Efficacy of Three Kayviruses Against Staphylococcus aureus Strains Isolated from COVID-19 Patients
Abstract
1. Introduction
2. Results
2.1. Phage Host Range Analysis
2.2. Biofilm Eradication by Phages
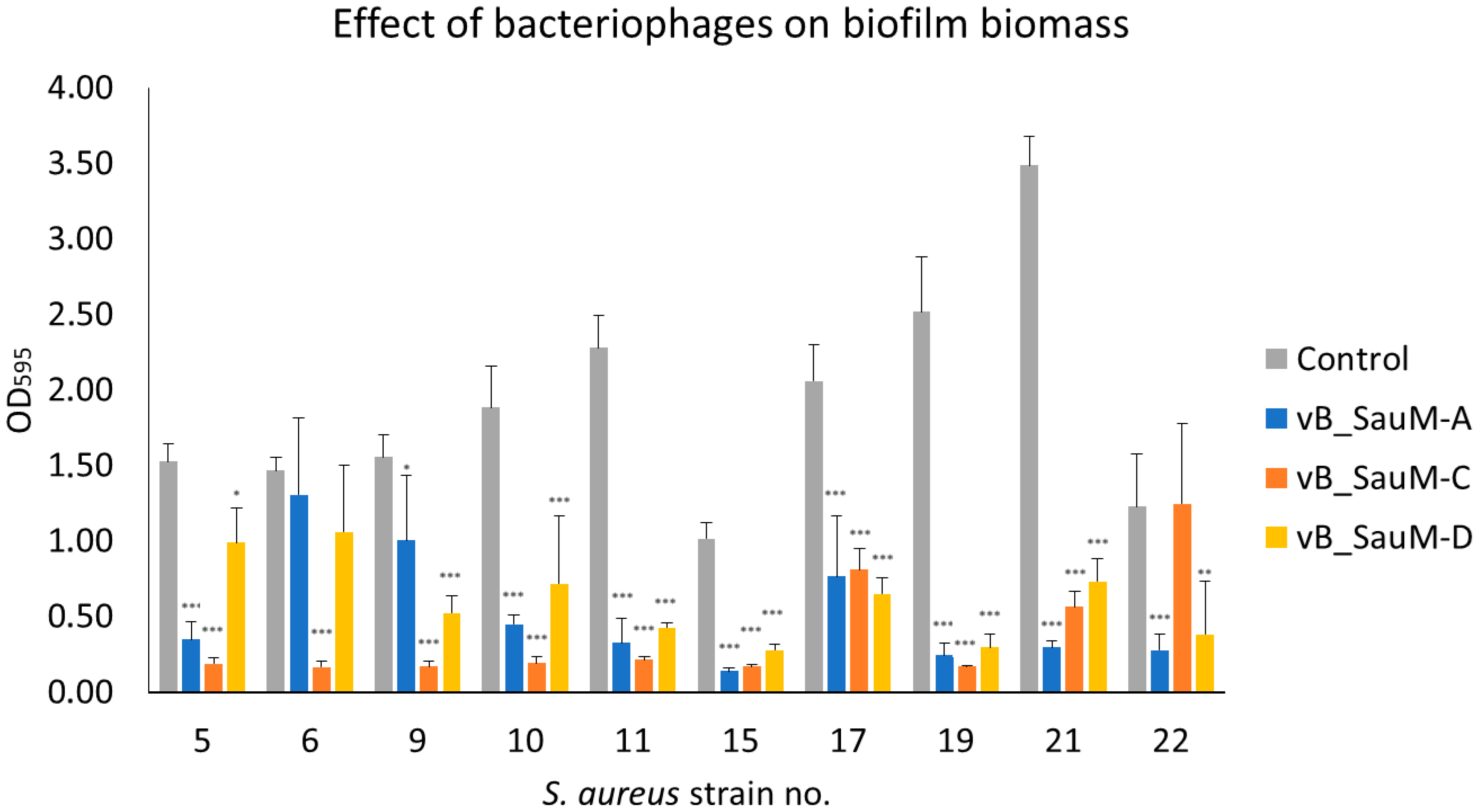
2.2.1. Biofilm Biomass Assessment Using Crystal Violet Staining
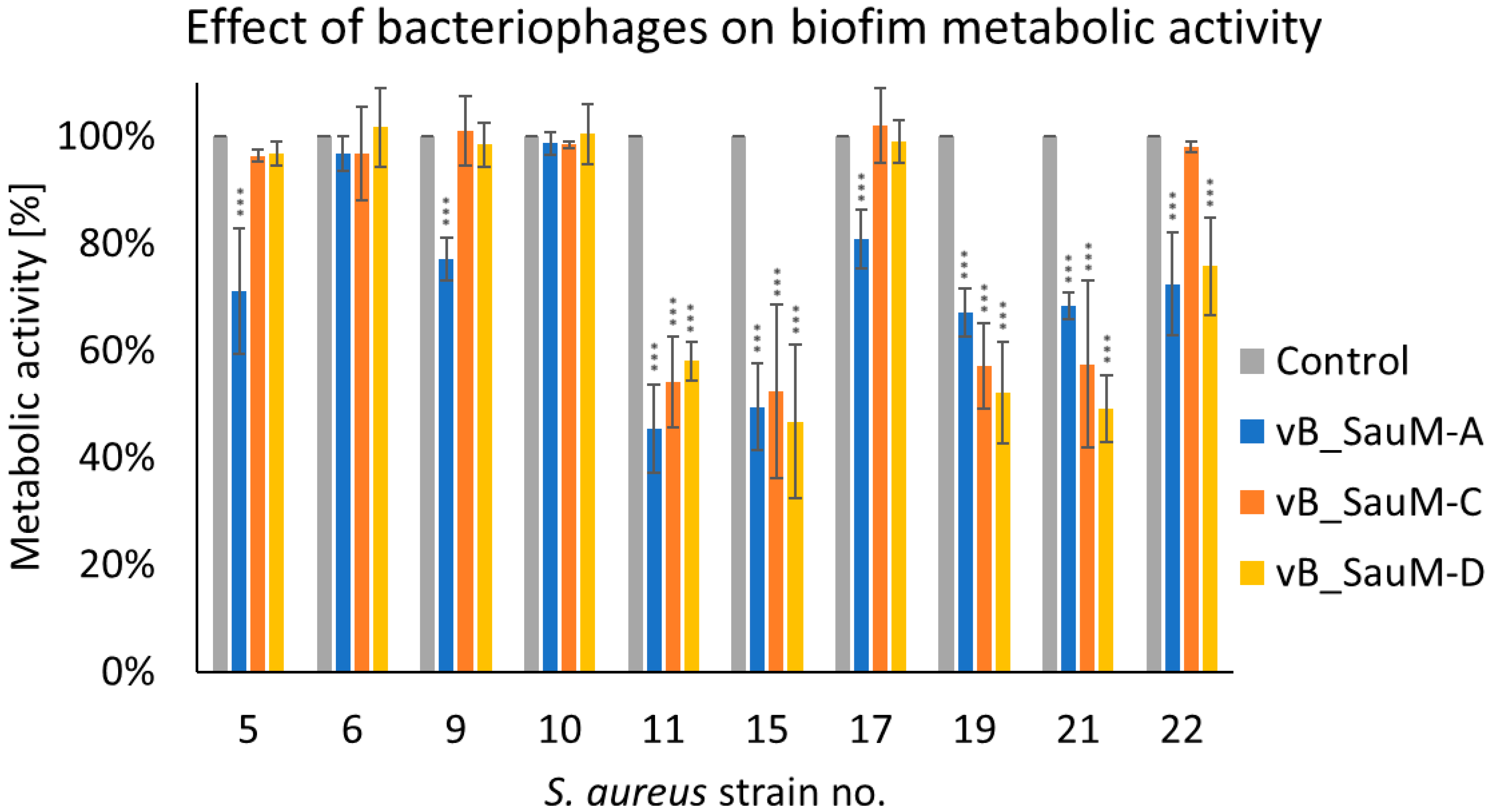
2.2.2. Assessment of Biofilm Metabolic Activity
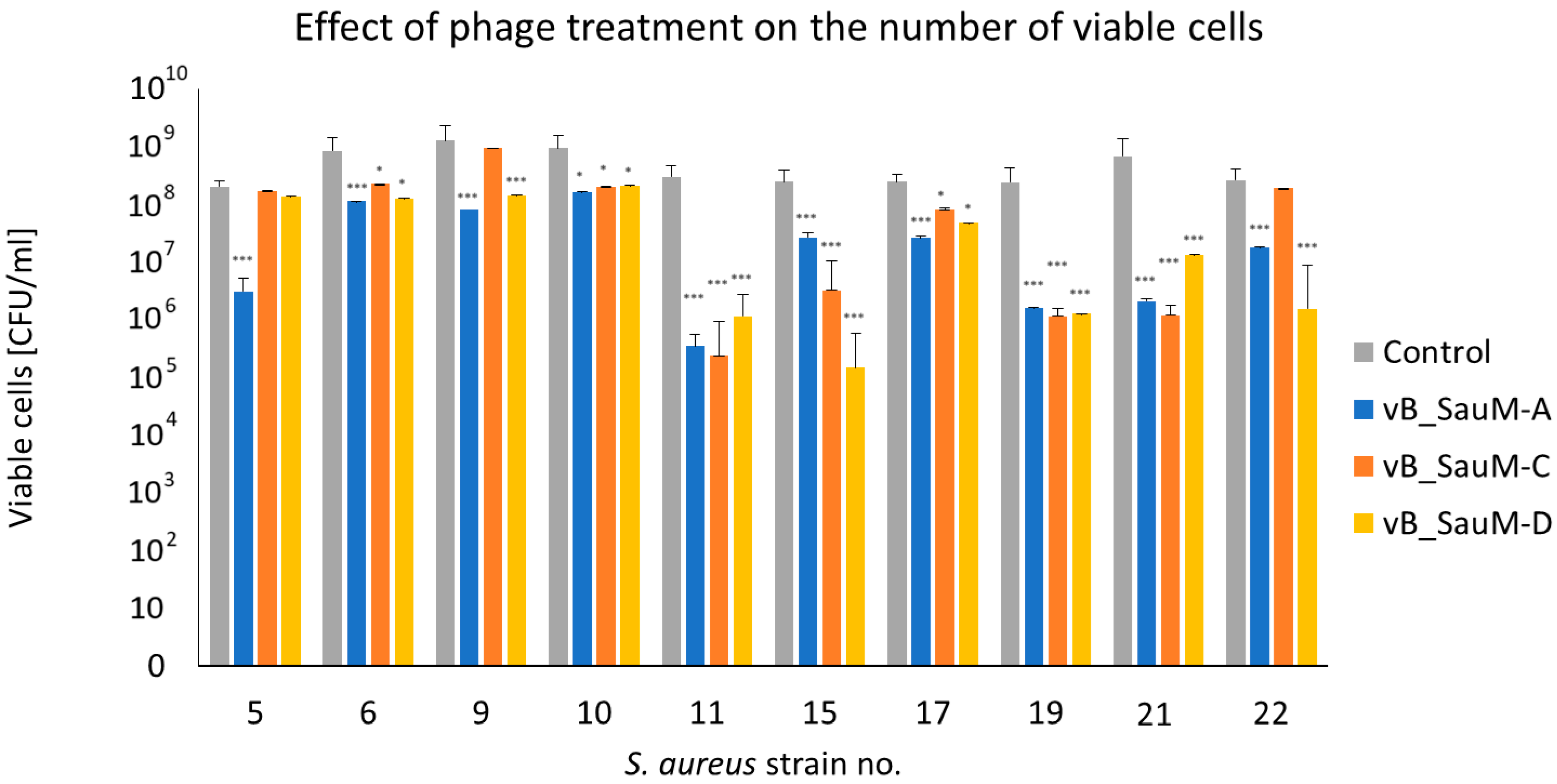
2.2.3. Assessment of Number of Viable Cells in Biofilm Using CFU/mL Count
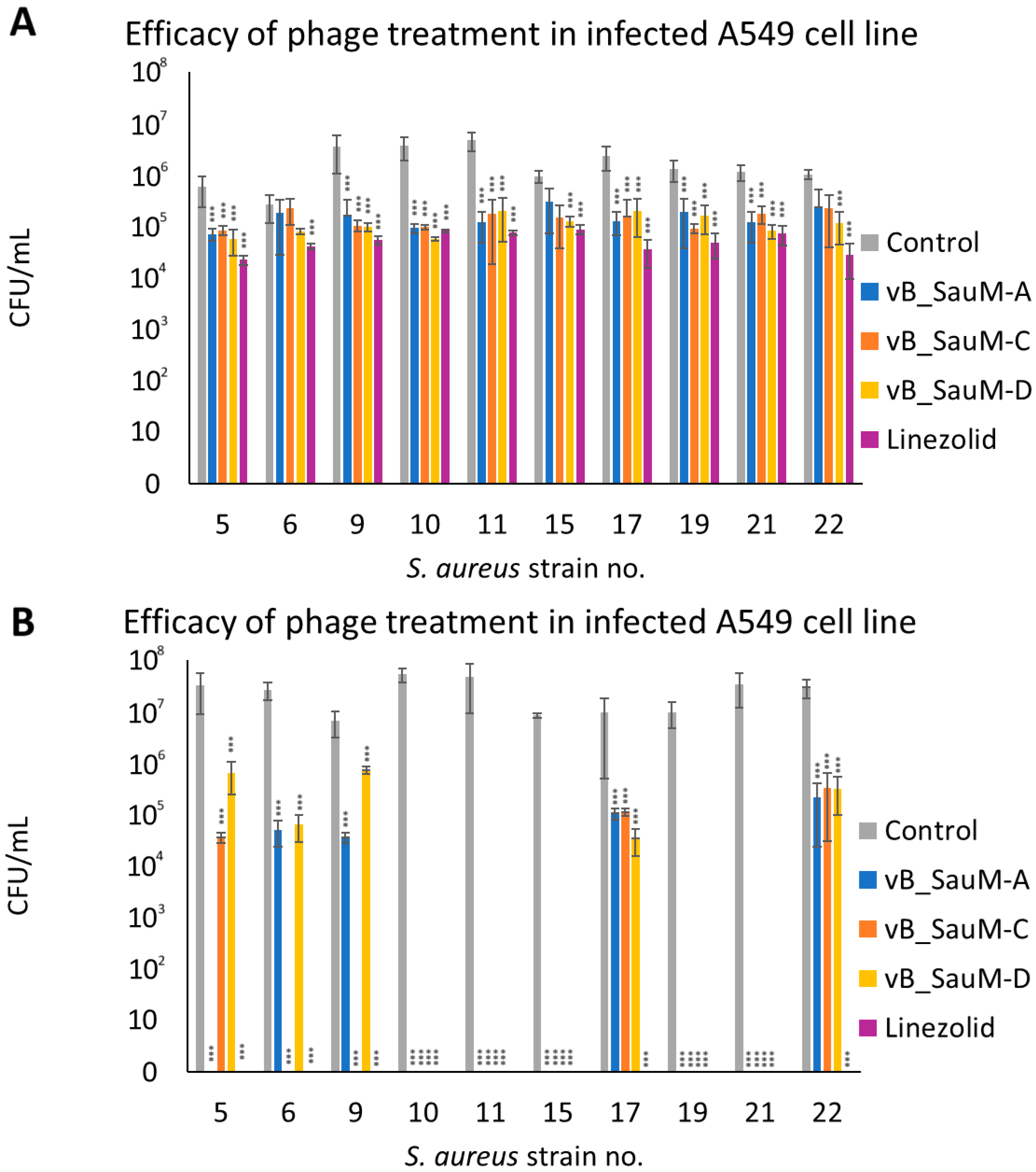
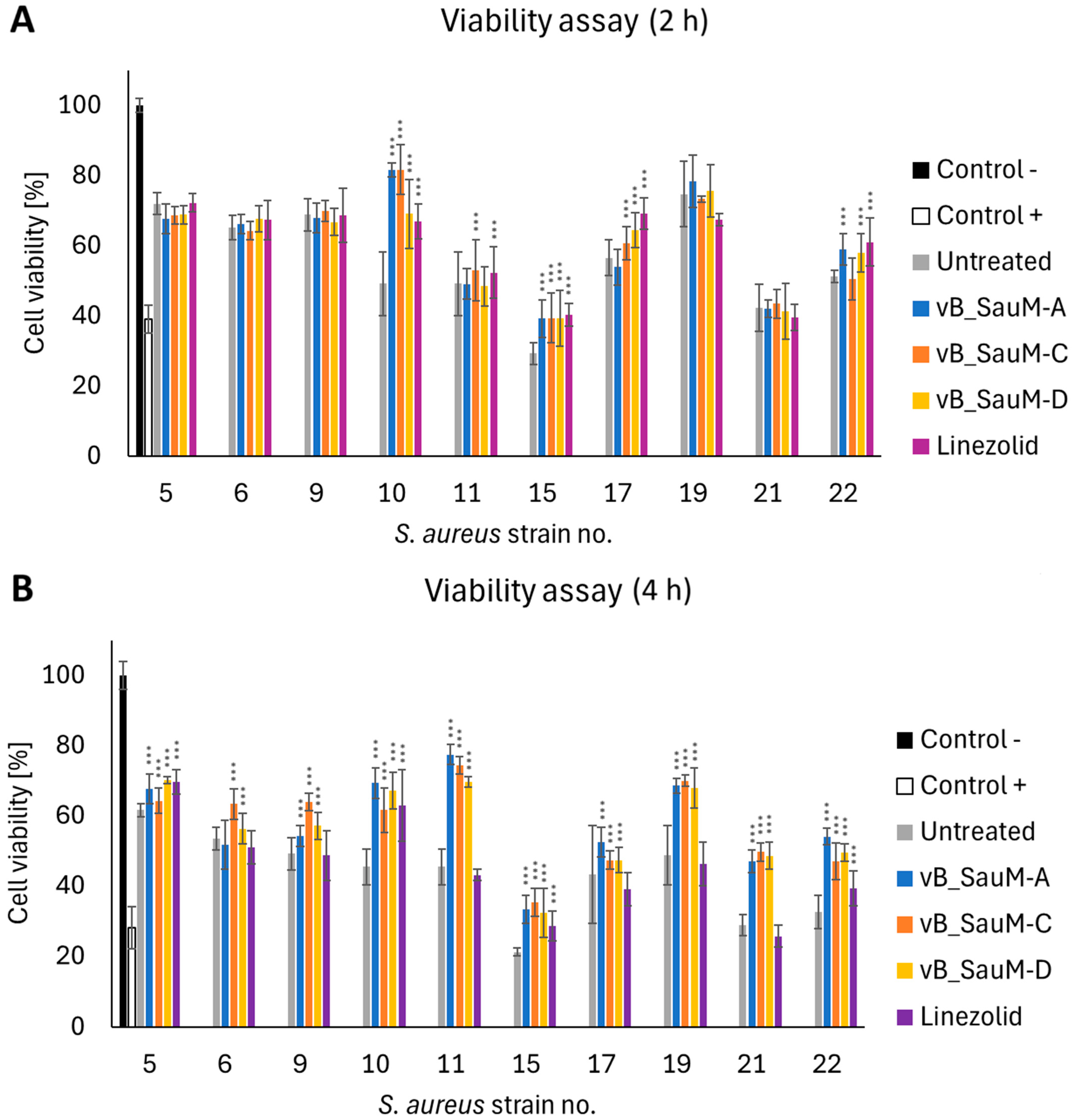
2.3. Analysis of Phage Treatment Efficacy of Infected Human Cell Line Using CFU/mL Count
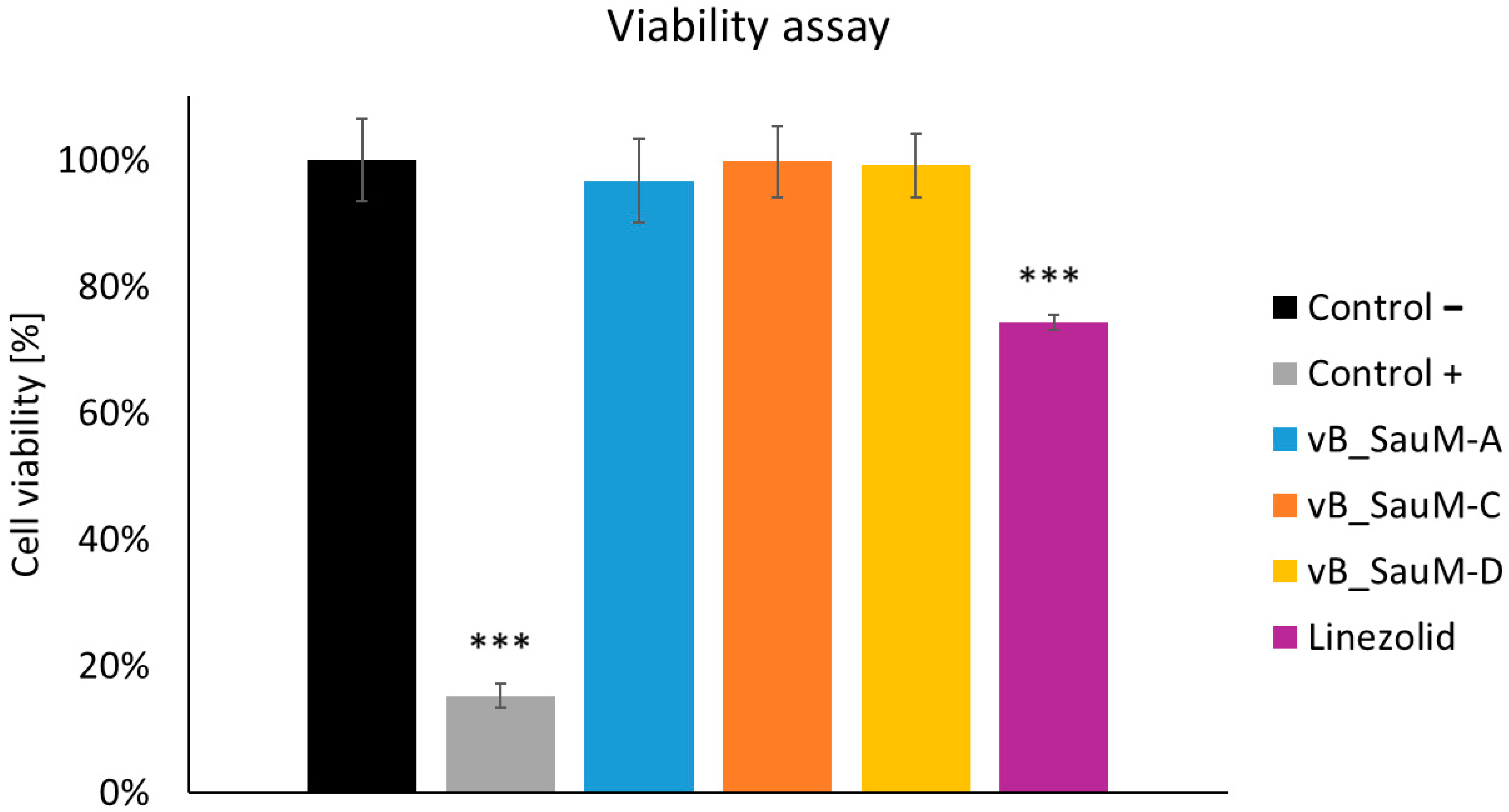
2.4. Analysis of Phage Treatment Influence on Viability of Human Cells Using Neutral Red Staining
3. Discussion
4. Materials and Methods
4.1. Staphylococcus Aureus Strains
4.2. Bacteriophages
4.3. Human Cell Line and Culture Conditions
4.4. Phage Host Range Analysis
4.5. Biofilm Eradication by Phages
4.5.1. Biofilm Biomass Assessment Using Crystal Violet Staining
4.5.2. Assessment of Biofilm Metabolic Activity
4.5.3. Assessment of Number of Viable Cells in Biofilm Using CFU/mL Count
4.6. Analysis of Phage Treatment Efficacy of Infected Human Cell Lines Using CFU/mL Count
4.7. Analysis of Phage Treatment Influence on Viability of Human Cells Using Neutral Red Staining
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patton, M.J.; Orihuela, C.J.; Harrod, K.S.; Bhuiyan, M.A.N.; Dominic, P.; Kevil, C.G.; Fort, D.; Liu, V.X.; Farhat, M.; Koff, J.L.; et al. COVID-19 Bacteremic Co-Infection Is a Major Risk Factor for Mortality, ICU Admission, and Mechanical Ventilation. Crit. Care 2023, 27, 34. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Coronavirus Disease 2019, Superinfections, and Antimicrobial Development: What Can We Expect? Clin. Infect. Dis. 2020, 71, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Liu, Y.H.; Wang, C.-Y.; Wang, Y.-H.; Hsueh, S.-C.; Yen, M.-Y.; Ko, W.-C.; Hsueh, P.-R. Asymptomatic Carrier State, Acute Respiratory Disease, and Pneumonia Due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Facts and Myths. J. Microbiol. Immunol. Infect. 2020, 53, 404–412. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus Infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Changchien, C.-H.; Chen, S.-W.; Chen, Y.-Y.; Chu, C. Antibiotic Susceptibility and Genomic Variations in Staphylococcus aureus Associated with Skin and Soft Tissue Infection (SSTI) Disease Groups. BMC Infect. Dis. 2016, 16, 276. [Google Scholar] [CrossRef]
- Kumaran, D.; Taha, M.; Yi, Q.; Ramirez-Arcos, S.; Diallo, J.-S.; Carli, A.; Abdelbary, H. Does Treatment Order Matter? Investigating the Ability of Bacteriophage to Augment Antibiotic Activity against Staphylococcus aureus Biofilms. Front. Microbiol. 2018, 9, 127. [Google Scholar] [CrossRef]
- Papastefan, S.T.; Buonpane, C.; Ares, G.; Benyamen, B.; Helenowski, I.; Hunter, C.J. Impact of Decolonization Protocols and Recurrence in Pediatric MRSA Skin and Soft-Tissue Infections. J. Surg. Res. 2019, 242, 70–77. [Google Scholar] [CrossRef]
- Clark, S.B.; Hicks, M.A. Staphylococcal Pneumonia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The Clinical Impact of Bacterial Biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef]
- Steele, A.; Stacey, H.J.; de Soir, S.; Jones, J.D. The Safety and Efficacy of Phage Therapy for Superficial Bacterial Infections: A Systematic Review. Antibiotics 2020, 9, 754. [Google Scholar] [CrossRef]
- Bichet, M.C.; Chin, W.H.; Richards, W.; Lin, Y.-W.; Avellaneda-Franco, L.; Hernandez, C.A.; Oddo, A.; Chernyavskiy, O.; Hilsenstein, V.; Neild, A.; et al. Bacteriophage Uptake by Mammalian Cell Layers Represents a Potential Sink That May Impact Phage Therapy. iScience 2021, 24, 102287. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Moreno, S.; Buttimer, C.; Bottacini, F.; Chanishvili, N.; Ross, P.; Hill, C.; Coffey, A. Insights into Gene Transcriptional Regulation of Kayvirus Bacteriophages Obtained from Therapeutic Mixtures. Viruses 2022, 14, 626. [Google Scholar] [CrossRef]
- Totten, K.M.C.; Cunningham, S.A.; Gades, N.M.; Etzioni, A.; Patel, R. Pharmacokinetic Assessment of Staphylococcal Phage K Following Parenteral and Intra-Articular Administration in Rabbits. Front. Pharmacol. 2022, 13, 840165. [Google Scholar] [CrossRef] [PubMed]
- Łubowska, N.; Grygorcewicz, B.; Kosznik-Kwaśnicka, K.; Zauszkiewicz-Pawlak, A.; Węgrzyn, A.; Dołęgowska, B.; Piechowicz, L. Characterization of the Three New Kayviruses and Their Lytic Activity Against Multidrug-Resistant Staphylococcus aureus. Microorganisms 2019, 7, 471. [Google Scholar] [CrossRef]
- McCallin, S.; Sarker, S.A.; Sultana, S.; Oechslin, F.; Brüssow, H. Metagenome Analysis of Russian and Georgian Pyophage Cocktails and a Placebo-Controlled Safety Trial of Single Phage versus Phage Cocktail in Healthy Staphylococcus aureus Carriers. Environ. Microbiol. 2018, 20, 3278–3293. [Google Scholar] [CrossRef]
- Botka, T.; Pantůček, R.; Mašlaňová, I.; Benešík, M.; Petráš, P.; Růžičková, V.; Havlíčková, P.; Varga, M.; Žemličková, H.; Koláčková, I.; et al. Lytic and Genomic Properties of Spontaneous Host-Range Kayvirus Mutants Prove Their Suitability for Upgrading Phage Therapeutics against Staphylococci. Sci. Rep. 2019, 9, 5475. [Google Scholar] [CrossRef] [PubMed]
- Kutter, E. Phage Host Range and Efficiency of Plating. In Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions; Clokie, M.R.J., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 141–149. ISBN 978-1-60327-164-6. [Google Scholar]
- Borgogna, T.R.; Hisey, B.; Heitmann, E.; Obar, J.J.; Meissner, N.; Voyich, J.M. Secondary Bacterial Pneumonia by Staphylococcus aureus Following Influenza A Infection Is SaeR/S Dependent. J. Infect. Dis. 2018, 218, 809–813. [Google Scholar] [CrossRef]
- Adelman, M.W.; Bhamidipati, D.R.; Hernandez-Romieu, A.C.; Babiker, A.; Woodworth, M.H.; Robichaux, C.; Murphy, D.J.; Auld, S.C.; Kraft, C.S.; Jacob, J.T.; et al. Secondary Bacterial Pneumonias and Bloodstream Infections in Patients Hospitalized with COVID-19. Ann. Am. Thorac. Soc. 2021, 18, 1584–1587. [Google Scholar] [CrossRef]
- Westblade, L.F.; Simon, M.S.; Satlin, M.J. Bacterial Coinfections in Coronavirus Disease 2019. Trends Microbiol. 2021, 29, 930–941. [Google Scholar] [CrossRef]
- Scott, H.; Zahra, A.; Fernandes, R.; Fries, B.C.; Thode, H.C.; Singer, A.J. Bacterial Infections and Death among Patients with Covid-19 versus Non Covid-19 Patients with Pneumonia. Am. J. Emerg. Med. 2022, 51, 1–5. [Google Scholar] [CrossRef]
- Losier, A.; Gupta, G.; Caldararo, M.; Dela Cruz, C.S. The Impact of Coronavirus Disease 2019 on Viral, Bacterial, and Fungal Respiratory Infections. Clin. Chest Med. 2023, 44, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Adalbert, J.R.; Varshney, K.; Tobin, R.; Pajaro, R. Clinical Outcomes in Patients Co-Infected with COVID-19 and Staphylococcus aureus: A Scoping Review. BMC Infect. Dis. 2021, 21, 985. [Google Scholar] [CrossRef] [PubMed]
- Arientová, S.; Jícha, Z.; Beran, O.; Holub, M. Decreased Quality of Care for Staphylococcus aureus Bacteremia during the COVID-19 Pandemic. BMC Infect. Dis. 2022, 22, 631. [Google Scholar] [CrossRef]
- Böing, C.W.; Froböse, N.J.; Schaumburg, F.; Kampmeier, S. Impact of the COVID-19 Pandemic on the Management of Staphylococcus aureus Bloodstream Infections in a Tertiary Care Hospital. Pathogens 2023, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.A.; Banerji, A.; Bernstein, J.A.; Bilgicer, B.; Blumenthal, K.; Castells, M.; Ein, D.; Lang, D.M.; Phillips, E. Cephalosporin Allergy: Current Understanding and Future Challenges. J. Allergy Clin. Immunol. Pract. 2019, 7, 2105–2114. [Google Scholar] [CrossRef]
- Kaminsky, L.W.; Dalessio, S.; Al-Shaikhly, T.; Al-Sadi, R. Penicillin Allergy Label Increases Risk of Worse Clinical Outcomes in COVID-19. J. Allergy Clin. Immunol. Pract. 2021, 9, 3629–3637.e2. [Google Scholar] [CrossRef]
- Rose, M.; Trubiano, J. Assessing Low-Risk Penicillin Allergies in Critical COVID-19, A Novel Perspective on an Emerging Antibiotic Allergy Opportunity. J. Allergy Clin. Immunol. Pract. 2023, 11, 636–637. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin Resistant Staphylococcus aureus Infections: A Review of Case Updating and Clinical Features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef]
- Braun, J.; Eckes, S.; Rommens, P.M.; Schmitz, K.; Nickel, D.; Ritz, U. Toxic Effect of Vancomycin on Viability and Functionality of Different Cells Involved in Tissue Regeneration. Antibiotics 2020, 9, 238. [Google Scholar] [CrossRef]
- Mayr, H.O.; Regenbrecht, N.; Mayr, M.F.; Riedel, B.; Hart, M.L.; Schmal, H.; Seidenstuecker, M. Effect of Vancomycin, Gentamicin and Clindamycin on Cartilage Cells In Vitro. Biomedicines 2023, 11, 3143. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Z.; Zhao, J.; Zhu, Z.; Yang, C.; Liu, Y. Prospects of Inhaled Phage Therapy for Combatting Pulmonary Infections. Front. Cell. Infect. Microbiol. 2021, 11, 758392. [Google Scholar] [CrossRef]
- Köhler, T.; Luscher, A.; Falconnet, L.; Resch, G.; McBride, R.; Mai, Q.-A.; Simonin, J.L.; Chanson, M.; Maco, B.; Galiotto, R.; et al. Personalized Aerosolised Bacteriophage Treatment of a Chronic Lung Infection Due to Multidrug-Resistant Pseudomonas Aeruginosa. Nat. Commun. 2023, 14, 3629. [Google Scholar] [CrossRef]
- Kaźmierczak, N.; Grygorcewicz, B.; Roszak, M.; Bochentyn, B.; Piechowicz, L. Comparative Assessment of Bacteriophage and Antibiotic Activity against Multidrug-Resistant Staphylococcus aureus Biofilms. Int. J. Mol. Sci. 2022, 23, 1274. [Google Scholar] [CrossRef] [PubMed]
- Piechowicz, L.; Kosznik-Kwaśnicka, K.; Jarzembowski, T.; Daca, A.; Necel, A.; Bonawenturczak, A.; Werbowy, O.; Stasiłojć, M.; Pałubicka, A. Staphylococcus aureus Co-Infection in COVID-19 Patients: Virulence Genes and Their Influence on Respiratory Epithelial Cells in Light of Risk of Severe Secondary Infection. Int. J. Mol. Sci. 2024, 25, 10050. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Ramachandran, A.; Thanki, A.M.; Vukusic, F.B.I.; Barylski, J.; Clokie, M.R.J. Bacteriophages Are More Virulent to Bacteria with Human Cells than They Are in Bacterial Culture; Insights from HT-29 Cells. Sci. Rep. 2018, 8, 5091. [Google Scholar] [CrossRef] [PubMed]
- Kosznik-Kwaśnicka, K.; Stasiłojć, M.; Stasiłojć, G.; Kaźmierczak, N.; Piechowicz, L. The Influence of Bacteriophages on the Metabolic Condition of Human Fibroblasts in Light of the Safety of Phage Therapy in Staphylococcal Skin Infections. Int. J. Mol. Sci. 2023, 24, 5961. [Google Scholar] [CrossRef]
- Goh, S.; Chang, B.J.; Riley, T.V. Effect of Phage Infection on Toxin Production by Clostridium Difficile. J. Med. Microbiol. 2005, 54, 129–135. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Animal and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Kaźmierczak, N.; Grygorcewicz, B.; Piechowicz, L. Biofilm Formation and Prevalence of Biofilm-Related Genes Among Clinical Strains of Multidrug-Resistant Staphylococcus aureus. Microb. Drug Resist. 2021, 27, 956–964. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral Red Uptake Assay for the Estimation of Cell Viability/Cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
| S. aureus Strain | Mean Phage Titer and SD | |||||
|---|---|---|---|---|---|---|
| vB_SauM-A | vB_SauM-C | vB_SauM-D | ||||
| Host strain | 3.00 × 1010 | - | 2.00 × 1010 | - | 2.60 × 1010 | - |
| COVID-19 1 | - | - | - | - | - | - |
| COVID-19 2 | - | - | 4.00 × 107 | ±1.74 × 107 | 5.33 × 108 | ±1.22 × 108 |
| COVID-19 3 | - | - | - | - | 5.60 × 107 | ±4.21 × 107 |
| COVID-19 4 | - | - | - | - | 7.33 × 108 | ±4.64 × 108 |
| COVID-19 5 | 4.48 × 1011 | ±2.95 × 1011 | 6.93 × 109 | ±3.35 × 109 | 1.76 × 109 | ±1.18 × 109 |
| COVID-19 6 | 1.34 × 109 | ±6.02 × 108 | 1.39 × 109 | ±9.10 × 108 | 3.60 × 109 | ±3.47 × 10 |
| COVID-19 7 | - | - | - | - | - | - |
| COVID-19 8 | - | - | - | - | - | - |
| COVID-19 9 | 1.58 × 1011 | ±1.40 × 1011 | 2.93 × 109 | ±1.97 × 109 | 3.07 × 109 | ±4.62 × 108 |
| COVID-19 10 | 1.53 × 1011 | ±1.48 × 1011 | 2.61 × 109 | ±2.60 × 109 | 4.31 × 109 | ±3.28 × 109 |
| COVID-19 11 | 1.54 × 1011 | ±1.32 × 1011 | 3.07 × 109 | ±4.62 × 108 | 1.80 × 109 | ±3.12 × 108 |
| COVID-19 12 | - | - | - | - | - | - |
| COVID-19 13 | - | - | 1.47 × 109 | ±8.33 × 108 | - | - |
| COVID-19 14 | - | - | - | - | - | - |
| COVID-19 15 | 1.27 × 1011 | ±1.12 × 1011 | 4.95 × 108 | ±4.72 × 108 | 1.48 × 109 | ±1.86 × 109 |
| COVID-19 16 | - | - | - | - | - | - |
| COVID-19 17 | 7.69 × 1010 | ±9.27 × 1010 | 9.67 × 108 | ± | - | - |
| COVID-19 18 | 8.41 × 1010 | ±7.39 × 1010 | - | - | 1.43 × 109 | ±1.58 × 109 |
| COVID-19 19 | 9.35 × 1010 | ±8.22 × 1010 | 9.55 × 108 | ±1.60 × 109 | 1.01 × 109 | ±1.21 × 109 |
| COVID-19 20 | 1.12 × 109 | ±5.25 × 108 | - | - | - | - |
| COVID-19 21 | 5.61 × 1010 | ±5.09 × 1010 | 4.93 × 108 | ±2.60 × 108 | 6.00 × 108 | ±2.40 × 108 |
| COVID-19 22 | 1.44 × 1011 | ±1.24 × 1011 | 6.53 × 108 | ±2.44 × 108 | 1.05 × 109 | ±3.72 × 108 |
| COVID-19 23 | - | - | 4.59 × 108 | ±7.46 × 108 | 3.17 × 108 | ±1.09 × 108 |
| COVID-19 24 | - | - | - | - | - | - |
| COVID-19 25 | - | - | - | - | - | - |
| COVID-19 26 | - | - | 4.79 × 108 | ±7.29 × 108 | - | - |
| S. aureus Strain | EOP with SD | ||
|---|---|---|---|
| vB_SauM-A | vB_SauM-C | vB_SauM-D | |
| Host strain | 100% | 100% | 100% |
| COVID-19 2 | - | 0.20 ± 0.09% | 2.05 ± 0.47% |
| COVID-19 3 | - | - | 0.22 ± 0.16% |
| COVID-19 4 | - | - | 2.82 ± 1.78% |
| COVID-19 5 | 1493.33 ± 66.85% | 34.67 ± 16.77% | 6.77 ± 4.53% |
| COVID-19 6 | 4.47 ± 0.013% | 6.93 ± 4.55% | 13.85 ± 3.35% |
| COVID-19 9 | 525.33 ± 31.36% | 14.67 ± 9.87% | 11.79 ± 1.78% |
| COVID-19 10 | 511.33 ± 33.08% | 13.07 ± 12.99% | 16.56 ± 2.63% |
| COVID-19 11 | 512.69 ± 29.46% | 15.33 ± 2.31% | 6.92 ± 1.20% |
| COVID-19 13 | - | 7.33 ± 4.16% | - |
| COVID-19 15 | 423.22 ± 20.05% | 2.47 ± 1.36% | 5.68 ± 0.17% |
| COVID-19 17 | 256.20 ± 20.69% | 4.83 ± 0.94% | - |
| COVID-19 18 | 280.33 ± 16.50% | - | 5.49 ± 6.08% |
| COVID-19 19 | 311.56 ± 18.34% | 4.77 ± 0.99% | 3.89 ± 0.66% |
| COVID-19 20 | 3.73 ± 0.12% | - | - |
| COVID-19 21 | 186.91 ± 11.27% | 2.47 ± 1.30% | 2.31 ± 0.92% |
| COVID-19 22 | 481.58 ± 27.71% | 3.27 ± 1.22% | 4.05 ± 1.43% |
| COVID-19 23 | - | 2.29 ± 0.73% | 1.22 ± 0.42% |
| COVID-19 26 | - | 2.39 ± 0.64% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piechowicz, L.; Kosznik-Kwaśnicka, K.; Kaźmierczak, N.; Grzenkowicz, M.; Stasiłojć, M.; Necel, A.; Werbowy, O.; Pałubicka, A. Efficacy of Three Kayviruses Against Staphylococcus aureus Strains Isolated from COVID-19 Patients. Antibiotics 2025, 14, 257. https://doi.org/10.3390/antibiotics14030257
Piechowicz L, Kosznik-Kwaśnicka K, Kaźmierczak N, Grzenkowicz M, Stasiłojć M, Necel A, Werbowy O, Pałubicka A. Efficacy of Three Kayviruses Against Staphylococcus aureus Strains Isolated from COVID-19 Patients. Antibiotics. 2025; 14(3):257. https://doi.org/10.3390/antibiotics14030257
Chicago/Turabian StylePiechowicz, Lidia, Katarzyna Kosznik-Kwaśnicka, Natalia Kaźmierczak, Milena Grzenkowicz, Małgorzata Stasiłojć, Agnieszka Necel, Olesia Werbowy, and Anna Pałubicka. 2025. "Efficacy of Three Kayviruses Against Staphylococcus aureus Strains Isolated from COVID-19 Patients" Antibiotics 14, no. 3: 257. https://doi.org/10.3390/antibiotics14030257
APA StylePiechowicz, L., Kosznik-Kwaśnicka, K., Kaźmierczak, N., Grzenkowicz, M., Stasiłojć, M., Necel, A., Werbowy, O., & Pałubicka, A. (2025). Efficacy of Three Kayviruses Against Staphylococcus aureus Strains Isolated from COVID-19 Patients. Antibiotics, 14(3), 257. https://doi.org/10.3390/antibiotics14030257







