Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023
Abstract
1. Introduction
2. Results
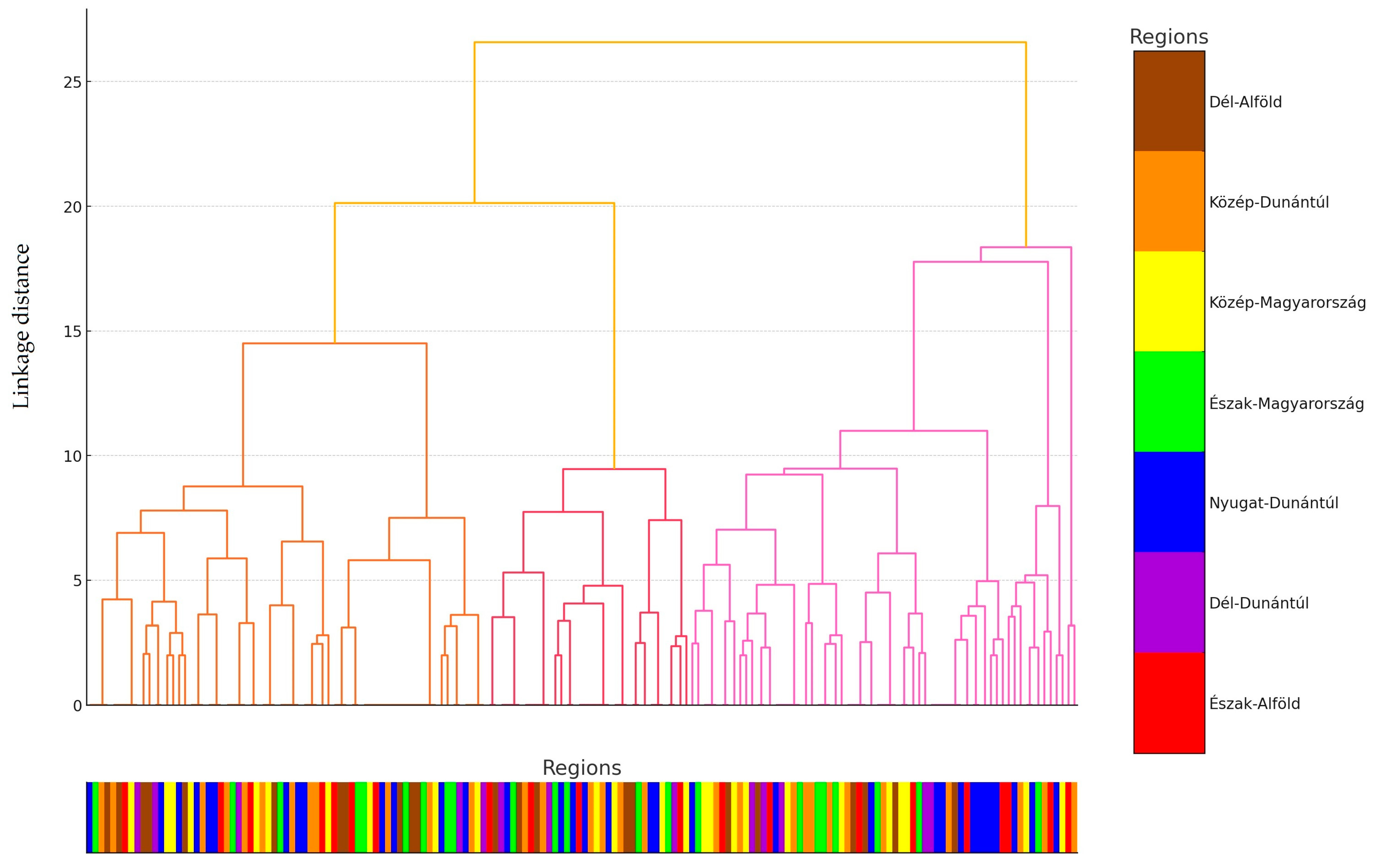
2.1. Regional Distribution and Origin of Samples Received
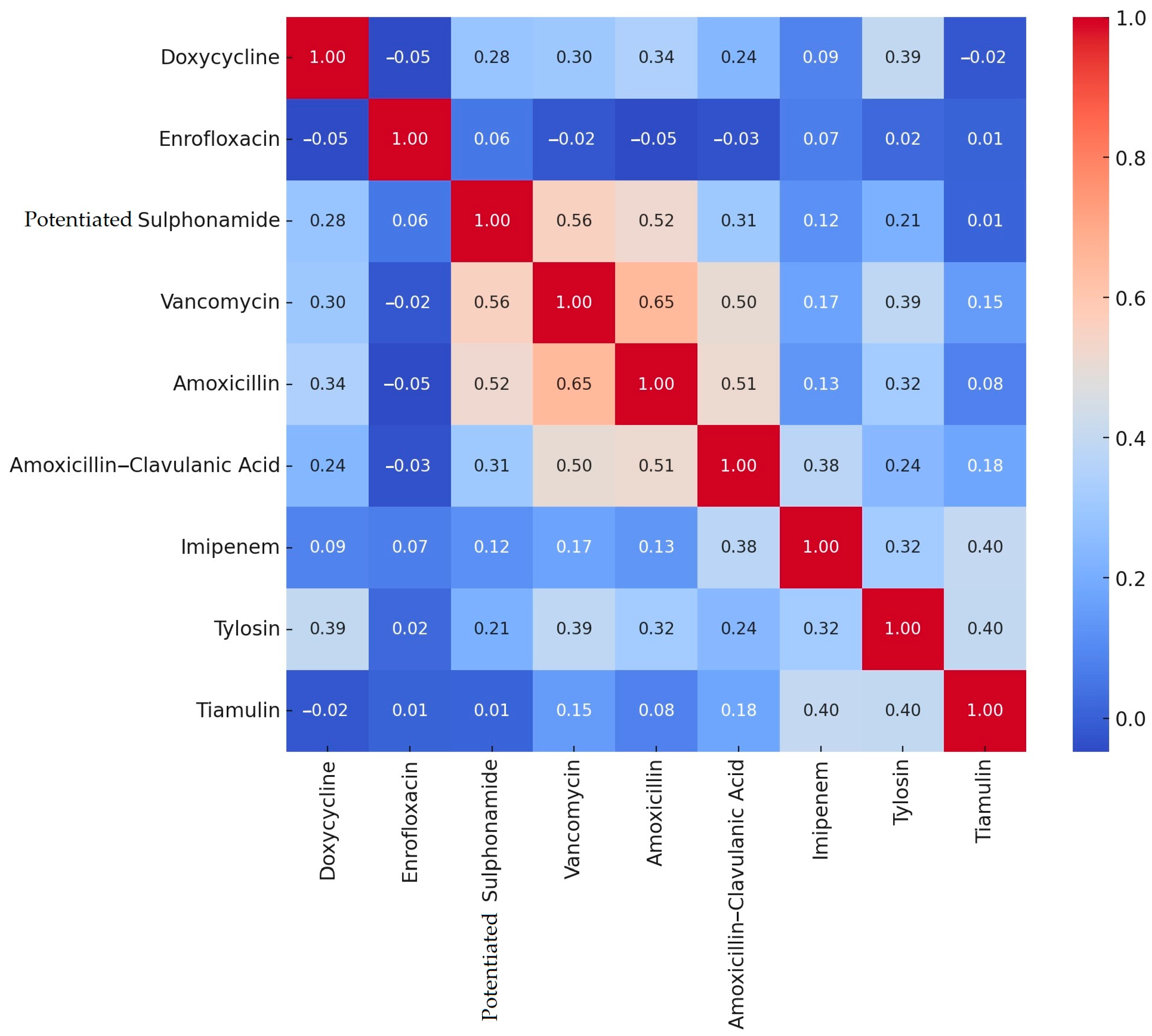
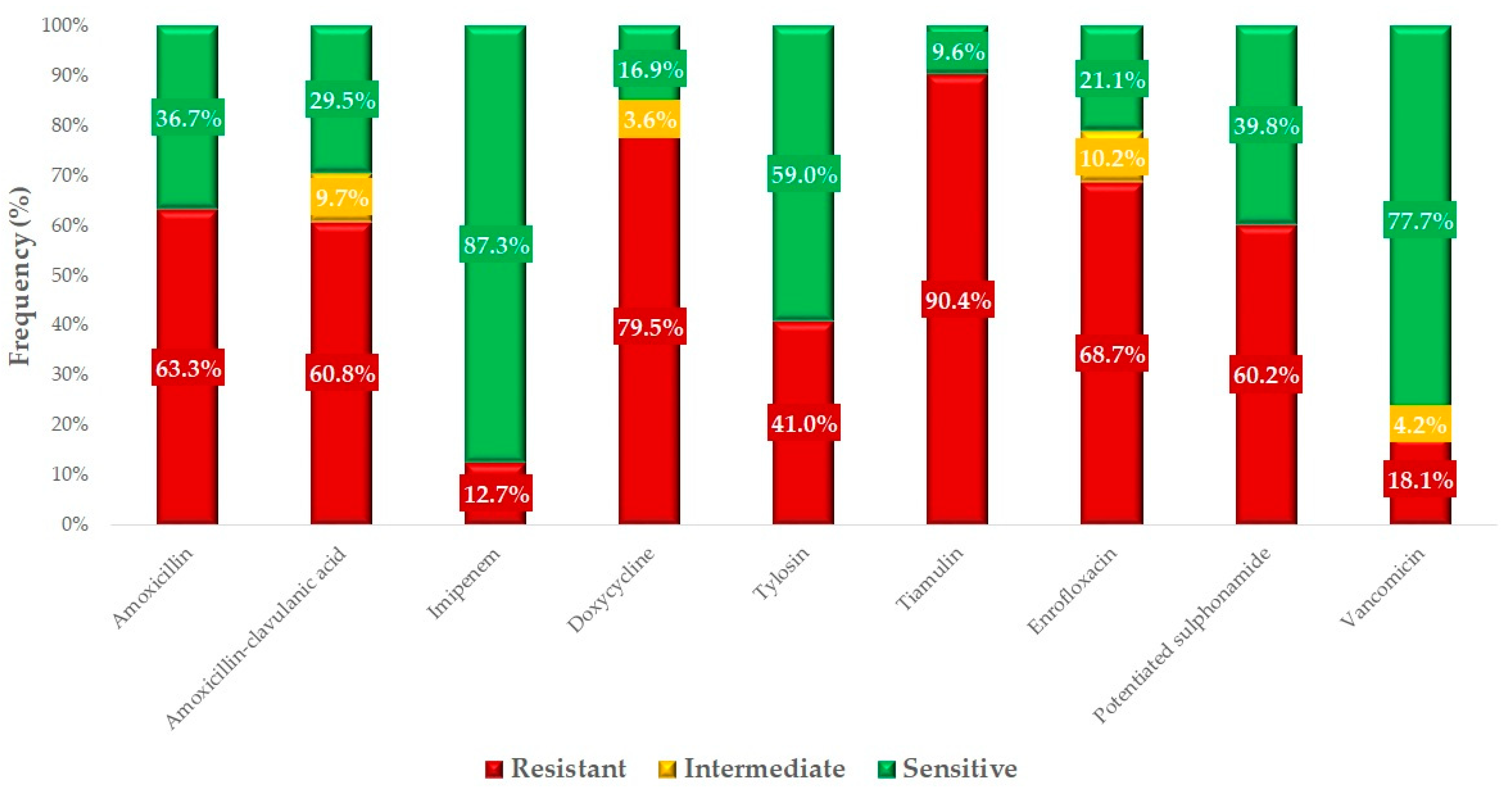
2.2. Antimicrobial Susceptibility Testing
3. Discussion
4. Materials and Methods
4.1. The Origin of Strains and Human Data
4.2. Minimum Inhibitory Concentration (MIC) Determination
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Infectious Diseases Society of America (IDSA); Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; et al. Combating Antimicrobial Resistance: Policy Recommendations to Save Lives. Clin. Infect. Dis. 2011, 52 (Suppl. S5), S397–S428. [Google Scholar] [CrossRef] [PubMed]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and Economic Impact of Antibiotic Resistance in Developing Countries: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 Laying Down the General Principles and Requirements of Food Law, Establishing the European Food Safety Authority and Laying Down Procedures in Matters of Food Safety. Off. J. Eur. Union 2002, 31, 1–24.
- Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the Monitoring of Zoonoses and Zoonotic Agents, Amending Council Decision 90/424/EEC and Repealing Council Directive 92/117/EEC. Off. J. Eur. Union 2003, 325, 31–40.
- Commission Implementing Decision (EU) 2020/1729 of 17 November 2020 on the Monitoring and Reporting of Antimicrobial Resistance in Zoonotic and Commensal Bacteria and Repealing Implementing Decision 2013/652/EU (Notified under Document C(2020) 7894) (Only the English Version Is Authentic) (Text with EEA Relevance). Off. J. Eur. Union 2020, 387, 8–21.
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Coculescu, B. Antimicrobial Resistance Induced by Genetic Changes. J. Med. Life 2009, 2, 114–123. [Google Scholar] [PubMed]
- Soucy, S.M.; Huang, J.; Gogarten, J.P. Horizontal Gene Transfer: Building the Web of Life. Nat. Rev. Genet. 2015, 16, 472–482. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J. Socioeconomic Enablers for Contagion: Factors Impelling the Antimicrobial Resistance Epidemic. Antibiotics 2019, 8, 86. [Google Scholar] [CrossRef]
- Collignon, P.; Beggs, J.J.; Walsh, T.R.; Gandra, S.; Laxminarayan, R. Anthropological and Socioeconomic Factors Contributing to Global Antimicrobial Resistance: A Univariate and Multivariable Analysis. Lancet Planet. Health 2018, 2, e398–e405. [Google Scholar] [CrossRef]
- Kovács, D.; Palkovicsné Pézsa, N.; Farkas, O.; Jerzsele, Á. Usage of Antibiotic Alternatives in Pig Farming: Literature Review. Magy. Állatorvosok Lapja 2021, 143, 281–282. [Google Scholar]
- Essősy, M.; Fodor, I.; Ihnáth, Z.; Karancsi, Z.; Kovács, D.; Szalai, K.V.; Szentmiklósi, D.; Jerzsele, Á. The Possibilities of Antibiotic-Free Broiler-Hen Fattening, with Special Reference to the Use of Pre- and Probiotics. Magy. Állatorvosok Lapja 2020, 142, 397–407. [Google Scholar]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-kott, A.F.; et al. Alternatives to Antibiotics for Organic Poultry Production: Types, Modes of Action and Impacts on Bird’s Health and Production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils—Animal Health Aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Kerek, Á.; Szabó, Á.; Dobra, P.F.; Bárdos, K.; Ózsvári, L.; Fehérvári, P.; Bata, Z.; Molnár-Nagy, V.; Jerzsele, Á. Determining the In Vivo Efficacy of Plant-Based and Probiotic-Based Antibiotic Alternatives against Mixed Infection with Salmonella enterica and Escherichia coli in Domestic Chickens. Vet. Sci. 2023, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Jerzsele, Á.; Somogyi, Z.; Szalai, M.; Kovács, D. Effects of Fermented Wheat Germ Extract on Artificial Salmonella Typhimurium Infection in Broiler Chickens. Magy. Állatorvosok Lapja 2020, 142, 77–85. [Google Scholar]
- Hetényi, N.; Bersényi, A.; Hullár, I. Physiological Effects of Medium-Chain Fatty Acids and Triglycerides, and Their Potential Use in Poultry and Swine Nutrition: A Literature Review. Magy. Állatorvosok Lapja 2024, 146, 651–659. [Google Scholar] [CrossRef]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Kerek, Á.; Csanády, P.; Tuska-Szalay, B.; Kovács, L.; Jerzsele, Á. In Vitro Efficacy of Hungarian Propolis against Bacteria, Yeast, and Trichomonas gallinae Isolated from Pigeons—A Possible Antibiotic Alternative? Resources 2023, 12, 101. [Google Scholar] [CrossRef]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity situation of large-scale poultry farms in Hungary according to the databases of National Food Chain Safety Office Centre for Disease Control and Biosecurity Audit System of Poultry Product Board of Hungary in the period of 2021–2022. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Kovács, L.; Hejel, P.; Farkas, M.; László, L. Könyves László Study Report on the Effect of a Litter Treatment Product Containing Bacillus licheniformis and Zeolite in Male Fattening Turkey Flock. Magy. Állatorvosok Lapja 2024, 146, 291–305. [Google Scholar] [CrossRef]
- Benmazouz, I.; Kövér, L.; Kardos, G. The Rise of Antimicrobial Resistance in Wild Birds: Potential AMR Sources and Wild Birds as AMR Reservoirs and Disseminators: Literature Review. Magy. Állatorvosok Lapja 2024, 146, 91–105. [Google Scholar] [CrossRef]
- Mag, P.; Németh, K.; Somogyi, Z.; Jerzsele, Á. Antibacterial therapy based on pharmacokinetic/ pharmacodynamic models in small animal medicine-1. Literature review. Magy. Állatorvosok Lapja 2023, 145, 419–438. [Google Scholar] [CrossRef]
- Geenen, P.L.; Graat, E.A.M.; Haenen, A.; Hengeveld, P.D.; Van Hoek, A.H.A.M.; Huijsdens, X.W.; Kappert, C.C.; Lammers, G.A.C.; Van Duijkeren, E.; Van De Giessen, A.W. Prevalence of Livestock-Associated MRSA on Dutch Broiler Farms and in People Living and/or Working on These Farms. Epidemiol. Infect. 2013, 141, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.M.; Stefani, L.M.; Lucheis, S.B.; Okano, W.; Cruz, J.C.M.; Souza, G.V.; Casagrande, T.A.C.; Bastos, P.A.S.; Pinheiro, R.R.; Arruda, M.M.; et al. Methicillin-Resistant Staphylococcus aureus in Poultry and Poultry Meat: A Meta-Analysis. J. Food Prot. 2018, 81, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.F.; Pidwill, G.R.; Carnell, O.T.; Surewaard, B.G.J.; Shamarina, D.; Sutton, J.A.F.; Jeffery, C.; Derré-Bobillot, A.; Archambaud, C.; Siggins, M.K.; et al. Commensal Bacteria Augment Staphylococcus aureus Infection by Inactivation of Phagocyte-Derived Reactive Oxygen Species. PLoS Pathog. 2021, 17, e1009880. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Chambers, H.F. Reemergence of Antibiotic-Resistant Staphylococcus aureus in the Genomics Era. J. Clin. Investig. 2009, 119, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- van Belkum, A. Staphylococcal Colonization and Infection: Homeostasis versus Disbalance of Human (Innate) Immunity and Bacterial Virulence. Curr. Opin. Infect. Dis. 2006, 19, 339–344. [Google Scholar] [CrossRef]
- Weems, J.J. The Many Faces of Staphylococcus Aureus Infection. Recognizing and Managing Its Life-Threatening Manifestations. Postgrad. Med. 2001, 110, 24–26, 29–31, 35–36. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Ito, T.; Hiramatsu, K. A New Class of Genetic Element, Staphylococcus Cassette Chromosome Mec, Encodes Methicillin Resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2000, 44, 1549–1555. [Google Scholar] [CrossRef]
- Kim, Y.J.; Oh, D.H.; Song, B.R.; Heo, E.J.; Lim, J.S.; Moon, J.S.; Park, H.J.; Wee, S.H.; Sung, K. Molecular Characterization, Antibiotic Resistance, and Virulence Factors of Methicillin-Resistant Staphylococcus aureus Strains Isolated from Imported and Domestic Meat in Korea. Foodborne Pathog. Dis. 2015, 12, 390–398. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Vink, C.; Kalenic, S.; Friedrich, A.W.; Bruggeman, C.A.; Stobberingh, E.E. The Molecular Evolution of Methicillin-Resistant Staphylococcus aureus. Clin. Microbiol. Infect. 2007, 13, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Neela, V.; Zafrul, A.M.; Mariana, N.S.; Van Belkum, A.; Liew, Y.K.; Rad, E.G. Prevalence of ST9 Methicillin-Resistant Staphylococcus aureus among Pigs and Pig Handlers in Malaysia. J. Clin. Microbiol. 2009, 47, 4138–4140. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Loeffen, F.; Bakker, J.; Klaassen, C.; Wulf, M. Methicillin-Resistant Staphylococcus aureus in Pig Farming. Emerg. Infect. Dis. J. 2005, 11, 1965. [Google Scholar] [CrossRef]
- Suepaul, S.; Stegger, M.; Boyen, F.; Georges, K.; Butaye, P. The Diversity and Zoonotic Potential of Staphylococcus pseudintermedius in Humans and Pet Dogs in Trinidad and Tobago. Antibiotics 2023, 12, 1266. [Google Scholar] [CrossRef]
- Moses, I.B.; Santos, F.F.; Gales, A.C. Human Colonization and Infection by Staphylococcus pseudintermedius: An Emerging and Underestimated Zoonotic Pathogen. Microorganisms 2023, 11, 581. [Google Scholar] [CrossRef]
- Kelesidis, T.; Tsiodras, S. Staphylococcus intermedius Is Not Only a Zoonotic Pathogen, but May Also Cause Skin Abscesses in Humans after Exposure to Saliva. Int. J. Infect. Dis. 2010, 14, e838–e841. [Google Scholar] [CrossRef] [PubMed]
- Khairullah, A.R.; Widodo, A.; Riwu, K.H.P.; Yanestria, S.M.; Moses, I.B.; Effendi, M.H.; Fauzia, K.A.; Fauziah, I.; Hasib, A.; Kusala, M.K.J.; et al. Spread of Livestock-Associated Methicillin-Resistant Staphylococcus aureus in Poultry and Its Risks to Public Health: A Comprehensive Review. Open Vet. J. 2024, 14, 2116–2128. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.A.J.W. Methicillin-Resistant Staphylococcus aureus in Food Products: Cause for Concern or Case for Complacency? Clin. Microbiol. Infect. 2010, 16, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sergelidis, D.; Angelidis, A.S. Methicillin-resistant Staphylococcus aureus: A Controversial Food-borne Pathogen. Lett. Appl. Microbiol. 2017, 64, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.A.; Ullah, H.; Tabassum, S.; Fatima, B.; Woodley, T.A.; Ramadan, H.; Jackson, C.R. Staphylococci in Poultry Intestines: A Comparison between Farmed and Household Chickens. Poult. Sci. 2020, 99, 4549–4557. [Google Scholar] [CrossRef]
- Lindsay, J.A.; Holden, M.T.G. Staphylococcus aureus: Superbug, Super Genome? Trends Microbiol. 2004, 12, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; DeLeo, F.R. Mobile Genetic Elements of Staphylococcus aureus. Cell Mol. Life Sci. 2010, 67, 3057–3071. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.E.; Contente-Cuomo, T.; Buchhagen, J.; Liu, C.M.; Watson, L.; Pearce, K.; Foster, J.T.; Bowers, J.; Driebe, E.M.; Engelthaler, D.M.; et al. Multidrug-Resistant Staphylococcus aureus in US Meat and Poultry. Clin. Infect. Dis. 2011, 52, 1227–1230. [Google Scholar] [CrossRef]
- Public Health Agency of Canada. Foodbook Report. Available online: https://www.canada.ca/en/public-health/services/publications/food-nutrition/foodbook-report.html (accessed on 8 July 2023).
- Public Health Agency of Canada. Public Health Notice—Outbreak of Salmonella Illnesses Linked to Raw Turkey and Raw Chicken. Available online: https://www.canada.ca/en/public-health/services/public-health-notices/2018/outbreak-salmonella-illnesses-raw-turkey-raw-chicken.html (accessed on 8 July 2023).
- Outbreak of Multidrug-Resistant Salmonella Infections Linked to Raw Turkey Products|Multidrug-Resistant Salmonella Infections Linked to Raw Turkey Products|July 2018|Salmonella|CDC. Available online: https://www.cdc.gov/salmonella/reading-07-18/index.html (accessed on 8 July 2023).
- Outbreak of Salmonella Infections Linked to Butterball Brand Ground Turkey|Outbreak of Salmonella Infections Linked to Butterball Ground Turkey|March 2019|Salmonella|CDC. Available online: https://www.cdc.gov/salmonella/schwarzengrund-03-19/index.html (accessed on 8 July 2023).
- Demczuk, W.; Soule, G.; Clark, C.; Ackermann, H.-W.; Easy, R.; Khakhria, R.; Rodgers, F.; Ahmed, R. Phage-Based Typing Scheme for Salmonella enterica serovar Heidelberg, a Causative Agent of Food Poisonings in Canada. J. Clin. Microbiol. 2003, 41, 4279–4284. [Google Scholar] [CrossRef]
- Routh, J.A.; Pringle, J.; Mohr, M.; Bidol, S.; Arends, K.; Adams-Cameron, M.; Hancock, W.T.; Kissler, B.; Rickert, R.; Folster, J.; et al. Nationwide Outbreak of Multidrug-Resistant Salmonella Heidelberg Infections Associated with Ground Turkey: United States, 2011. Epidemiol. Infect 2015, 143, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- CDC. Salmonella Hadar Infections Associated with Turkey Burgers—Salmonella. Available online: https://www.cdc.gov/salmonella/2011/turkey-burger-4-4-2011.html (accessed on 8 July 2023).
- Government of Canada, Public Services and Procurement Canada. Information Archivée Dans le Web. Available online: https://publications.gc.ca/collections/collection_2020/aspc-phac/HP2-4-2018-eng-4.pdf (accessed on 9 July 2023).
- Miller, E.A.; Elnekave, E.; Flores-Figueroa, C.; Johnson, A.; Kearney, A.; Munoz-Aguayo, J.; Tagg, K.A.; Tschetter, L.; Weber, B.P.; Nadon, C.A.; et al. Emergence of a Novel Salmonella enterica serotype Reading Clonal Group Is Linked to Its Expansion in Commercial Turkey Production, Resulting in Unanticipated Human Illness in North America. mSphere 2020, 5, e00056-20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Critically Important Antimicrobials for Human Medicine; 6th rev.; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151552-8.
- Health Canada. Categorization of Antimicrobial Drugs Based on Importance in Human Medicine. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html (accessed on 9 July 2023).
- Benrabia, I.; Hamdi, T.M.; Shehata, A.A.; Neubauer, H.; Wareth, G. Methicillin-Resistant Staphylococcus aureus (MRSA) in Poultry Species in Algeria: Long-Term Study on Prevalence and Antimicrobial Resistance. Vet. Sci. 2020, 7, 54. [Google Scholar] [CrossRef]
- Rafiq, K.; Islam, M.R.; Siddiky, N.A.; Samad, M.A.; Chowdhury, S.; Hossain, K.M.M.; Rume, F.I.; Hossain, M.K.; Mahbub-E-Elahi, A.; Ali, M.Z.; et al. Antimicrobial Resistance Profile of Common Foodborne Pathogens Recovered from Livestock and Poultry in Bangladesh. Antibiotics 2022, 11, 1551. [Google Scholar] [CrossRef] [PubMed]
- Fuda, C.C.S.; Fisher, J.F.; Mobashery, S. Beta-Lactam Resistance in Staphylococcus aureus: The Adaptive Resistance of a Plastic Genome. Cell Mol. Life Sci. 2005, 62, 2617–2633. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Cariou, N.; Salandre, O.; Le Guennec, J.; Nemeghaire, S.; Butaye, P. Genotyping and Antimicrobial 662 Resistance of Staphylococcus Aureus Isolates from Diseased Turkeys. Avian Pathol 2013, 42, 572–580, 66. [Google Scholar] [CrossRef]
- Moawad, A.A.; Hotzel, H.; Awad, O.; Roesler, U.; Hafez, H.M.; Tomaso, H.; Neubauer, H.; El-Adawy, H. Evolution of Antibiotic Resistance of Coagulase-Negative staphylococci Isolated from Healthy Turkeys in Egypt: First Report of Linezolid Resistance. Microorganisms 2019, 7, 476. [Google Scholar] [CrossRef] [PubMed]
- Gardete, S.; Tomasz, A. Mechanisms of Vancomycin Resistance in Staphylococcus aureus. J. Clin. Investig. 2014, 124, 2836–2840. [Google Scholar] [CrossRef] [PubMed]
- El-Adawy, H.; Ahmed, M.; Hotzel, H.; Monecke, S.; Schulz, J.; Hartung, J.; Ehricht, R.; Neubauer, H.; Hafez, H.M. Characterization of Methicillin-Resistant Staphylococcus aureus Isolated from Healthy Turkeys and Broilers Using DNA Microarrays. Front. Microbiol. 2016, 7, 2019. [Google Scholar] [CrossRef] [PubMed]
- Laczay, P.; Semjén, G.; Lehel, J.; Nagy, G. Pharmacokinetics and Bioavailability of Doxycycline in Fasted and Nonfasted Broiler Chickens. Acta Vet. Hung. 2001, 49, 31–37. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, X.; Liu, Y.; Zhang, Y.; Xu, M.; Wang, S.; Sun, Y.; Zhou, T.; Chen, L. In Vitro Antimicrobial Activity and Resistance Mechanisms of the New Generation Tetracycline Agents, Eravacycline, Omadacycline, and Tigecycline against Clinical Staphylococcus aureus Isolates. Front. Microbiol. 2022, 13, 1043736. [Google Scholar] [CrossRef] [PubMed]
- Sköld, O. Sulfonamide Resistance: Mechanisms and Trends. Drug Resist. Updates 2000, 3, 155–160. [Google Scholar] [CrossRef]
- Jones, R.N. Review of the in Vitro Spectrum of Activity of Imipenem. Am. J. Med. 1985, 78, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, I.; Alramany, D.; Harb, G.; Nicolau, D. 1733. Stability of Imipenem/Cilastatin/Relebactam under Different Temperature and Administration Conditions. Open Forum Infect. Dis. 2022, 9, ofac492.1363. [Google Scholar] [CrossRef]
- Sornsuvit, C.; Wientong, P.; Uitrakul, S.; Okonogi, S.; Katip, W. Influence of Concentration and Temperature on Stability of Imipenem Focused on Solutions for Extended Infusion. Dose Response 2021, 19, 15593258211059325. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yeh, K.-S.; Liu, H.-T.; Lin, J.-H. Staphylococcus aureus Isolated from Pork and Chicken Carcasses in Taiwan: Prevalence and Antimicrobial Susceptibility. J. Food Prot. 2009, 72, 608–611. [Google Scholar] [CrossRef]
- Nemeghaire, S.; Argudín, M.A.; Haesebrouck, F.; Butaye, P. Molecular Epidemiology of Methicillin-Resistant Staphylococcus sciuri in Healthy Chickens. Vet. Microbiol. 2014, 171, 357–363. [Google Scholar] [CrossRef]
- Bahraminia, F.; Emadi, S.R.; Emaneini, M.; Farzaneh, N.; Rad, M.; Khoramian, B. A High Prevalence of Tylosin Resistance among Staphylococcus aureus Strains Isolated from Bovine Mastitis. Vet. Res. Forum 2017, 8, 121–125. [Google Scholar]
- Barnácz, F.; Kerek, Á.; Csirmaz, B.; Román, I.L.; Gál, C.; Horváth, Á.; Hajduk, E.; Szabó, Á.; Jerzsele, Á.; Kovács, L. The Status of Antimicrobial Resistance in Domestic Poultry with Different Breeding Purposes in Hungary between 2022–2023. Magy. Állatorvosok Lapja 2024, 146, 339–356. [Google Scholar] [CrossRef]
- Kerek, Á.; Szabó, Á.; Jerzsele, Á. Antimicrobial Susceptibility Profiles of Pasteurella multocida Isolates from Clinical Cases of Waterfowl in Hungary between 2022 and 2023. Vet. Sci. 2024, 11, 194. [Google Scholar] [CrossRef]
- Pintér, K.; Ádám, K.; Tibor, M. Antibiotic Susceptibility of Pasteurella multocida Strains, Genetic Background of Antimicrobial Resistance Literature Review. Magy. Állatorvosok Lapja 2023, 147, 239–256. [Google Scholar] [CrossRef]
- Sonola, V.S.; Misinzo, G.; Matee, M.I. Occurrence of Multidrug-Resistant Staphylococcus aureus among Humans, Rodents, Chickens, and Household Soils in Karatu, Northern Tanzania. Int. J. Environ. Res. Public Health 2021, 18, 8496. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Wang, T.; Onodera, Y.; Uchida, Y.; Sato, K. Mechanism of Quinolone Resistance in Staphylococcus aureus. J. Infect. Chemother. 2000, 6, 131–139. [Google Scholar] [CrossRef]
- Nazarchuk, O.A.; Nahaichuk, V.I.; Osadchuk, N.I.; Dmytriiev, D.V.; Dmytriiev, K.D.; Turzhanska, O.S. Prognostic Parameters of the Susceptibility of Staphylococcus spp. to Aminoglycosides and Doxycycline. Wiad. Lek. 2020, 73, 1615–1619. [Google Scholar] [CrossRef]
- CLSI Standards M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-Test? On Assumptions for Hypothesis Tests and Multiple Interpretations of Decision Rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Sibson, R. SLINK: An Optimally Efficient Algorithm for the Single-Link Cluster Method. Comput. J. 1973, 16, 30–34. [Google Scholar] [CrossRef]


| Antibiotics | Respiratory–Cloaca | Broiler–Breeding | 3 Young–4 Adult | 5 Small–6 Medium |
|---|---|---|---|---|
| p-Values | ||||
| Doxycycline | 0.0067 * | 0.0067 * | 0.0067 * | 0.0265 * |
| Enrofloxacin | 0.2669 | 0.9473 | 0.9473 | 0.1141 |
| 1 Potentiated sulphonamide | 0.0097 * | 0.1932 | 0.1932 | 0.0020 * |
| Vancomycin | 0.3505 | 0.0137 * | 0.0137 * | 0.0421 * |
| Amoxicillin | 0.8926 | 0.0321 * | 0.0321 * | 0.0729 |
| 2 Amoxicillin–clavulanic acid | 0.5078 | 0.0043 * | 0.0043 * | 0.2733 |
| Imipenem | 0.3624 | 0.0157 * | 0.0157 * | 0.0248 * |
| Tilozin | 0.9904 | 0.0001 * | 0.0001 * | 0.0006 * |
| Tiamulin | 0.4776 | 0.0180 * | 0.0180 * | 0.2706 |
| Antibiotics | 1 BP * | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | MIC50 | MIC90 | 2 ECOFF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/mL | µg/mL | ||||||||||||||||||||||||
| Amoxicillin | 0.5 | 1 | 0 | 4 | 11 | 22 | 23 | 13 | 11 | 15 | 14 | 16 | 3 | 12 | 0 | 1 | 6 | 11 | 3 | 1 | 256 | 0.5 | |||
| 0.6% | 0.0% | 2.4% | 6.6% | 13.3% | 13.9% | 7.8% | 6.6% | 9.0% | 8.4% | 9.6% | 1.8% | 7.2% | 0.0% | 0.6% | 3.6% | 6.6% | 1.8% | ||||||||
| Doxycycline | 0.5 | 1 | 1 | 2 | 6 | 3 | 1 | 3 | 11 | 6 | 10 | 28 | 18 | 21 | 13 | 21 | 10 | 11 | 2 | 32 | 0.5 | ||||
| 0.6% | 0.6% | 1.2% | 3.6% | 1.8% | 0.6% | 1.8% | 6.6% | 3.6% | 6.0% | 16.9% | 10.8% | 12.7% | 7.8% | 12.7% | 6.0% | 6.6% | |||||||||
| 3 Amoxicillin-clavulanic acid | 1 | 1 | 0 | 3 | 11 | 18 | 16 | 16 | 17 | 19 | 19 | 22 | 17 | 6 | 1 | 0.25 | 16 | 0.5 | |||||||
| 0.6% | 0.0% | 1.8% | 6.6% | 10.8% | 9.6% | 9.6% | 10.2% | 11.4% | 11.4% | 13.3% | 10.2% | 3.6% | 0.6% | ||||||||||||
| Tiamulin | 4 | 2 | 3 | 5 | 6 | 2 | 3 | 13 | 22 | 35 | 29 | 18 | 14 | 14 | 64 | 512 | 2 | ||||||||
| 1.2% | 1.8% | 3.0% | 3.6% | 1.2% | 1.8% | 7.8% | 13.3% | 21.1% | 17.5% | 10.8% | 8.4% | 8.4% | |||||||||||||
| Enrofloxacin | 4 | 3 | 1 | 8 | 6 | 7 | 10 | 6 | 11 | 20 | 8 | 18 | 21 | 35 | 7 | 5 | 16 | 64 | 0.5 | ||||||
| 1.8% | 0.6% | 4.8% | 3.6% | 4.2% | 6.0% | 3.6% | 6.6% | 12.0% | 4.8% | 10.8% | 12.7% | 21.1% | 4.2% | 3.0% | |||||||||||
| 4 Potentiated sulphonamide | 4 | 8 | 7 | 19 | 14 | 18 | 20 | 23 | 8 | 12 | 37 | 4 | 64 | 0.25 | |||||||||||
| 4.8% | 4.2% | 11.4% | 8.4% | 10.8% | 12.0% | 13.9% | 4.8% | 7.2% | 22.3% | ||||||||||||||||
| Imipenem | 8 | 1 | 0 | 0 | 3 | 10 | 24 | 20 | 10 | 21 | 17 | 16 | 9 | 14 | 13 | 6 | 2 | 0.063 | 8 | 0.125 | |||||
| 0.6% | 0.0% | 0.0% | 1.8% | 6.0% | 14.5% | 12.0% | 6.0% | 12.7% | 10.2% | 9.6% | 5.4% | 8.4% | 7.8% | 3.6% | 1.2% | ||||||||||
| Vancomycin | 32 | 1 | 16 | 37 | 26 | 38 | 8 | 3 | 3 | 4 | 5 | 0 | 0 | 18 | 7 | 1 | 256 | 2 | |||||||
| 0.6% | 9.6% | 22.3% | 15.7% | 22.9% | 4.8% | 1.8% | 1.8% | 2.4% | 3.0% | 0.0% | 0.0% | 10.8% | 4.2% | ||||||||||||
| Tilozin | 64 | 1 | 2 | 1 | 8 | 11 | 13 | 35 | 9 | 10 | 3 | 4 | 1 | 1 | 6 | 6 | 16 | 39 | 4 | 1024 | 2 | ||||
| 0.6% | 1.2% | 0.6% | 4.8% | 6.6% | 7.8% | 21.1% | 5.4% | 6.0% | 1.8% | 2.4% | 0.6% | 0.6% | 3.6% | 3.6% | 9.6% | 23.5% | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, L.; Szabó, Á.; Barnácz, F.; Csirmaz, B.; Jerzsele, Á.; Kerek, Á. Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics 2025, 14, 200. https://doi.org/10.3390/antibiotics14020200
Kovács L, Szabó Á, Barnácz F, Csirmaz B, Jerzsele Á, Kerek Á. Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics. 2025; 14(2):200. https://doi.org/10.3390/antibiotics14020200
Chicago/Turabian StyleKovács, László, Ábel Szabó, Franciska Barnácz, Bence Csirmaz, Ákos Jerzsele, and Ádám Kerek. 2025. "Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023" Antibiotics 14, no. 2: 200. https://doi.org/10.3390/antibiotics14020200
APA StyleKovács, L., Szabó, Á., Barnácz, F., Csirmaz, B., Jerzsele, Á., & Kerek, Á. (2025). Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Turkeys in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics, 14(2), 200. https://doi.org/10.3390/antibiotics14020200







