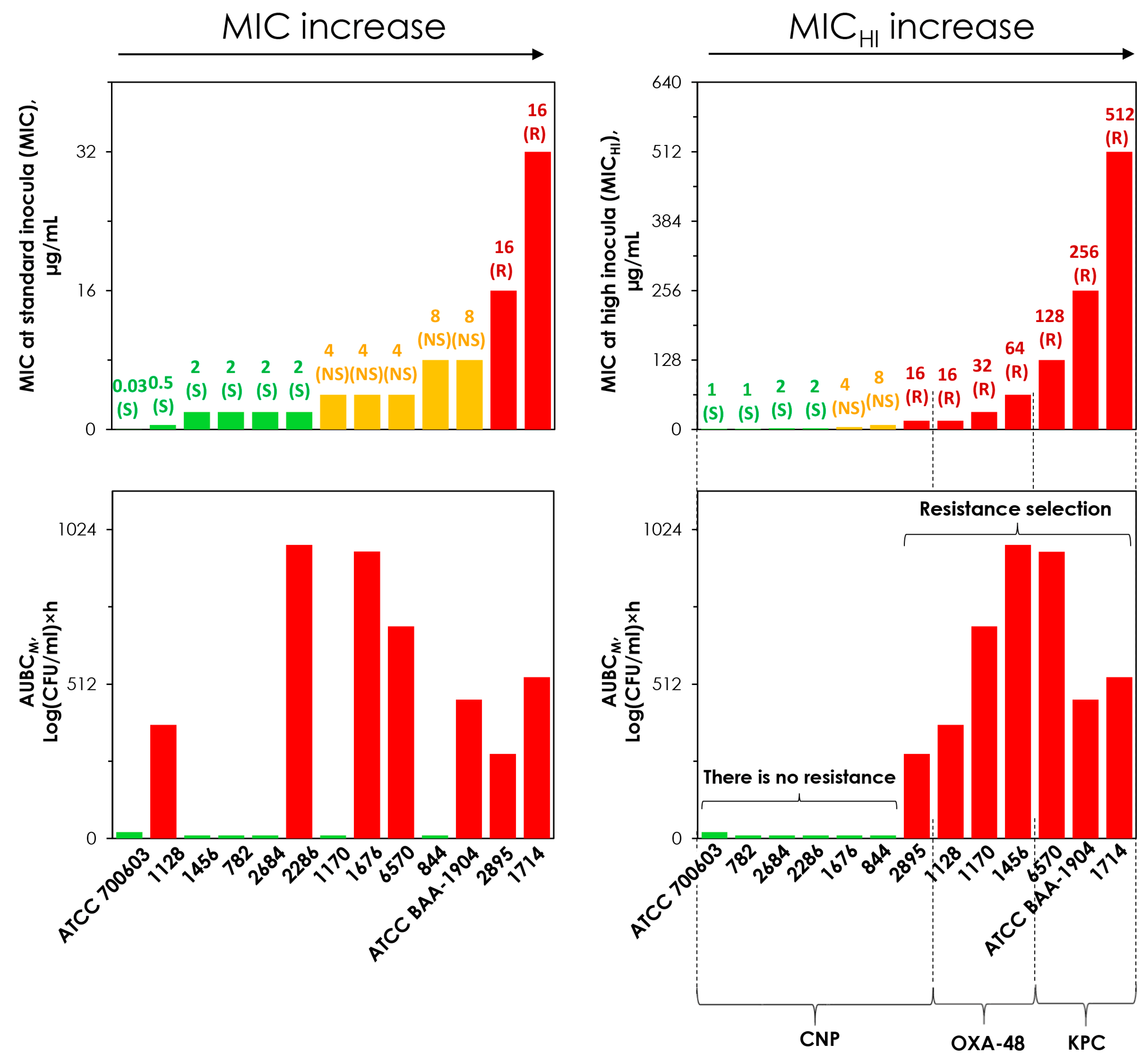

| № | K. pneumoniae Strain | Carbapenemases | Meropenem MIC, µg/mL | Meropenem MICHI, µg/mL | MICHI/MIC Ratio |
|---|---|---|---|---|---|
| 1 | 782 | None | 1 (S) | 2 (S) | 2 |
| 2 | 2684 | None | 2 (S) | 2 (S) | 1 |
| 3 | 2286 | None | 2 (S) | 2 (S) | 1 |
| 4 | 1676 | None | 4 (NS) | 4 (NS) | 1 |
| 5 | 844 | None | 8 (NS) | 8 (NS) | 1 |
| 6 | 2895 | None | 16 (R) | 16 (R) | 1 |
| 7 | 6570 | KPC | 4 (NS) | 128 (R) | 32 |
| 8 | ATCC BAA-1904 | KPC | 8 (NS) | 256 (R) | 64 |
| 9 | 1714 | KPC | 32 (R) | 512 (R) | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golikova, M.V.; Alieva, K.N.; Strukova, E.N.; Savelieva, J.R.; Kondratieva, D.A.; Dovzhenko, S.A.; Kobrin, M.B.; Ageevets, V.A.; Avdeeva, A.A.; Zinner, S.H. Predicting the Effect of Meropenem Against Klebsiella pneumoniae Using Minimum Inhibitory Concentrations Determined at High Inocula. Antibiotics 2025, 14, 258. https://doi.org/10.3390/antibiotics14030258
Golikova MV, Alieva KN, Strukova EN, Savelieva JR, Kondratieva DA, Dovzhenko SA, Kobrin MB, Ageevets VA, Avdeeva AA, Zinner SH. Predicting the Effect of Meropenem Against Klebsiella pneumoniae Using Minimum Inhibitory Concentrations Determined at High Inocula. Antibiotics. 2025; 14(3):258. https://doi.org/10.3390/antibiotics14030258
Chicago/Turabian StyleGolikova, Maria V., Kamilla N. Alieva, Elena N. Strukova, Julia R. Savelieva, Daria A. Kondratieva, Svetlana A. Dovzhenko, Mikhail B. Kobrin, Vladimir A. Ageevets, Alisa A. Avdeeva, and Stephen H. Zinner. 2025. "Predicting the Effect of Meropenem Against Klebsiella pneumoniae Using Minimum Inhibitory Concentrations Determined at High Inocula" Antibiotics 14, no. 3: 258. https://doi.org/10.3390/antibiotics14030258
APA StyleGolikova, M. V., Alieva, K. N., Strukova, E. N., Savelieva, J. R., Kondratieva, D. A., Dovzhenko, S. A., Kobrin, M. B., Ageevets, V. A., Avdeeva, A. A., & Zinner, S. H. (2025). Predicting the Effect of Meropenem Against Klebsiella pneumoniae Using Minimum Inhibitory Concentrations Determined at High Inocula. Antibiotics, 14(3), 258. https://doi.org/10.3390/antibiotics14030258






