A National Surveillance of the Antibiotic Susceptibility of Acinetobacter baumannii in Saudi Arabia
Abstract
1. Introduction
2. Results
2.1. Isolates Distribution by Participating Hospitals
2.2. Sources of Isolates
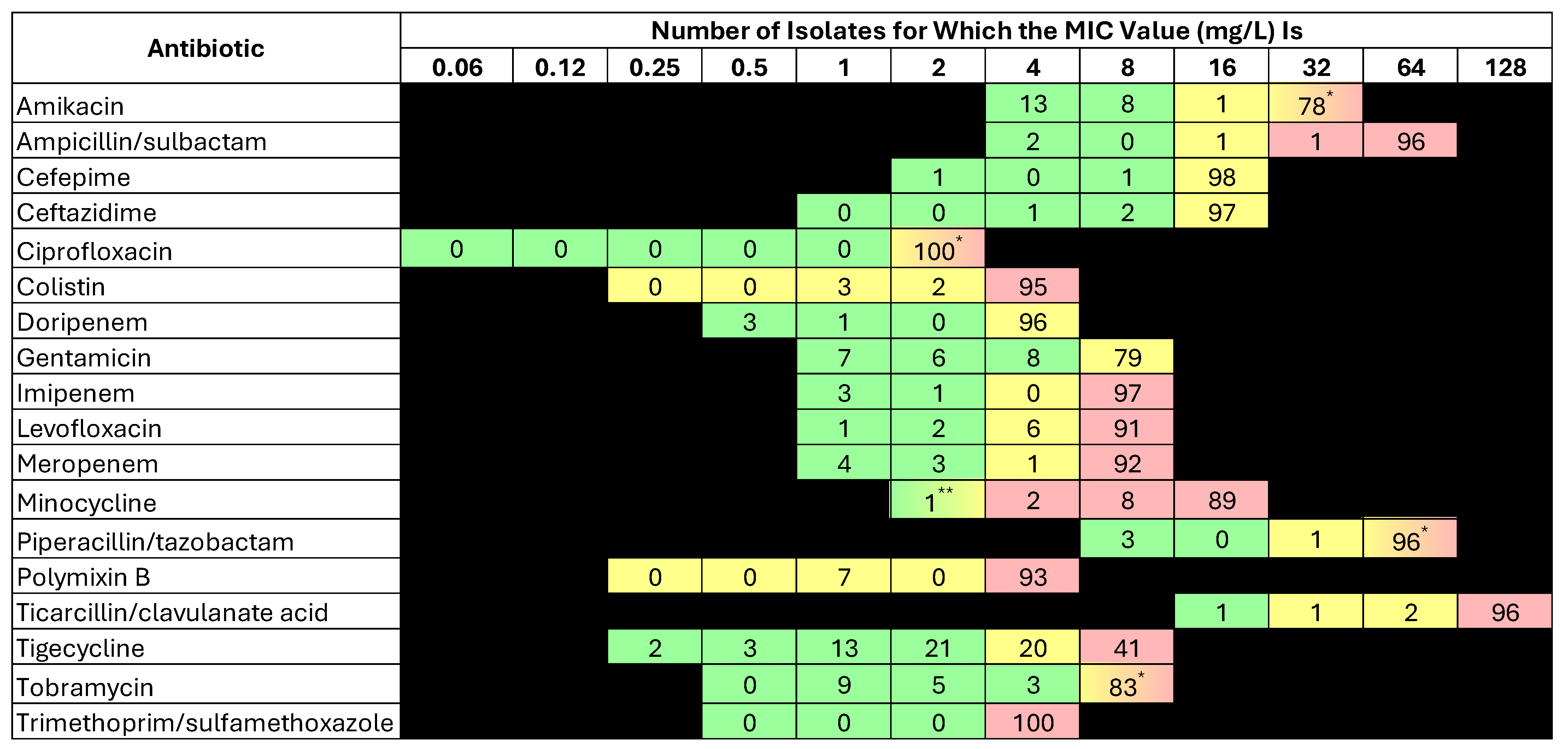
2.3. Susceptibility Results
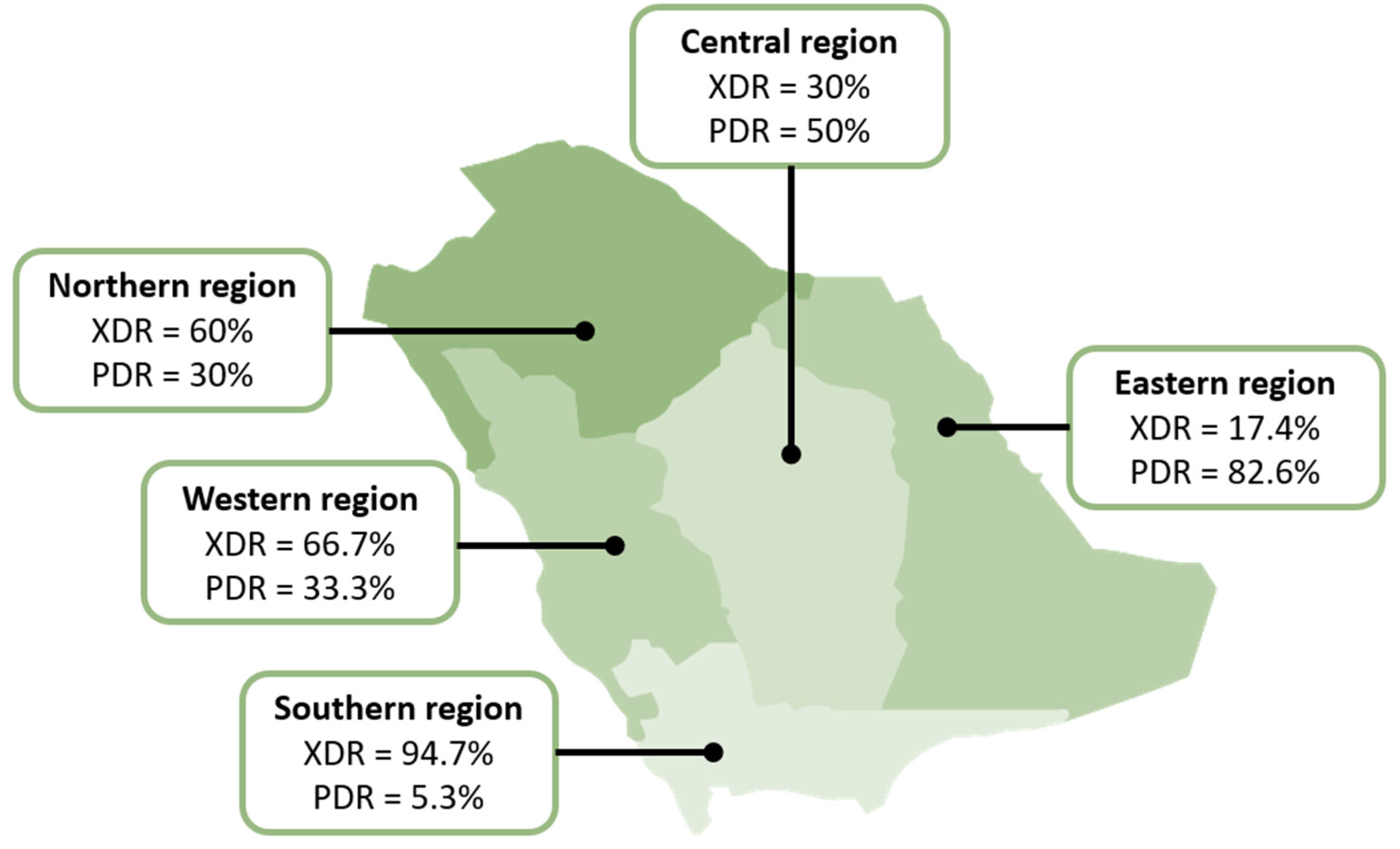
2.4. Distribution of Drug-Resistant Isolates
3. Materials and Methods
3.1. Study Period and Collection Process
3.2. Bacterial Isolates
3.3. Antimicrobial Susceptibility Testing
3.4. Definitions
3.5. Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| BMD | Broth microdilution |
| CLSI | Clinical Laboratory Standards Institute |
| ICU | Intensive care unit |
| MDR | Multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| XDR | Extensive drug-resistant |
| PDR | Pandrug-resistant |
| WHO | World Health Organization |
References
- Thabit, A.K.; Crandon, J.L.; Nicolau, D.P. Antimicrobial resistance: Impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin. Pharmacother. 2015, 16, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Thertieth Informational Supplement [Document M100-S33]; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2023. [Google Scholar]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 11 February 2025).
- Thabit, A.K.; Alabbasi, A.Y.; Alnezary, F.S.; Almasoudi, I.A. An Overview of Antimicrobial Resistance in Saudi Arabia (2013–2023) and the Need for National Surveillance. Microorganisms 2023, 11, 2086. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K.; Development, C.M.; CLSI Methods Development and Standardization Working Group of the Subcommittee on Antimicrobial Susceptibility Testing. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Thertieth Informational Supplement [Document M100-S35]; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2025. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 13.0. Available online: http://www.eucast.org (accessed on 11 February 2025).
- Shah, M.W.; Yasir, M.; Farman, M.; Jiman-Fatani, A.A.; Almasaudi, S.B.; Alawi, M.; El-Hossary, D.; Azhar, E.I. Antimicrobial susceptibility and molecular characterization of clinical strains of Acinetobacter baumannii in Western Saudi Arabia. Microb. Drug Resist. 2019, 25, 1297–1305. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Product Information: TYGACIL®. Tigecycline Intravenous Injection. Pfizer, Inc.: Philadelphia, PA, USA, 2020. [Google Scholar]
- Kharaba, A.; Abdelaziz Hussein, M.A.; Al-Hameed, F.M.; Mandourah, Y.; Almekhlafi, G.A.; Algethamy, H.; Hamdan, A.; Azem, M.A.; Fatani, J.; al Beshabshe, A.; et al. Acinetobacter baumannii in Saudi Arabia: The New Growing Threat. Saudi Crit. Care J. 2019, 3, 54–57. [Google Scholar] [CrossRef]
- Jung, J.; Park, W. Acinetobacter species as model microorganisms in environmental microbiology: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2533–2548. [Google Scholar] [CrossRef]
- Cavallo, I.; Oliva, A.; Pages, R.; Sivori, F.; Truglio, M.; Fabrizio, G.; Pasqua, M.; Pimpinelli, F.; Di Domenico, E.G. Acinetobacter baumannii in the critically ill: Complex infections get complicated. Front. Microbiol. 2023, 14, 1196774. [Google Scholar] [CrossRef]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019, 39, 10–39. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, T.; Ardebili, A. Plastic binding feature of polymyxins: The effect on MIC susceptibility measurements. Infect. Drug Resist. 2019, 12, 2649–2653. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C.A.; Nicolau, D.P. To add or not to add polysorbate 80: Impact on colistin MICs for clinical strains of Enterobacteriaceae and Pseudomonas aeruginosa and quality controls. J. Clin. Microbiol. 2014, 52, 3810. [Google Scholar] [CrossRef]
- Alam, M.Z.; Alam, Q.; Jiman-Fatani, A.A.; Shukri, H.A.; Haque, A. A surveillance study on the prevalence and antimicrobial resistance pattern among different groups of bacteria isolated from Western province of Saudi Arabia. Biomed. Res. 2017, 28, 898–906. [Google Scholar]
- AlAmri, A.M.; AlQurayan, A.M.; Sebastian, T.; AlNimr, A.M. Molecular surveillance of multidrug-resistant Acinetobacter baumannii. Curr. Microbiol. 2020, 77, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Said, K.B.; Alsolami, A.; Khalifa, A.M.; Khalil, N.A.; Moursi, S.; Osman, A.; Fahad, D.; Rakha, E.; Rashidi, M.; Moussa, S.; et al. A multi-point surveillance for antimicrobial resistance profiles among clinical isolates of Gram-negative bacteria recovered from major Ha’il hospitals, Saudi Arabia. Microorganisms 2021, 9, 2024. [Google Scholar] [CrossRef]
- Kabrah, A. Extended-spectrum beta-lactamase and carbapenem-resistant Gram-negative pathogens in Makkah, Saudi Arabia. Ethiop. J. Health Sci. 2022, 32, 1221–1230. [Google Scholar] [CrossRef]
- Saleem, M.; Syed Khaja, A.S.; Hossain, A.; Alenazi, F.; Said, K.B.; Moursi, S.A.; Almalaq, H.A.; Mohamed, H.; Rakha, E. Molecular characterization and antibiogram of Acinetobacter baumannii clinical isolates recovered from the patients with ventilator-associated pneumonia. Healthcare 2022, 10, 2210. [Google Scholar] [CrossRef]
- Marie, M.A.; Krishnappa, L.G.; Alzahrani, A.J.; Mubaraki, M.A.; Alyousef, A.A. A prospective evaluation of synergistic effect of sulbactam and tazobactam combination with meropenem or colistin against multidrug resistant Acinetobacter baumannii. Bosn. J. Basic Med. Sci. 2015, 15, 24–29. [Google Scholar] [CrossRef][Green Version]
- Anand, A.; Verma, A.; Kaur, S.; Kathayat, P.; Manoj, R.M.; Aakanksha, A.; Turzin, J.K.; Satapathy, P.; Khatib, M.N.; Gaidhane, S.; et al. An overview of sulbactam-durlobactam approval and implications in advancing therapeutics for hospital-acquired and ventilator-associated pneumonia by Acinetobacter baumannii-calcoaceticus complex: A narrative review. Health Sci. Rep. 2024, 7, e70066. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Hackel, M.A.; McLeod, S.M.; Miller, A.A. In Vitro Activity of Sulbactam-Durlobactam against Global Isolates of Acinetobacter baumannii-calcoaceticus Complex Collected from 2016 to 2021. Antimicrob. Agents Chemother. 2022, 66, e0078122. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.H.; Brune, A.; Nastro, M.; Vay, C.; Famiglietti, A. In vitro synergistic activity of the sulbactam/avibactam combination against extensively drug-resistant Acinetobacter baumannii. J. Med. Microbiol. 2020, 69, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Pasteran, F.; Cedano, J.; Baez, M.; Albornoz, E.; Rapoport, M.; Osteria, J.; Montana, S.; Le, C.; Ra, G.; Bonomo, R.A.; et al. A New Twist: The Combination of Sulbactam/Avibactam Enhances Sulbactam Activity against Carbapenem-Resistant Acinetobacter baumannii (CRAB) Isolates. Antibiotics 2021, 10, 577. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Hospital | ||||||
|---|---|---|---|---|---|---|---|
| King Abdulaziz University Hospital | King Fahad Hospital of the University | King Khaled Hospital | King Fahad Specialist Hospital | King Fahad Hospital | King Fahad Specialist Hospital | Maternity and Children Hospital of King Salman Medical City | |
| City | Jeddah | Dammam | Najran | Qassim | Albaha | Tabuk | Madinah |
| Region | Western | Eastern | Southern | Central | Southern | Northern | Western |
| Bed capacity | 879 | 633 | 330 | 600 | 320 | 205 | 500 |
| Number of ICUs | 5 (99 beds) | 5 (77 beds) | 4 (56 beds) | 4 (49 beds) | 4 (64 beds) | 2 (43 beds) | 3 (117 beds) |
| N (%) of isolates | 16 (16) | 23 (23) | 17 (17) | 20 (20) | 2 (2) | 20 (20) | 2 (2) |
| Source | n (%) | XDR * | PDR * |
|---|---|---|---|
| Respiratory | 43 (43) | 22 (51.2) | 19 (44.2) |
| Skin or soft tissue | 16 (16) | 9 (56.3) | 4 (25) |
| Urine | 15 (15) | 10 (66.7) | 5 (33.3) |
| Body fluid | 14 (14) | 4 (30.8) | 8 (61.5) |
| Blood | 8 (8) | 3 (37.5) | 5 (62.5) |
| Eye | 2 (2) | 1 (50) | 1 (50) |
| Cerebrospinal fluid | 1 (1) | 1 (100) | 0 (0) |
| Ear | 1 (1) | 1 (100) | 0 (0) |
| p-value | - | 0.514/0.400 ** | 0.425 |
| Antibiotic | % Susceptibility | MIC50 | MIC90 | MIC Range |
|---|---|---|---|---|
| Amikacin | 22 | >32 | >32 | ≤4–>32 |
| Ampicillin/sulbactam | 2 | >64/32 | >64/32 | ≤4/2–>64/32 |
| Cefepime | 2 | >16 | >16 | ≤2–>16 |
| Ceftazidime | 3 | >16 | >16 | 4–>16 |
| Ciprofloxacin | 0 | >2 | >2 | - |
| Colistin * | 5 | >4 | >4 | 1–>4 |
| Doripenem | 4 | >4 | >4 | ≤0.5–>4 |
| Gentamicin | 23 | >8 | >8 | ≤1–>8 |
| Imipenem | 4 | >8 | >8 | ≤1–>8 |
| Levofloxacin | 3 | >8 | >8 | ≤1–>8 |
| Meropenem | 7 | >8 | >8 | ≤1–>8 |
| Minocycline | 3 | >16 | >16 | ≤2–>16 |
| Piperacillin/tazobactam | 3 | >64/4 | >64/4 | ≤8/4–>64/4 |
| Polymixin B * | 1 | >4 | >4 | 1–>4 |
| Ticarcillin/clavulanate acid | 1 | >128/2 | >128/2 | ≤16/2–>128/2 |
| Tigecycline | 39 | 4 | >8 | ≤0.25–>8 |
| Tobramycin | 25 | >8 | >8 | ≤1–>8 |
| Trimethoprim/sulfamethoxazole | 0 | >4/76 | >4/76 | >4/76–>4–76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thabit, A.K.; Alharbi, F.S.; Jawah, A.F.; Alghamdi, A.M.; Miaji, M.Y.; Alturki, F.; Hosin, N.; Bazuqamah, M.; Almutairi, M.S.; Alhamed, H.; et al. A National Surveillance of the Antibiotic Susceptibility of Acinetobacter baumannii in Saudi Arabia. Antibiotics 2025, 14, 209. https://doi.org/10.3390/antibiotics14020209
Thabit AK, Alharbi FS, Jawah AF, Alghamdi AM, Miaji MY, Alturki F, Hosin N, Bazuqamah M, Almutairi MS, Alhamed H, et al. A National Surveillance of the Antibiotic Susceptibility of Acinetobacter baumannii in Saudi Arabia. Antibiotics. 2025; 14(2):209. https://doi.org/10.3390/antibiotics14020209
Chicago/Turabian StyleThabit, Abrar K., Feras S. Alharbi, Anas F. Jawah, Ammar M. Alghamdi, Musaab Y. Miaji, Fatimah Alturki, Nehal Hosin, Mohammed Bazuqamah, Masaad Saeed Almutairi, Hamad Alhamed, and et al. 2025. "A National Surveillance of the Antibiotic Susceptibility of Acinetobacter baumannii in Saudi Arabia" Antibiotics 14, no. 2: 209. https://doi.org/10.3390/antibiotics14020209
APA StyleThabit, A. K., Alharbi, F. S., Jawah, A. F., Alghamdi, A. M., Miaji, M. Y., Alturki, F., Hosin, N., Bazuqamah, M., Almutairi, M. S., Alhamed, H., Elhendawy, A., Atallah, D., Humadi, A. A., Alfifi, K. A., Alfadel, K., & Eljaaly, K., on behalf of the Saudi AntiMicrobial Surveillance (SAMS) Study Group. (2025). A National Surveillance of the Antibiotic Susceptibility of Acinetobacter baumannii in Saudi Arabia. Antibiotics, 14(2), 209. https://doi.org/10.3390/antibiotics14020209







