A Snapshot of Antimicrobial Resistance in Semi-Wild Oryx: Baseline Data from Qatar
Abstract
1. Introduction
2. Results
2.1. Demographic Data
2.2. Detection of Phenotypically Antimicrobial Resistant E. coli
2.3. Screening Genetic Determinants of Resistance Using AMR Gene-Specific PCR Analyses
2.4. Whole Genome Sequencing Using the Oxford Nanopore Technology (ONT)
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Escherichia coli Isolation and Identification
4.3. Phenotypic Antibiotic Susceptibility Testing (AST)
4.4. WGS Using Oxford Nanopore Technology (ONT)
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Resistance Phenotype | Frequency | Percentage (%) | Multiple Antimicrobial Resistance Indices (MARI) |
|---|---|---|---|
| CIP; TE | 3 | 3 | 0.14 |
| AMP; TE | 4 | 4 | 0.14 |
| * AMP; SXT; CTX; TE | 1 | 1 | 0.26 |
| AMP; SXT | 1 | 1 | 0.14 |
| * AMP; SXT; TE | 1 | 1 | 0.2 |
| No resistance | 72 | 72 | Not applicable |
| Resistant to only one antibiotic | 18 | 18 | Not applicable |
| Antibiotic | Target Gene | Primer Sequence (5′–3′) | PCR Fragment Size (bp) | References |
|---|---|---|---|---|
| Tetracycline (TE) | tetA | F: GCT ACA TCC TGC TTG CCT TC | 210 | [36] |
| R: CAT AGA TCG CCG TGA AGA GG | ||||
| tetB | F: TTG GTT AGG GGC AAG TTT TG | 659 | [36] | |
| R: GTA ATG GGC CAA TAA CAC CG | ||||
| tetC | F: CTT GAG AGC CTT CAA CCC AG | 418 | [37] | |
| R: ATG GTC GTC ATC TAC CTG CC | ||||
| tetE | F: AAA CCA CAT CCT CCA TAC GC | 278 | [37] | |
| R: AAA TAG GCC ACA ACC GTC AG | ||||
| Ampicillin (AMP) | blaTEM-1 | F: TCG CCG CAT ACA CTA TTC TCA GAA TGA | 431 | [38] |
| R: CTG ACT CCC CGT CGT GTA GAT A | ||||
| blaCTX-M | F: ACG CTC ACC GGC TCC AGA TTT AT | 688 | [39] | |
| R: CGATATCGTTGGTGGTGCCATA | ||||
| blaSHV | F: GGG TTA TTC TTA TTT GTC GCT | 567 | [38] | |
| R: TTAGCGTTGCCAAGTGCTC |
| Target Gene | PCR Conditions (Temperature/Duration) | ||||
|---|---|---|---|---|---|
| Initial Denaturation | Denaturation | Annealing | Extension | Final Extension | |
| tetA | 95 °C for 5 min | 94 °C for 1 min | 55 °C for 1 min | 72 °C for 1 min (35 cycles) | 72 °C for 5 min |
| tetB | 95 °C for 5 min | 94 °C for 1 min | 55 °C for 1 min | 72 °C for 1 min (35 cycles) | 72 °C for 5 min |
| tetC | 95 °C for 5 min | 94 °C for 1 min | 55 °C for 1 min | 72 °C for 1 min (35 cycles) | 72 °C for 5 min |
| tetE | 95 °C for 5 min | 94 °C for 1 min | 55 °C for 1 min | 72 °C for 1 min (35 cycles) | 72 °C for 5 min |
| blaTEM-1 | 96 °C for 5 min | 96 °C for 30 s | 44 °C for 45 s | 72 °C for 60 s (35 cycles) | 72 °C for 10 min |
| blaCTX-M | 95 °C for 5 min | 94 °C for 30 s | 60 °C for 40 s | 72 °C for 1 min (30 cycles) | 72 °C for 7 min |
| blaSHV | 95 °C for 5 min | 94 °C for 1 min | 58 °C for 1 min | 72 °C for 1 min (35 cycles) | 72 °C for 5 min |
References
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Salam, M.d.A.; Al-Amin, M.d.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Skarżyńska, M.; Leekitcharoenphon, P.; Hendriksen, R.S.; Aarestrup, F.M.; Wasyl, D. A Metagenomic Glimpse into the Gut of Wild and Domestic Animals: Quantification of Antimicrobial Resistance and More. PLoS ONE 2020, 15, e0242987. [Google Scholar] [CrossRef]
- Dolejska, M.; Literak, I. Wildlife Is Overlooked in the Epidemiology of Medically Important Antibiotic-Resistant Bacteria. Antimicrob. Agents Chemother. 2019, 63, e01167-19. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadidi, S.H.; Al mana, H.; Almoghrabi, S.Z.; El-Obeid, T.; AlAli, W.Q.; Eltai, N.O. Retail Chicken Carcasses as a Reservoir of Multidrug-Resistant Salmonella. Microb. Drug Resist. 2022, 28, 824–831. [Google Scholar] [CrossRef]
- Allen, S.E.; Boerlin, P.; Janecko, N.; Lumsden, J.S.; Barker, I.K.; Pearl, D.L.; Reid-Smith, R.J.; Jardine, C. Antimicrobial Resistance in Generic Escherichia coli Isolates from Wild Small Mammals Living in Swine Farm, Residential, Landfill, and Natural Environments in Southern Ontario, Canada. Appl. Env. Microbiol. 2011, 77, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Dagher, L.A.; Hassan, J.; Kharroubi, S.; Jaafar, H.; Kassem, I.I. Nationwide Assessment of Water Quality in Rivers across Lebanon by Quantifying Fecal Indicators Densities and Profiling Antibiotic Resistance of Escherichia coli. Antibiotics 2021, 10, 883. [Google Scholar] [CrossRef]
- Hassan, J.; Osman, M.; Xu, T.; Naas, T.; Schiff, S.J.; Mann, D.; Esseili, M.A.; Deng, X.; Kassem, I.I. Monitoring Sewage and Effluent Water Is an Effective Approach for the Detection of the Mobile Colistin Resistance Genes (Mcr) and Associated Bacterial Hosts in the Human Population and Environment in the USA. Environ. Pollut. 2025, 366, 125515. [Google Scholar] [CrossRef]
- Kassem, I.I. Chinks in the Armor: The Role of the Nonclinical Environment in the Transmission of Staphylococcus Bacteria. Am. J. Infect. Control 2011, 39, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Ewers, C.; Wieler, L.H. Extended-Spectrum Beta-Lactamases Producing E. coli in Wildlife, yet Another Form of Environmental Pollution? Front. Microbiol. 2011, 2, 246. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 2: Management Strategies and New Agents. Pharm. Ther. 2015, 40, 344–352. [Google Scholar]
- Arnold, K.E.; Williams, N.J.; Bennett, M. ‘Disperse Abroad in the Land’: The Role of Wildlife in the Dissemination of Antimicrobial Resistance. Biol. Lett. 2016, 12, 20160137. [Google Scholar] [CrossRef]
- Esposito, E.; Scarpellini, R.; Celli, G.; Marliani, G.; Zaghini, A.; Mondo, E.; Rossi, G.; Piva, S. Wild Birds as Potential Bioindicators of Environmental Antimicrobial Resistance: A Preliminary Investigation. Res. Vet. Sci. 2024, 180, 105424. [Google Scholar] [CrossRef]
- Bourdonnais, E.; Le Bris, C.; Brauge, T.; Midelet, G. Tracking Antimicrobial Resistance Indicator Genes in Wild Flatfish from the English Channel and the North Sea Area: A One Health Concern. Environ. Pollut. 2024, 343, 123274. [Google Scholar] [CrossRef]
- Tallon, A.K.; Smith, R.K.; Rush, S.; Naveda-Rodriguez, A.; Brooks, J.P. The Role of New World Vultures as Carriers of Environmental Antimicrobial Resistance. BMC Microbiol. 2024, 24, 487. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Hu, Y.; Liu, F.; Wang, Y.; Bi, Y.; Lv, N.; Li, J.; Zhu, B.; Gao, G.F. Metagenomic Analysis Reveals the Microbiome and Resistome in Migratory Birds. Microbiome 2020, 8, 26. [Google Scholar] [CrossRef]
- Osińska, M.; Nowakiewicz, A.; Zięba, P.; Gnat, S.; Łagowski, D.; Trościańczyk, A. A Rich Mosaic of Resistance in Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolated from Red Foxes (Vulpes Vulpes) in Poland as a Potential Effect of Increasing Synanthropization. Sci. Total Environ. 2022, 818, 151834. [Google Scholar] [CrossRef] [PubMed]
- Kassem, I.I.; Nasser, N.A.; Salibi, J. Prevalence and Loads of Fecal Pollution Indicators and the Antibiotic Resistance Phenotypes of Escherichia coli in Raw Minced Beef in Lebanon. Foods 2020, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, Animal and Environmental Contributors to Antibiotic Resistance in Low-Resource Settings: Integrating Behavioural, Epidemiological and One Health Approaches. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180332. [Google Scholar] [CrossRef]
- Kümmerer, K.; Henninger, A. Promoting Resistance by the Emission of Antibiotics from Hospitals and Households into Effluent. Clin. Microbiol. Infect. 2003, 9, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Atterby, C.; Börjesson, S.; Ny, S.; Järhult, J.D.; Byfors, S.; Bonnedahl, J. ESBL-Producing Escherichia coli in Swedish Gulls—A Case of Environmental Pollution from Humans? PLoS ONE 2017, 12, e0190380. [Google Scholar] [CrossRef]
- Radhouani, H.; Silva, N.; Poeta, P.; Torres, C.; Correia, S.; Igrejas, G. Potential Impact of Antimicrobial Resistance in Wildlife, Environment and Human Health. Front. Microbiol. 2014, 5, 23. [Google Scholar] [CrossRef]
- Qatar e-Nature Qatar E-Nature—Arabian Oryx, White Oryx. Available online: https://www.enature.qa/specie/arabian-oryx-white-oryx/ (accessed on 25 December 2024).
- Julien Meet The National Animal of Qatar: The Arabian Oryx. Available online: https://www.explorationjunkie.com/qatar-national-animal/ (accessed on 14 December 2024).
- Leo Arabian Oryx—Antelope IUCN. Antelope IUCN. Available online: https://antelopesg.org/arabian-oryx/ (accessed on 25 December 2024).
- WHO Qatar: National Antimicrobial Resistance Action Plan 2024–2030. Available online: https://www.who.int/publications/m/item/qatar--national-antimicrobial-resistance-action-plan-2024-2030 (accessed on 14 December 2024).
- Fernandes, R.; Abreu, R.; Serrano, I.; Such, R.; Garcia-Vila, E.; Quirós, S.; Cunha, E.; Tavares, L.; Oliveira, M. Resistant Escherichia coli Isolated from Wild Mammals from Two Rescue and Rehabilitation Centers in Costa Rica: Characterization and Public Health Relevance. Sci. Rep. 2024, 14, 8039. [Google Scholar] [CrossRef] [PubMed]
- Alhababi, D.A.; Eltai, N.O.; Nasrallah, G.K.; Farg, E.A.; Al Thani, A.A.; Yassine, H.M. Antimicrobial Resistance of Commensal Escherichia coli Isolated from Food Animals in Qatar. Microb. Drug Resist. 2020, 26, 420–427. [Google Scholar] [CrossRef]
- Erkorkmaz, B.A.; Zeevi, D.; Rudich, Y. Dust Storm-Driven Dispersal of Potential Pathogens and Antibiotic Resistance Genes in the Eastern Mediterranean. Sci. Total Environ. 2025, 958, 178021. [Google Scholar] [CrossRef]
- Gens, K.D.; Singer, R.S.; Dilworth, T.J.; Heil, E.L.; Beaudoin, A.L. Antimicrobials in Animal Agriculture in the United States: A Multidisciplinary Overview of Regulation and Utilization to Foster Collaboration: On Behalf Of the Society of Infectious Diseases Pharmacists. Open Forum Infect. Dis. 2022, 9, ofac542. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Eltai, N.; Al Thani, A.A.; Al-Hadidi, S.H.; Abdfarag, E.A.; Al-Romaihi, H.; Mahmoud, M.H.; Alawad, O.K.; Yassine, H.M. Antibiotic Resistance Profile of Commensal Escherichia coli Isolated from Healthy Sheep in Qatar. J. Infect. Dev. Ctries. 2020, 14, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.P. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; ISBN 9781684400324. [Google Scholar]
- Woh, P.Y.; Yeung, M.P.S.; Goggins, W.B. Multiple Antibiotic Resistance Index (MARI) of Human-Isolated Salmonella Species: A Practical Bacterial Antibiotic Surveillance Tool. J. Antimicrob. Chemother. 2023, 78, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded Curation, Support for Machine Learning, and Resistome Prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids. Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Ramírez-Bayard, I.E.; Mejía, F.; Medina-Sánchez, J.R.; Cornejo-Reyes, H.; Castillo, M.; Querol-Audi, J.; Martínez-Torres, A.O. Prevalence of Plasmid-Associated Tetracycline Resistance Genes in Multidrug-Resistant Escherichia coli Strains Isolated from Environmental, Animal and Human Samples in Panama. Antibiotics 2023, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; He, X.; Bu, Y.; Shi, P.; Miao, Y.; Zhou, H.; Shan, Z.; Zhang, X.-X. Environmental Fate of Tetracycline Resistance Genes Originating from Swine Feedlots in River Water. J. Environ. Sci. Health Part B 2014, 49, 624–631. [Google Scholar] [CrossRef]
- Eltai, N.O.; Al Thani, A.A.; Al-Ansari, K.; Deshmukh, A.S.; Wehedy, E.; Al-Hadidi, S.H.; Yassine, H.M. Molecular Characterization of Extended Spectrum β-Lactamases Enterobacteriaceae Causing Lower Urinary Tract Infection among Pediatric Population. Antimicrob. Resist. Infect. Control 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, M.C.; Shariff, M.; Nnajide, C.M.; Beri, K.; Okezie, U.M.; Iroha, I.R.; Esimone, C.O. Phenotypic and Molecular Characterization of β-Lactamases among Enterobacterial Uropathogens in Southeastern Nigeria. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 5843904. [Google Scholar] [CrossRef]
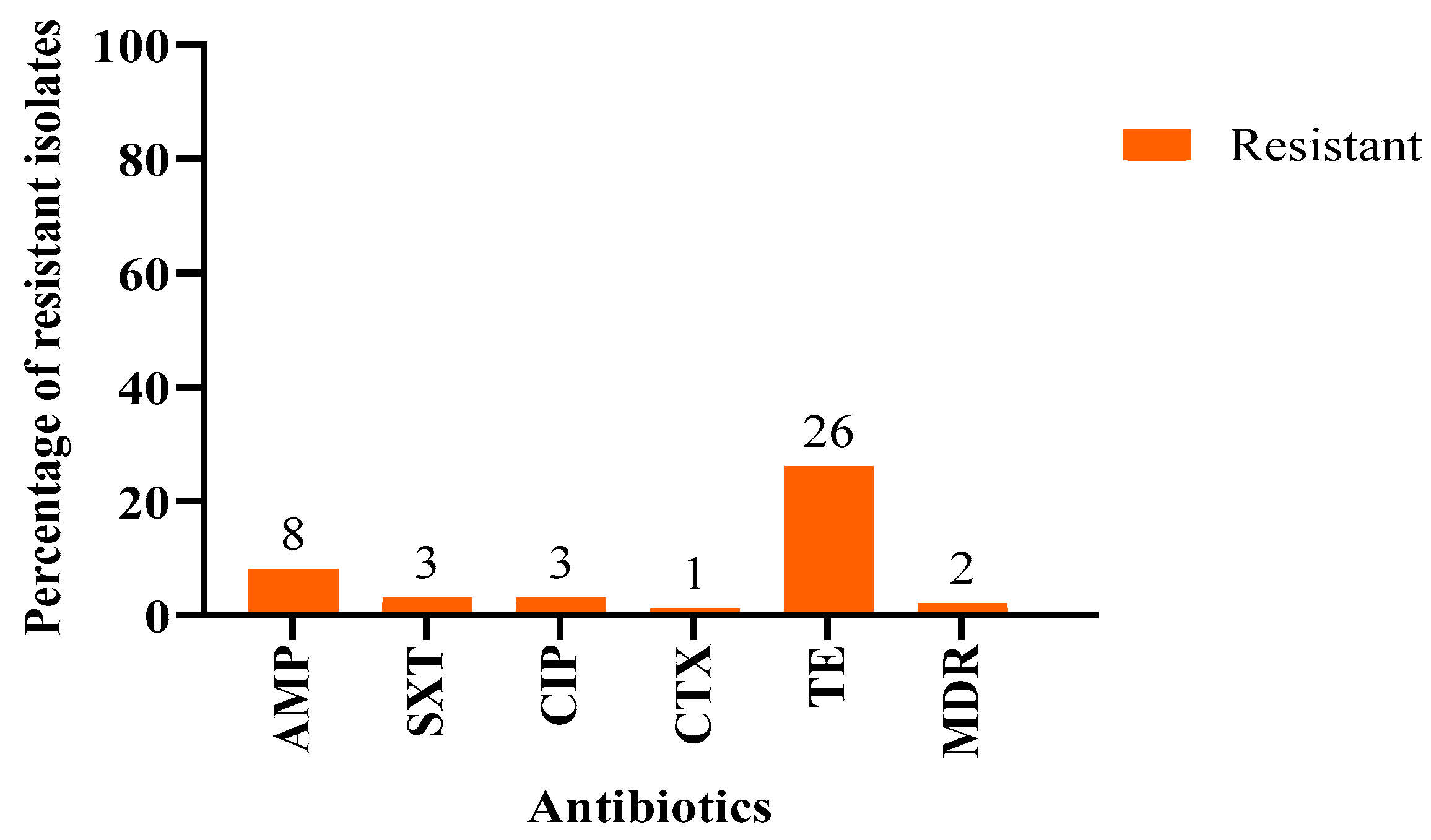
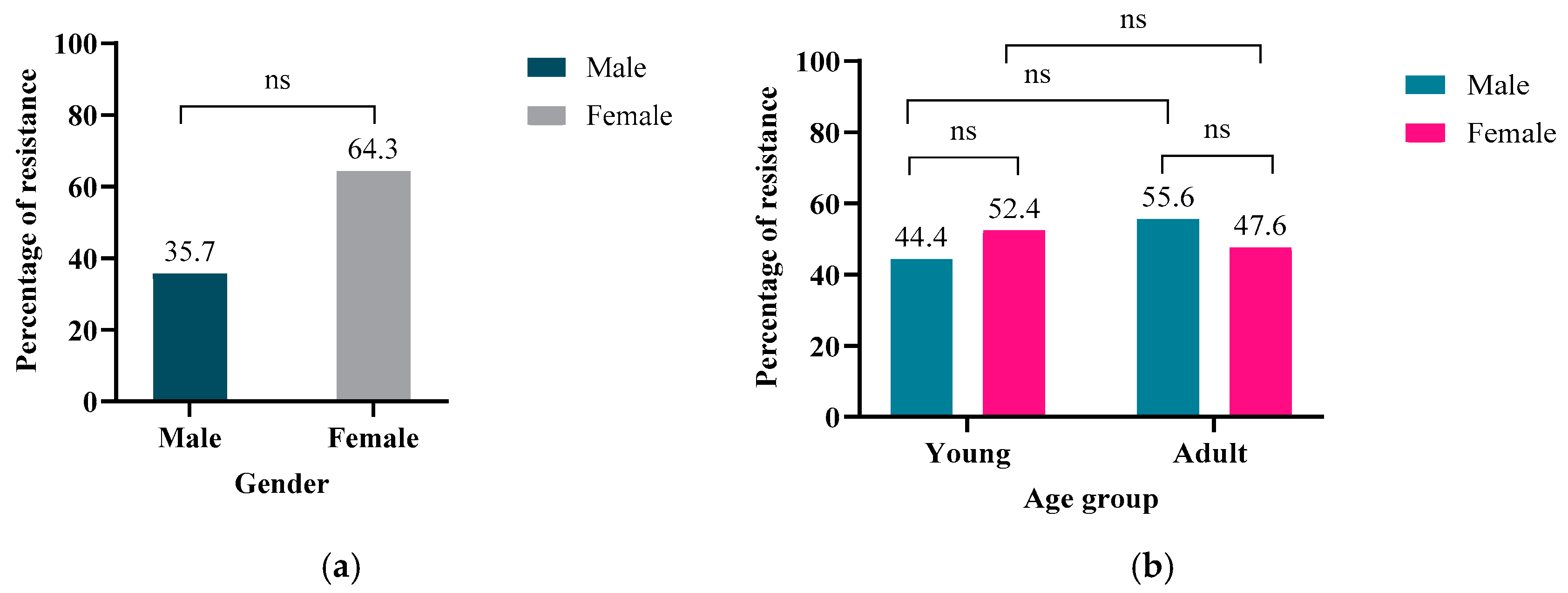



| No. | Antibiotic | Concentration | Susceptible (n, %) | Resistant (n, %) |
|---|---|---|---|---|
| 1 | Ampicillin (AMP) | 10 μg | 92 (92%) | 8 (8%) |
| 2 | Amoxicillin-clavulanic acid (AUG) | 30 μg | 100 (100%) | 0 (0%) |
| 3 | Piperacillin-tazobactam (TZP) | 25 μg | 100 (100%) | 0 (0%) |
| 4 | Ertapenem (ETP) | 10 μg | 100 (100%) | 0 (0%) |
| 5 | Meropenem (MRP) | 10 μg | 100 (100%) | 0 (0%) |
| 6 | Amikacin (AK) | 30 μg | 100 (100%) | 0 (0%) |
| 7 | Gentamicin (CN) | 10 μg | 100 (100%) | 0 (0%) |
| 8 | Fosfomycin (FOS) | 200 μg | 100 (100%) | 0 (0%) |
| 9 | Trimethoprim-sulfamethoxazole (SXT) | 25 μg | 97 (3%) | 3 (3%) |
| 10 | Ciprofloxacin (CIP) | 5 μg | 97 (3%) | 3 (3%) |
| 11 | Cefotaxime (CTX) | 30 μg | 99 (99%) | 1 (1%) |
| 12 | Ceftazidime (CAZ) | 30 μg | 100 (100%) | 0 (0%) |
| 13 | Nitrofurantoin (F) | 300 μg | 100 (100%) | 0 (0%) |
| 14 | Tetracycline (TE) | 30 μg | 74 (74%) | 26 (26%) |
| 15 | Colistin (Broth microdilution) | 0.25–16 mg/mL | 100 (100%) | 0 (0%) |
| Isolate No. | Phenotypic Resistance | AMR Genes | Drug Class | Resistance Mechanism | Accession Number | Bio Project Number |
|---|---|---|---|---|---|---|
| 91 | Tetracycline | acrS, kpnE | Tetracycline | Antibiotic efflux | JBKFEU000000000 | PRJNA1203416 |
| 11 | Tetracycline | soxR with mutation | Tetracycline | Antibiotic target alteration Antibiotic efflux | JBKFDZ000000000 | PRJNA1203247 |
| 34 | Ciprofloxacin | emrA | Fluoroquinolone antibiotic | Antibiotic efflux | JBKFEA000000000 | PRJNA1203252 |
| 32 | Ampicillin | acrAB-toIC with acrR mutation | Penicillins | Antibiotic target alteration Antibiotic efflux | JBKFEO000000000 | PRJNA1203389 |
| 29 | Tetracycline, ampicillin | kpnE | Tetracycline | Antibiotic efflux | ||
| fabl mutations | Multi-drug | Antibiotic target alteration | JBKFEP000000000 | PRJNA1203404 | ||
| 35 | Tetracycline | kpnE | Tetracycline | Antibiotic efflux | JBKFEQ000000000 | PRJNA1203405 |
| 47 | Ampicillin | qacG | Small MDR antibiotic efflux pump | Antibiotic efflux | JBKFES000000000 | PRJNA1203414 |
| 55 | Ampicillin | acrAB-toIC with marR mutations | Multi-drug | Antibiotic target alteration Antibiotic efflux | JBKFET000000000 | PRJNA1203415 |
| 92 | Ampicillin | acrAB-toIC with acrR mutation | penicillin | Antibiotic target alteration Antibiotic efflux | JBKFEV000000000 | PRJNA1203417 |
| 39 | Cefotaxime | soxR with mutation | Cephalosporin drug | antibiotic target alteration, antibiotic efflux | JBKFER000000000 | PRJNA1203408 |
| 78 | Ciprofloxacin | mdtE, emrB, AcrE,mdtF and marA | Fluoroquinolone | Antibiotic efflux | CP182564 | PRJNA1224957 |
| 06 | Ciprofloxacin | mdtE, emrB, AcrE, mdtF and marA | Fluoroquinolone | Antibiotic efflux | CP180189 | PRJNA1218299 |
| No. | Antibiotic | Antibiotic Class | Concentration | CLSI Susceptibility Range (mm) |
|---|---|---|---|---|
| 1 | Ampicillin (AMP) | Penicillin | 10 μg | ≥17 S/R 13≤ |
| 2 | Amoxicillin-clavulanic acid (AUG) | Penicillin | 30 μg | ≥18 S/R 13≤ |
| 3 | Piperacillin-tazobactam (TZP) | Penicillin-beta-lactamase inhibitor | 25 μg | ≥21 S/R 17≤ |
| 4 | Ertapenem (ETP) | Carbapenem | 10 μg | ≥22 S/R 18≤ |
| 5 | Meropenem (MRP) | Carbapenem | 10 μg | ≥23 S/R 19≤ |
| 6 | Amikacin (AK) | Aminoglycoside | 30 μg | ≥17 S/R 16≤ |
| 7 | Gentamicin (CN) | Aminoglycoside | 10 μg | ≥15 S/R 12≤ |
| 8 | Fosfomycin (FOS) | phosphonic acid derivative | 200 μg | ≥16 S/R 12≤ |
| 9 | Trimethoprim-sulfamethoxazole (SXT)-Sulfonamide, | 25 μg | ≥ 16 S/R 10 ≤ | |
| 10 | Ciprofloxacin (CIP) | Fluoroquinolone | 5 μg | ≥21 S/R 15≤ |
| 11 | Cefotaxime (CTX) | Cephalosporin | 30 μg | ≥26 S/R 22≤ |
| 12 | Ceftazidime (CAZ) | Cephalosporin | 30 μg | ≥21 S/R 17≤ |
| 13 | Nitrofurantoin (F) | Nitrofuran | 300 μg | ≥17 S/R 14≤ |
| 14 | Tetracycline (TE) | Tetracycline | 30 μg | ≥15 S/R 11≤ |
| 15 | Colistin (Broth microdilution) | Polymyxin | 0.25–16 mg/mL | ≤1 S/R 4≥ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushahidur Rahman, A.; Ahmed, S.E.; Osman, S.A.; Al-Haddad, R.A.; Almiski, A.; Kamar, R.; Abdelrahman, H.; Kassem, I.I.; Dogliero, A.; Eltai, N.O. A Snapshot of Antimicrobial Resistance in Semi-Wild Oryx: Baseline Data from Qatar. Antibiotics 2025, 14, 248. https://doi.org/10.3390/antibiotics14030248
Mushahidur Rahman A, Ahmed SE, Osman SA, Al-Haddad RA, Almiski A, Kamar R, Abdelrahman H, Kassem II, Dogliero A, Eltai NO. A Snapshot of Antimicrobial Resistance in Semi-Wild Oryx: Baseline Data from Qatar. Antibiotics. 2025; 14(3):248. https://doi.org/10.3390/antibiotics14030248
Chicago/Turabian StyleMushahidur Rahman, Asma, Salma E. Ahmed, Shayma A. Osman, Radhia A. Al-Haddad, Abdallah Almiski, Ristha Kamar, Hana Abdelrahman, Issmat I. Kassem, Andrea Dogliero, and Nahla O. Eltai. 2025. "A Snapshot of Antimicrobial Resistance in Semi-Wild Oryx: Baseline Data from Qatar" Antibiotics 14, no. 3: 248. https://doi.org/10.3390/antibiotics14030248
APA StyleMushahidur Rahman, A., Ahmed, S. E., Osman, S. A., Al-Haddad, R. A., Almiski, A., Kamar, R., Abdelrahman, H., Kassem, I. I., Dogliero, A., & Eltai, N. O. (2025). A Snapshot of Antimicrobial Resistance in Semi-Wild Oryx: Baseline Data from Qatar. Antibiotics, 14(3), 248. https://doi.org/10.3390/antibiotics14030248







