Prophage ϕSA169 Enhances Vancomycin Persistence in Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
1. Introduction
2. Results
2.1. Patients Infected by MRSA Containing ϕSA169-like Prophage Appear to Have Worse Clinical Outcomes
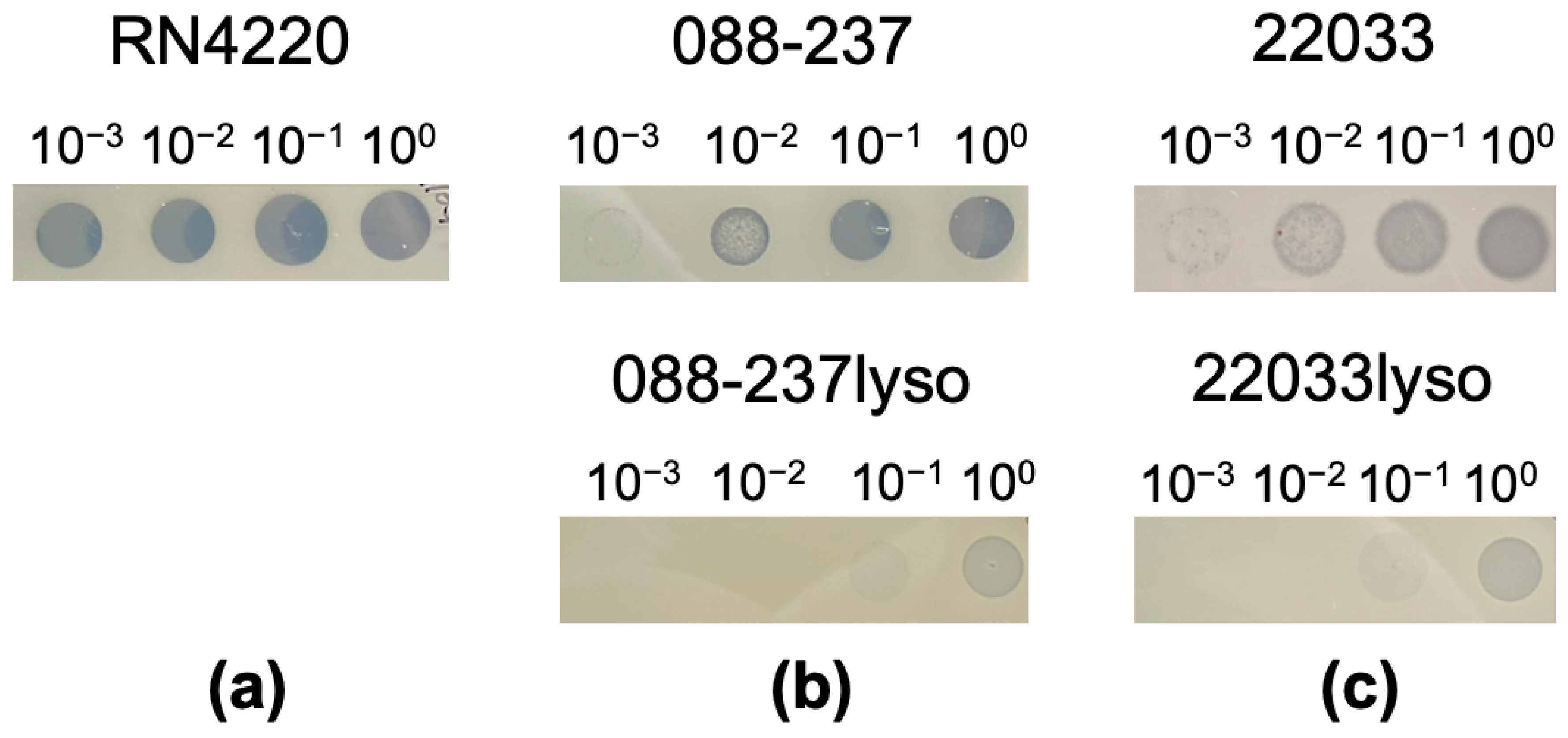
2.2. Successful Construction of Lysogens
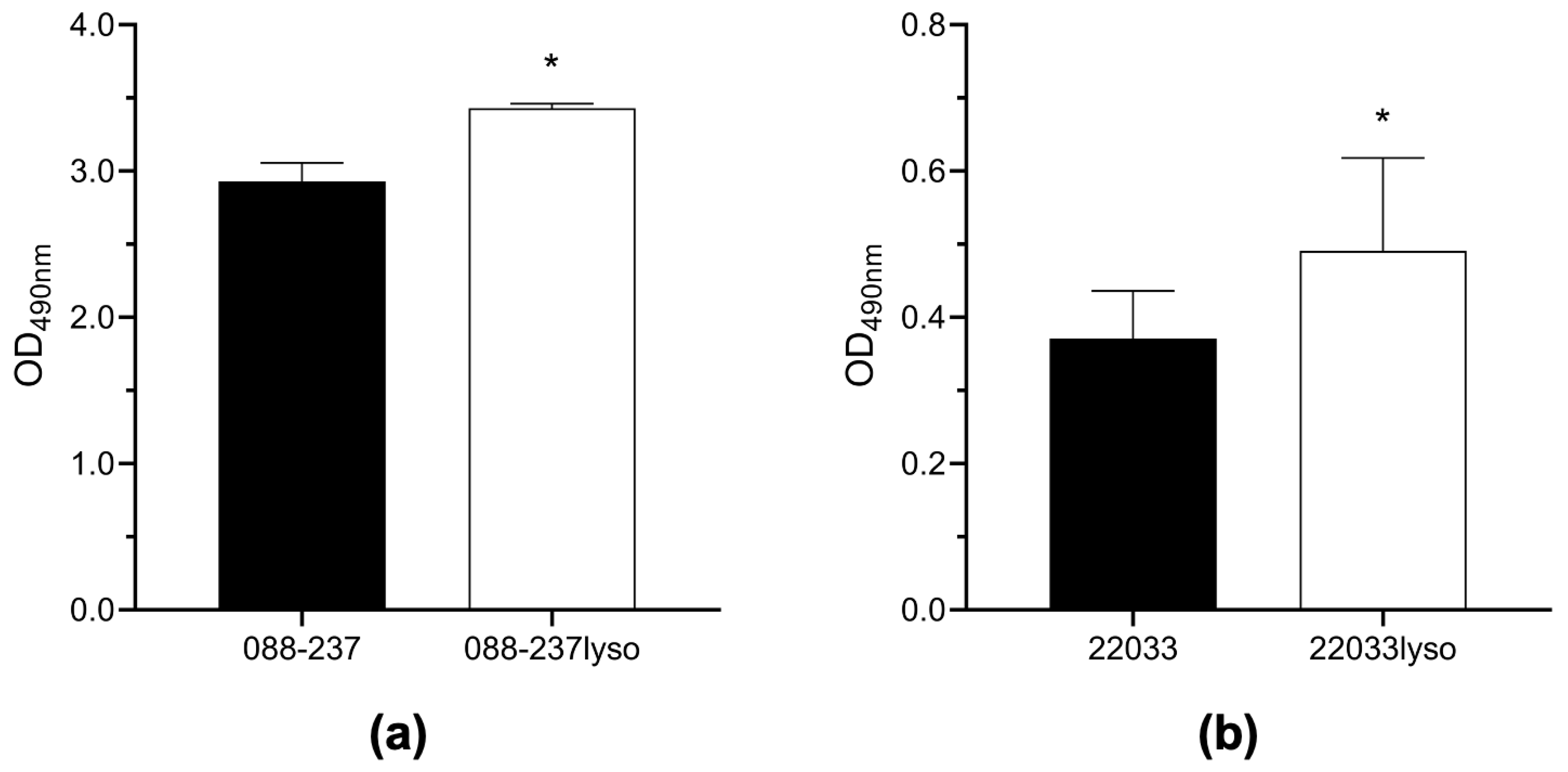
2.3. Prophage ϕSA169 Significantly Enhances Biofilm Formation in MRSA Strains
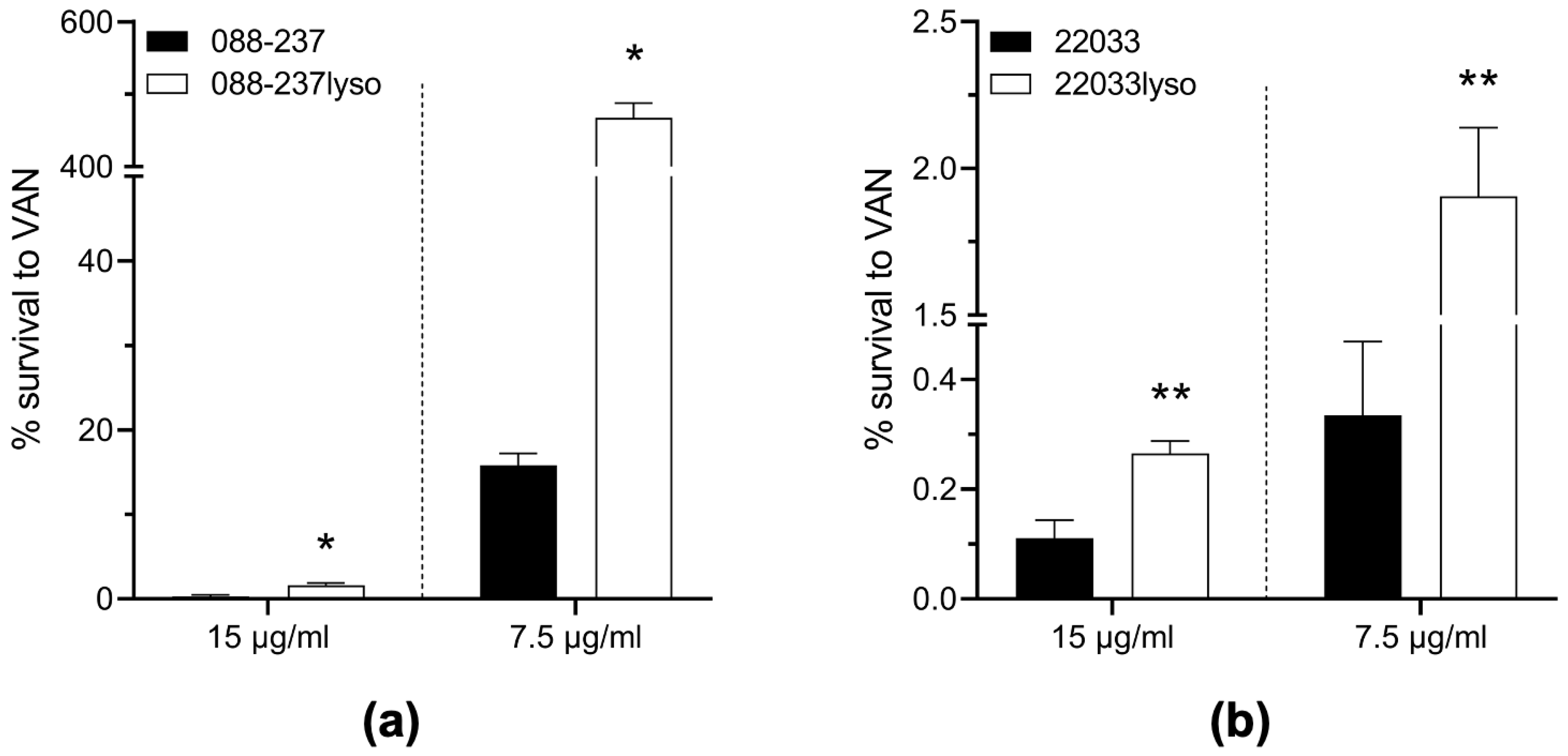
2.4. Prophage ϕSA169 Significantly Enhances MRSA Survival During VAN Exposure
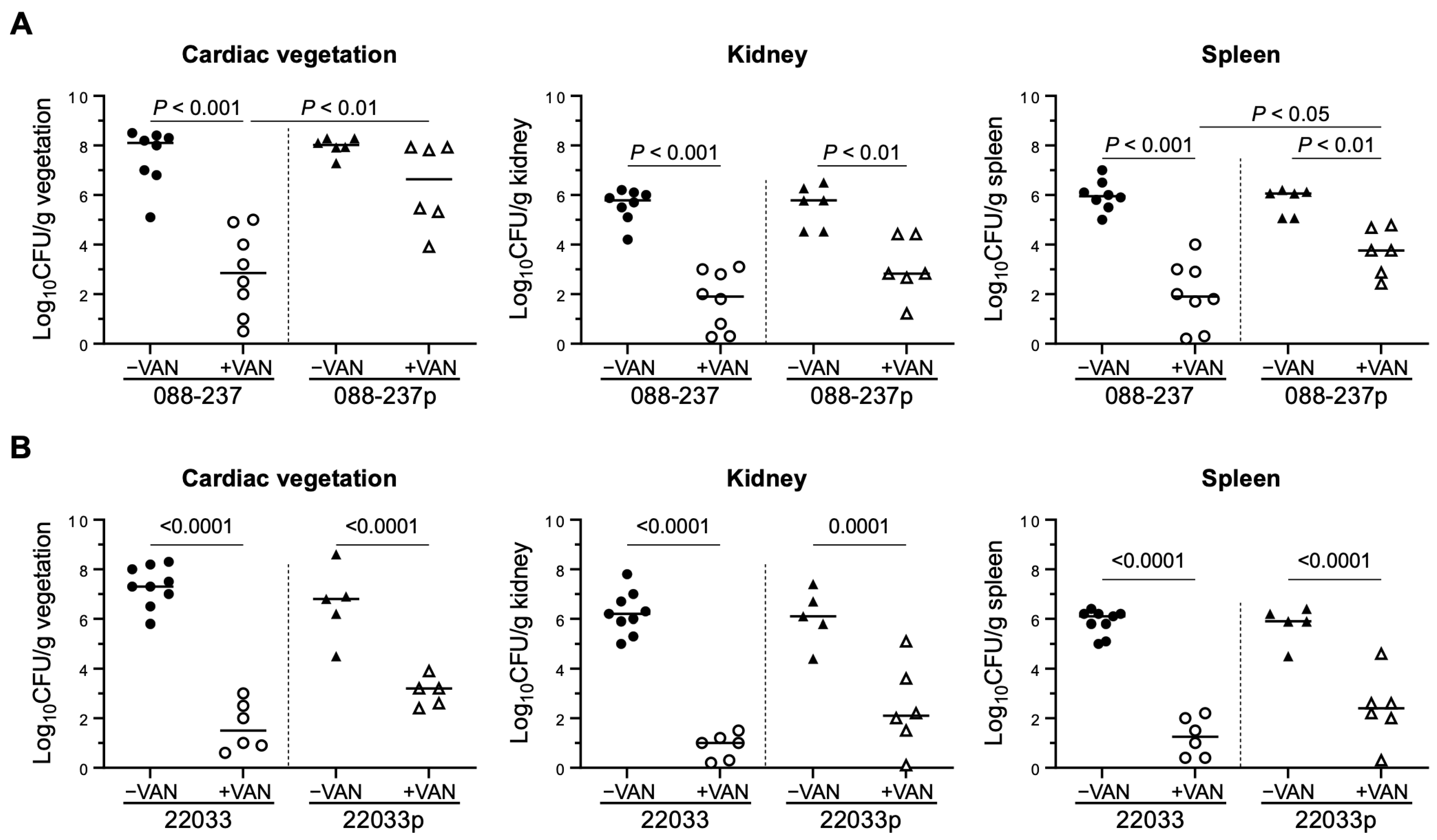
2.5. Prophage ϕSA169 Promotes MRSA VAN Persistence During Treatment in an Experimental IE Model
3. Discussion
4. Materials and Methods
4.1. Paired Clinical Isolate-Clinical Outcomes Data
4.2. Bacterial Strains and Growth Medium
4.3. Isolation and Amplification of Phage ϕSA169
4.4. Lysogen Construction and Superinfection Immunity Test
4.5. Confirmation of Lysogens
4.6. Determination of Antibiotics Minimum Inhibitory Concentrations (MICs)
4.7. Biofilm Formation
4.8. In Vitro VAN Killing Activity
4.9. Experimental IE Model in Rabbits
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deghorain, M.; Van Melderen, L. The Staphylococci phages family: An overview. Viruses 2012, 4, 3316–3335. [Google Scholar] [CrossRef] [PubMed]
- Goerke, C.; Pantucek, R.; Holtfreter, S.; Schulte, B.; Zink, M.; Grumann, D.; Broker, B.M.; Doskar, J.; Wolz, C. Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J. Bacteriol. 2009, 191, 3462–3468. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Wolz, C. Phages of Staphylococcus aureus and their impact on host evolution. Infect. Genet. Evol. 2014, 21, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Malachowa, N.; DeLeo, F.R. Mobile genetic elements of Staphylococcus aureus. Cell Mol. Life Sci. 2010, 67, 3057–3071. [Google Scholar] [CrossRef]
- Bae, T.; Baba, T.; Hiramatsu, K.; Schneewind, O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol. Microbiol. 2006, 62, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Chaguza, C.; Smith, J.T.; Bruce, S.A.; Gibson, R.; Martin, I.W.; Andam, C.P. Prophage-encoded immune evasion factors are critical for Staphylococcus aureus host infection, switching, and adaptation. Cell Genom. 2022, 2, 100194. [Google Scholar] [CrossRef]
- Rooijakkers, S.H.; Ruyken, M.; Roos, A.; Daha, M.R.; Presanis, J.S.; Sim, R.B.; van Wamel, W.J.; van Kessel, K.P.; van Strijp, J.A. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol. 2005, 6, 920–927. [Google Scholar] [CrossRef]
- van Wamel, W.J.; Rooijakkers, S.H.; Ruyken, M.; van Kessel, K.P.; van Strijp, J.A. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 2006, 188, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Chan, L.; Tattevin, P.; Kajikawa, O.; Martin, T.R.; Basuino, L.; Mai, T.T.; Marbach, H.; Braughton, K.R.; Whitney, A.R.; et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. USA 2010, 107, 5587–5592. [Google Scholar] [CrossRef]
- McCarthy, A.J.; Witney, A.A.; Lindsay, J.A. Staphylococcus aureus temperate bacteriophage: Carriage and horizontal gene transfer is lineage associated. Front. Cell Infect. Microbiol. 2012, 2, 6. [Google Scholar] [CrossRef]
- Ingmer, H.; Gerlach, D.; Wolz, C. Temperate phages of Staphylococcus aureus. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Haaber, J.; Penades, J.R.; Ingmer, H. Transfer of antibiotic resistance in Staphylococcus aureus. Trends Microbiol. 2017, 25, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Seidl, K.; Corvaglia, A.R.; Bayer, A.S.; Xiong, Y.Q.; Francois, P. Genome sequences of sequence type 45 (ST45) persistent methicillin-resistant Staphylococcus aureus (MRSA) bacteremia strain 300-169 and ST45 resolving MRSA bacteremia strain 301-188. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Sakoulas, G.; McIntyre, L.M.; Meka, V.G.; Arbeit, R.D.; Cabell, C.H.; Stryjewski, M.E.; Eliopoulos, G.M.; Reller, L.B.; Corey, G.R.; et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J. Infect. Dis. 2004, 190, 1140–1149. [Google Scholar] [CrossRef]
- Li, L.; Wang, G.; Li, Y.; Francois, P.; Bayer, A.S.; Chen, L.; Seidl, K.; Cheung, A.; Xiong, Y.Q. Impact of the novel prophage varphiSA169 on persistent methicillin-resistant Staphylococcus aureus endovascular infection. mSystems 2020, 5. [Google Scholar] [CrossRef]
- Seidl, K.; Chen, L.; Bayer, A.S.; Hady, W.A.; Kreiswirth, B.N.; Xiong, Y.Q. Relationship of agr expression and function with virulence and vancomycin treatment outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 5631–5639. [Google Scholar] [CrossRef] [PubMed]
- Abdelhady, W.; Chen, L.; Bayer, A.S.; Seidl, K.; Yeaman, M.R.; Kreiswirth, B.N.; Xiong, Y.Q. Early agr activation correlates with vancomycin treatment failure in multi-clonotype MRSA endovascular infections. J. Antimicrob. Chemother. 2015, 70, 1443–1452. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Otto, M. Improved understanding of factors driving methicillin-resistant Staphylococcus aureus epidemic waves. Clin. Epidemiol. 2013, 5, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Bondy-Denomy, J.; Qian, J.; Westra, E.R.; Buckling, A.; Guttman, D.S.; Davidson, A.R.; Maxwell, K.L. Prophages mediate defense against phage infection through diverse mechanisms. ISME J. 2016, 10, 2854–2866. [Google Scholar] [CrossRef] [PubMed]
- Waldron, D.E.; Lindsay, J.A. Sau1: A novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J. Bacteriol. 2006, 188, 5578–5585. [Google Scholar] [CrossRef]
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef]
- Yee, R.; Yuan, Y.; Tarff, A.; Brayton, C.; Gour, N.; Feng, J.; Zhang, Y. Eradication of Staphylococcus aureus biofilm infection by persister drug combination. Antibiotics 2022, 11, 1278. [Google Scholar] [CrossRef]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef]
- Baek, K.T.; Frees, D.; Renzoni, A.; Barras, C.; Rodriguez, N.; Manzano, C.; Kelley, W.L. Genetic variation in the Staphylococcus aureus 8325 strain lineage revealed by whole-genome sequencing. PLoS ONE 2013, 8, e77122. [Google Scholar] [CrossRef] [PubMed]
- Novick, R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 1967, 33, 155–166. [Google Scholar] [CrossRef]
- Abdelhady, W.; Bayer, A.S.; Seidl, K.; Moormeier, D.E.; Bayles, K.W.; Cheung, A.; Yeaman, M.R.; Xiong, Y.Q. Impact of vancomycin on sarA-mediated biofilm formation: Role in persistent endovascular infections due to methicillin-resistant Staphylococcus aureus. J. Infect. Dis. 2014, 209, 1231–1240. [Google Scholar] [CrossRef]
- Challagundla, L.; Reyes, J.; Rafiqullah, I.; Sordelli, D.O.; Echaniz-Aviles, G.; Velazquez-Meza, M.E.; Castillo-Ramirez, S.; Fittipaldi, N.; Feldgarden, M.; Chapman, S.B.; et al. Phylogenomic classification and the evolution of clonal complex 5 methicillin-resistant Staphylococcus aureus in the western hemisphere. Front. Microbiol. 2018, 9, 1901. [Google Scholar] [CrossRef]
- Jiang, J.H.; Cameron, D.R.; Nethercott, C.; Aires-de-Sousa, M.; Peleg, A.Y. Virulence attributes of successful methicillin-resistant Staphylococcus aureus lineages. Clin. Microbiol. Rev. 2023, 36, e0014822. [Google Scholar] [CrossRef]
- Bal, A.M.; Coombs, G.W.; Holden, M.T.G.; Lindsay, J.A.; Nimmo, G.R.; Tattevin, P.; Skov, R.L. Genomic insights into the emergence and spread of international clones of healthcare-, community- and livestock-associated meticillin-resistant Staphylococcus aureus: Blurring of the traditional definitions. J. Glob. Antimicrob. Resist. 2016, 6, 95–101. [Google Scholar] [CrossRef]
- Carrel, M.; Perencevich, E.N.; David, M.Z. USA300 methicillin-resistant Staphylococcus aureus, United States, 2000–2013. Emerg. Infect. Dis. 2015, 21, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Richter, S.S.; Heilmann, K.P.; Dohrn, C.L.; Riahi, F.; Tendolkar, S.; McDanel, J.S.; Doern, G.V. Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: Results from a nationwide surveillance study. Infect. Control Hosp. Epidemiol. 2014, 35, 285–292. [Google Scholar] [CrossRef]
- Wirtz, C.; Witte, W.; Wolz, C.; Goerke, C. Transcription of the phage-encoded Panton-Valentine leukocidin of Staphylococcus aureus is dependent on the phage life-cycle and on the host background. Microbiology 2009, 155, 3491–3499. [Google Scholar] [CrossRef]
- Alves, D.R.; Gaudion, A.; Bean, J.E.; Perez Esteban, P.; Arnot, T.C.; Harper, D.R.; Kot, W.; Hansen, L.H.; Enright, M.C.; Jenkins, A.T. Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl. Environ. Microbiol. 2014, 80, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Krausz, K.L.; Bose, J.L. Bacteriophage transduction in Staphylococcus aureus: Broth-based method. Methods Mol. Biol. 2016, 1373, 63–68. [Google Scholar] [CrossRef]
- Guerrero-Bustamante, C.A.; Hatfull, G.F. Bacteriophage tRNA-dependent lysogeny: Requirement of phage-encoded tRNA genes for establishment of lysogeny. mBio 2024, 15, e0326023. [Google Scholar] [CrossRef]
- Petrova, Z.O.; Broussard, G.W.; Hatfull, G.F. Mycobacteriophage-repressor-mediated immunity as a selectable genetic marker: Adephagia and BPs repressor selection. Microbiology 2015, 161, 1539–1551. [Google Scholar] [CrossRef][Green Version]
- Brown, S.; Mitarai, N.; Sneppen, K. Protection of bacteriophage-sensitive Escherichia coli by lysogens. Proc. Natl. Acad. Sci. USA 2022, 119, e2106005119. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, S.C.; Rose, W.E.; Patel, R.; Proctor, R.A.; Chambers, H.F.; Harrison, E.M.; Pak, Y.; Bayer, A.S. A combined phenotypic-genotypic predictive algorithm for in vitro detection of bicarbonate: Beta-lactam sensitization among methicillin-resistant Staphylococcus aureus (MRSA). Antibiotics 2021, 10, 1089. [Google Scholar] [CrossRef] [PubMed]
- Atshan, S.S.; Shamsudin, M.N.; Lung, L.T.; Sekawi, Z.; Ghaznavi-Rad, E.; Pei, C.P. Comparative characterisation of genotypically different clones of MRSA in the production of biofilms. J. Biomed. Biotechnol. 2012, 2012, 417247. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Abdelhady, W.; Donegan, N.P.; Seidl, K.; Cheung, A.; Zhou, Y.F.; Yeaman, M.R.; Bayer, A.S.; Xiong, Y.Q. Role of purine biosynthesis in persistent methicillin-resistant Staphylococcus aureus infection. J. Infect. Dis. 2018, 218, 1367–1377. [Google Scholar] [CrossRef]

| Strains | Characteristics | VAN MIC (μg/mL) | References |
|---|---|---|---|
| 088-237 | RB-MRSA, agr-II, SCCmec II, CC5 | 1.0 | [16,23] |
| 088-237lyso | 088-237 lysogenized with prophage ϕSA169 | 1.0 | This study |
| 22033 | RB-MRSA, agr-III, SCCmec IV, CC30 | 1.0 | [17] |
| 22033lyso | 22033 lysogenized with prophage ϕSA169 | 1.0 | This study |
| 300-169 | PB-MRSA, agr-I, SCCmec IV, CC45; donor of ϕSA169 | [16,23] | |
| RN4220 | MSSA, NCTC8325-4, α-hemolysin negative, β-hemolysin positive | [24,25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Berti, A.D.; Abdelhady, W.; Xiong, Y.Q. Prophage ϕSA169 Enhances Vancomycin Persistence in Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics 2025, 14, 191. https://doi.org/10.3390/antibiotics14020191
Li Y, Berti AD, Abdelhady W, Xiong YQ. Prophage ϕSA169 Enhances Vancomycin Persistence in Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics. 2025; 14(2):191. https://doi.org/10.3390/antibiotics14020191
Chicago/Turabian StyleLi, Yi, Andrew D. Berti, Wessam Abdelhady, and Yan Q. Xiong. 2025. "Prophage ϕSA169 Enhances Vancomycin Persistence in Methicillin-Resistant Staphylococcus aureus (MRSA)" Antibiotics 14, no. 2: 191. https://doi.org/10.3390/antibiotics14020191
APA StyleLi, Y., Berti, A. D., Abdelhady, W., & Xiong, Y. Q. (2025). Prophage ϕSA169 Enhances Vancomycin Persistence in Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics, 14(2), 191. https://doi.org/10.3390/antibiotics14020191







