Exploring Antimycobacterial Potential: Safety Evaluation and Active Compound Isolation from Gymnopilus junonius
Abstract
1. Introduction
2. Results
2.1. Preliminary Antimycobacterial Screening of an Ethanol Extract of G. junonius
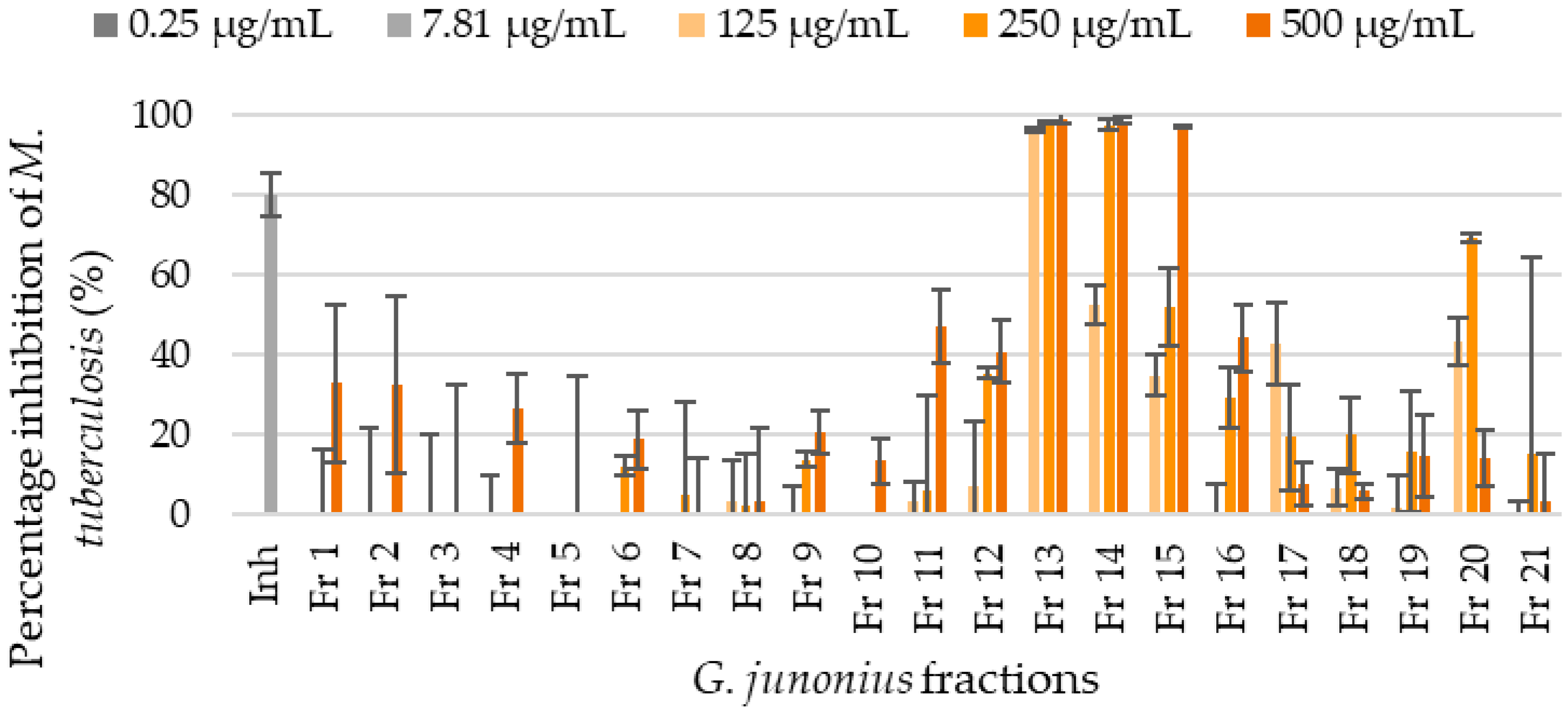
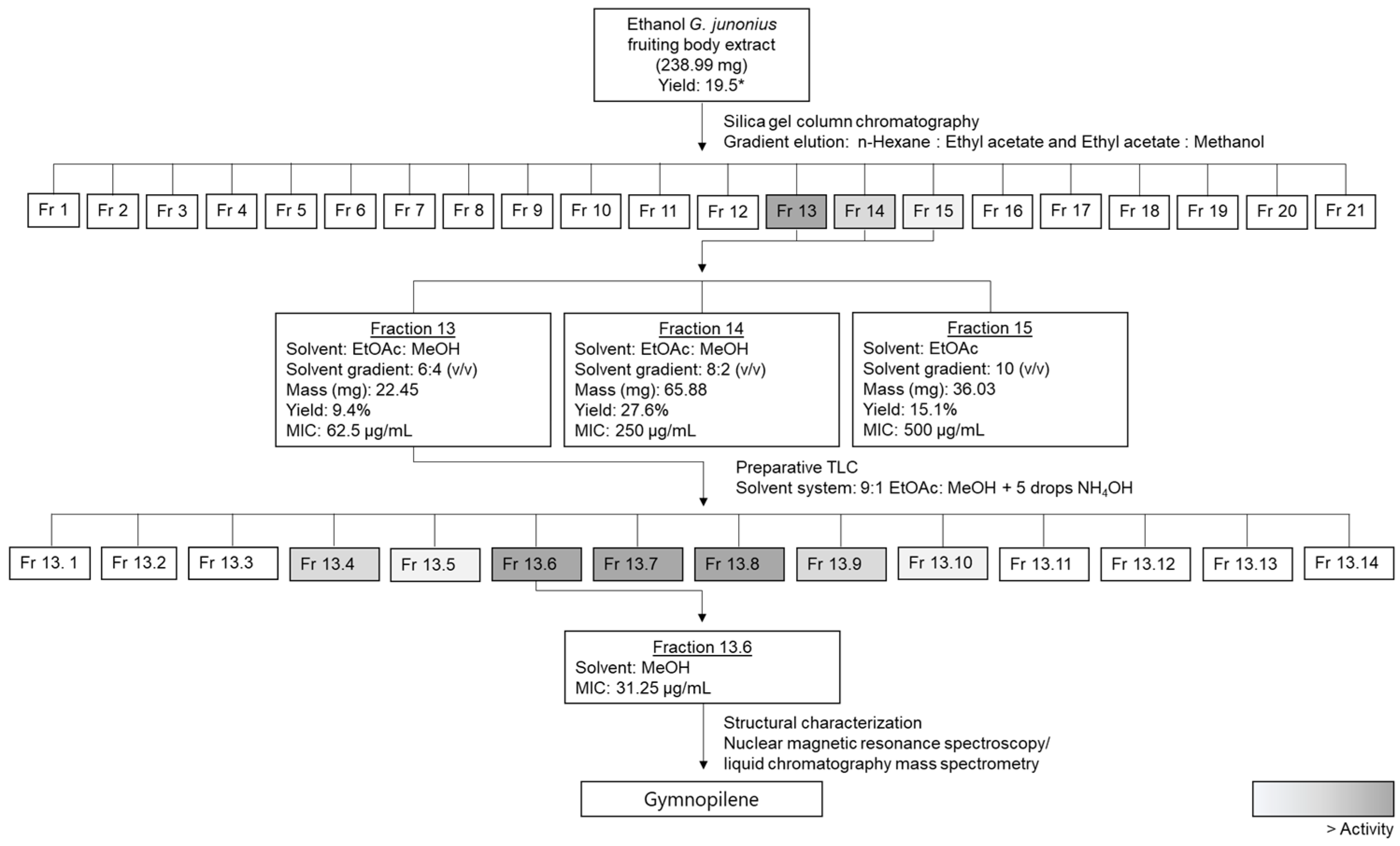
2.2. Bioassay-Guided Fractionation and Determination of the Antimycobacterial Activity of Isolated Fractions from a G. junonius Extract Using the Resazurin Microtiter Assay (REMA)
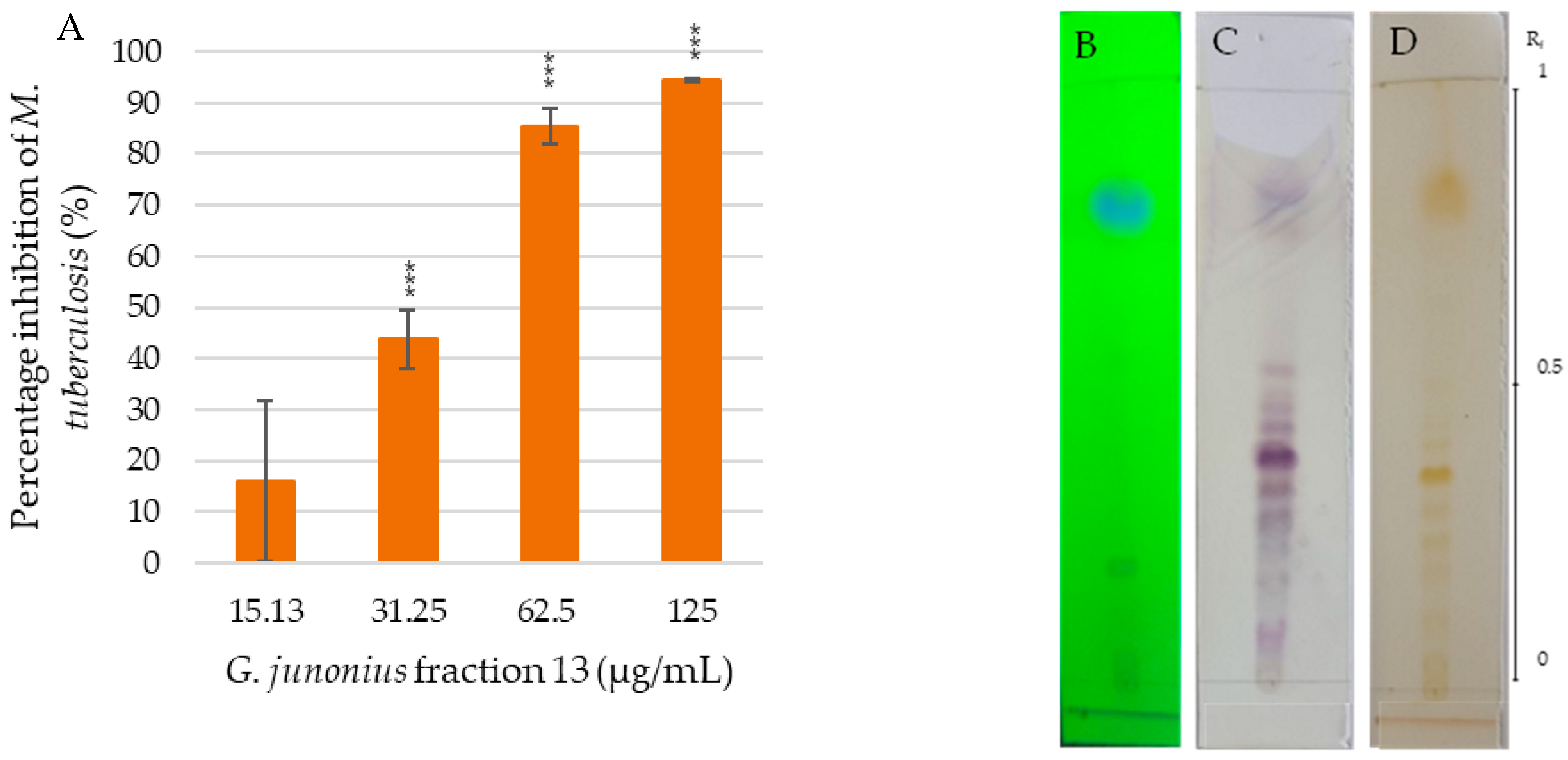
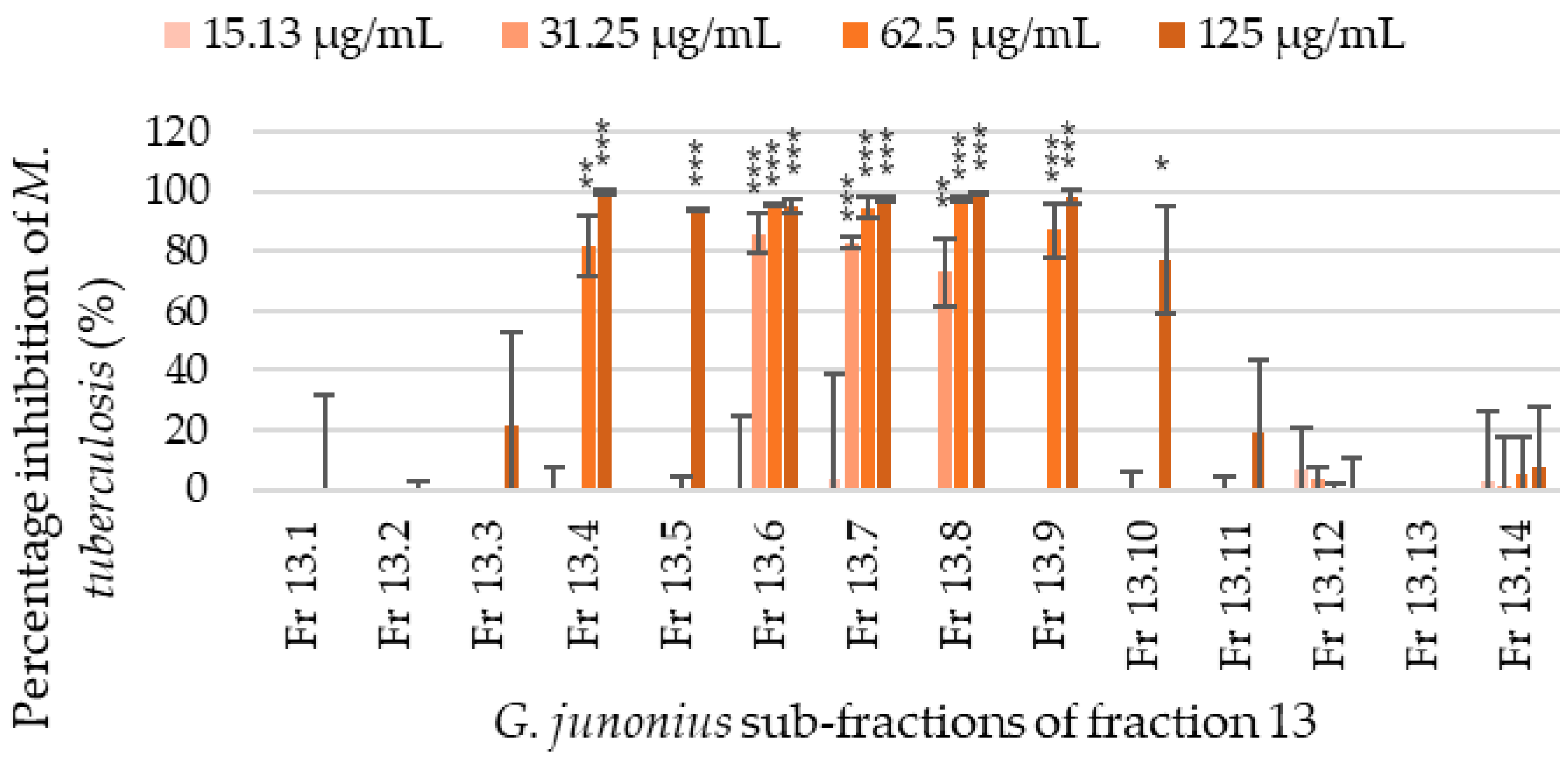
2.3. Isolation of Sub-Fractions from Fraction 13 Using Preparative TLC and Determination of Antimycobacterial Activity
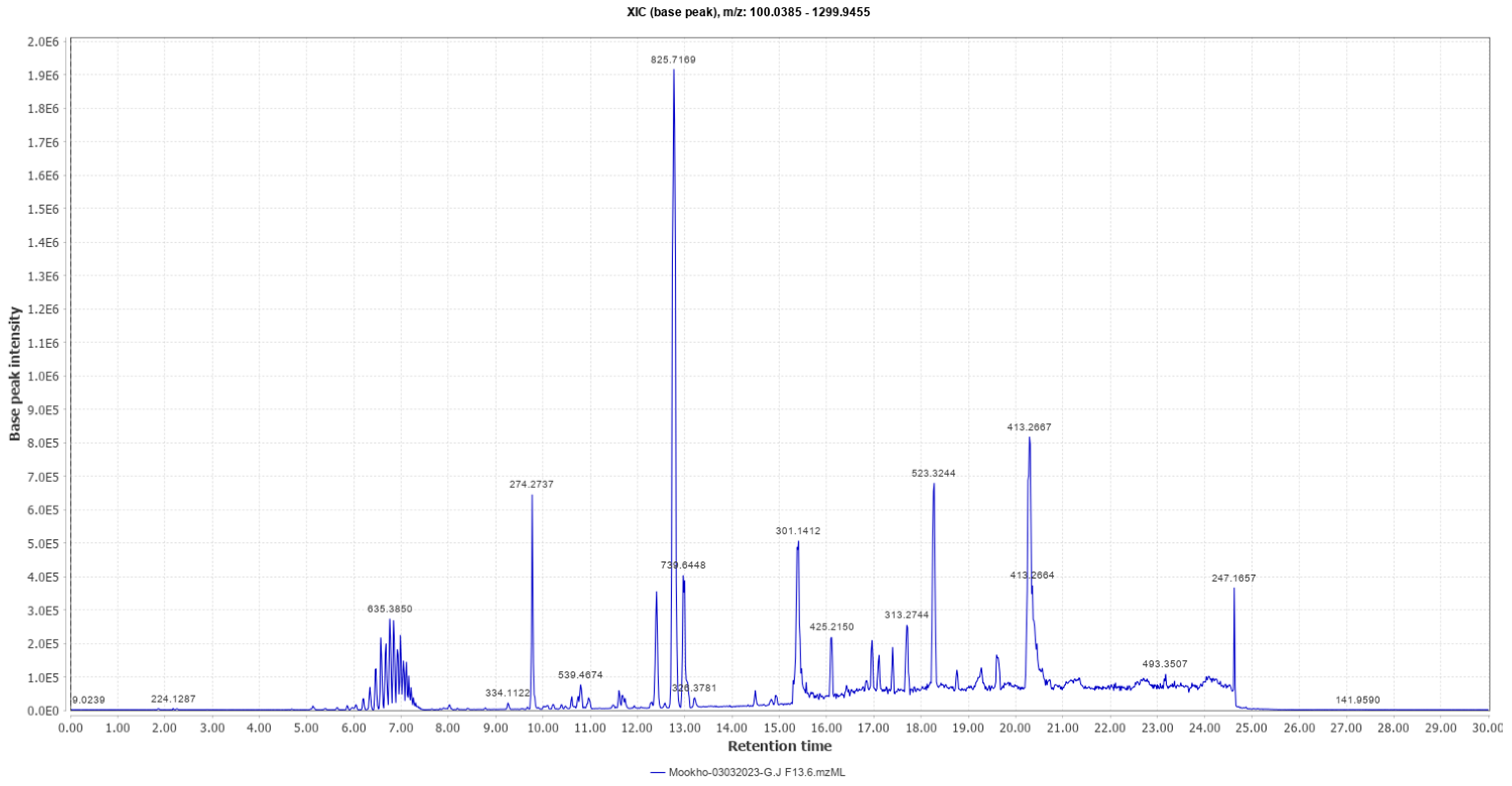
2.4. Structural Elucidation
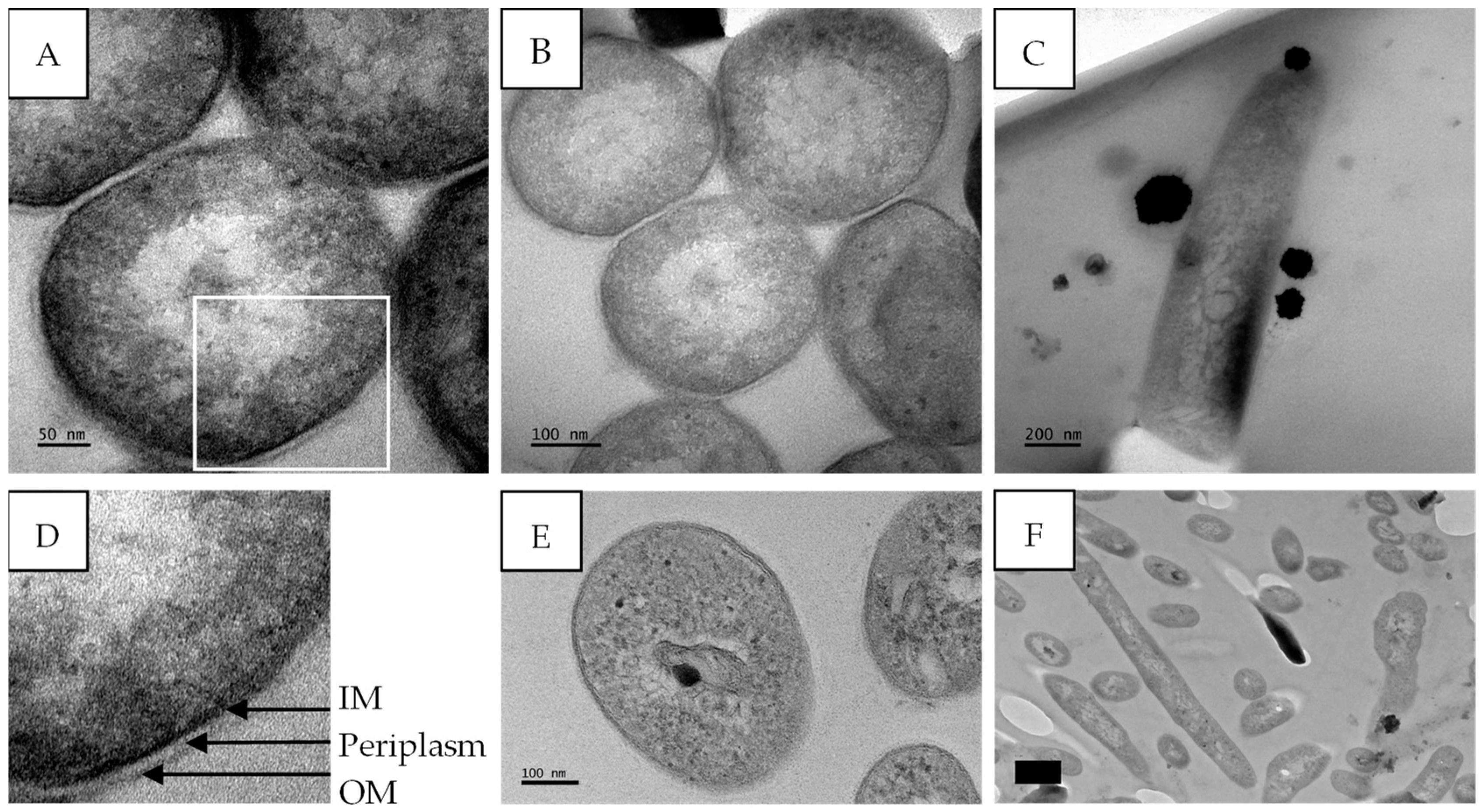
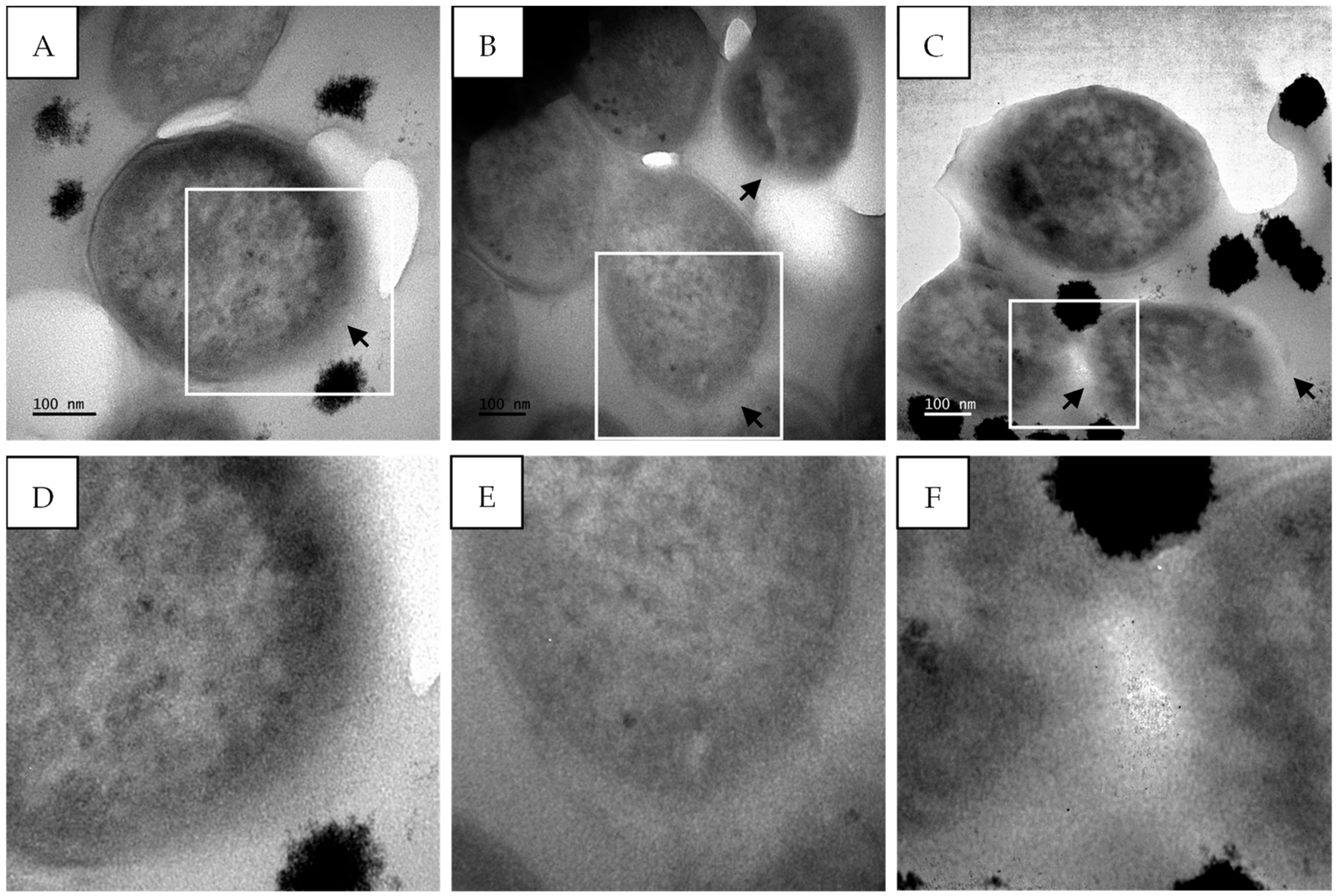
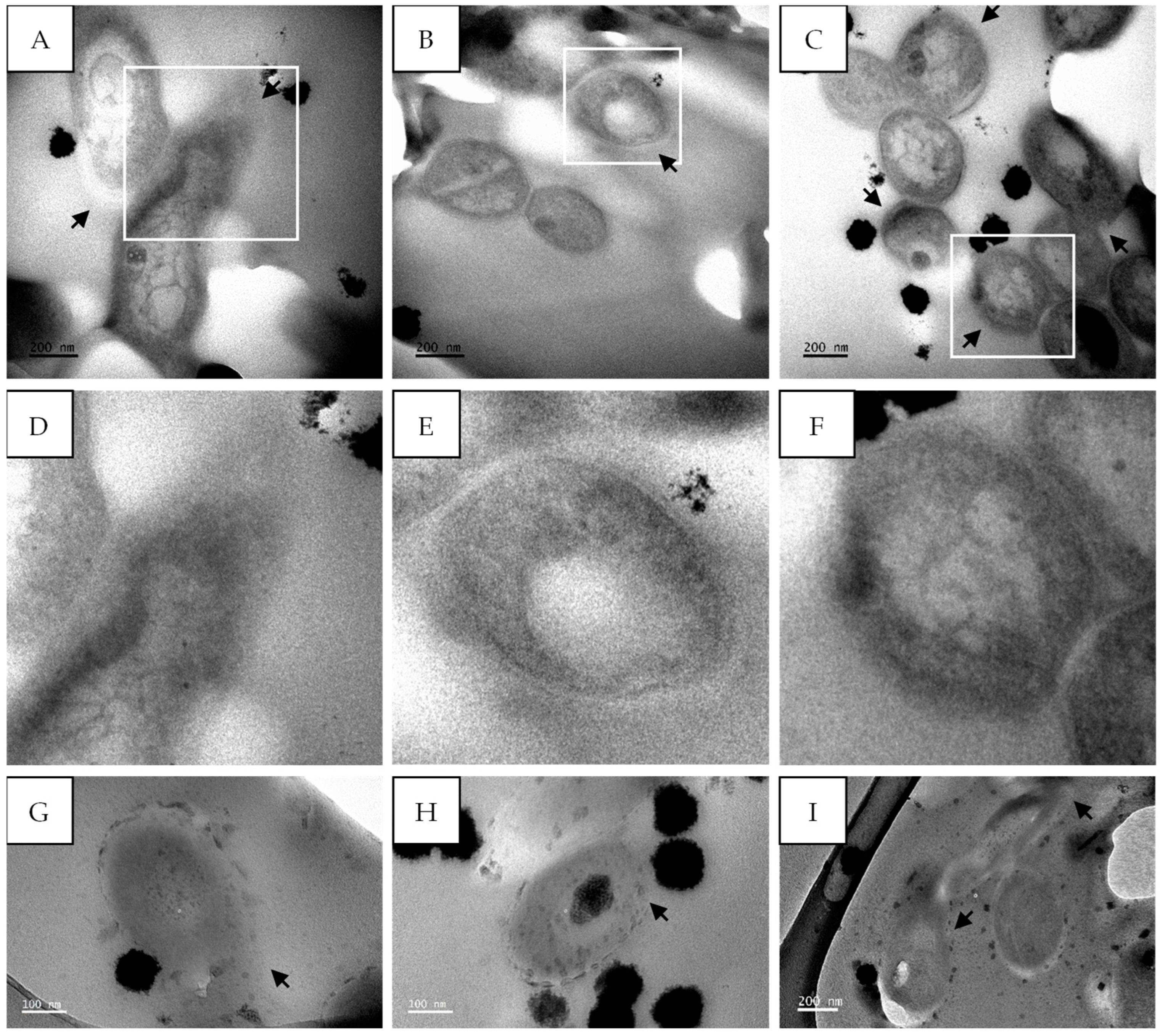
2.5. TEM
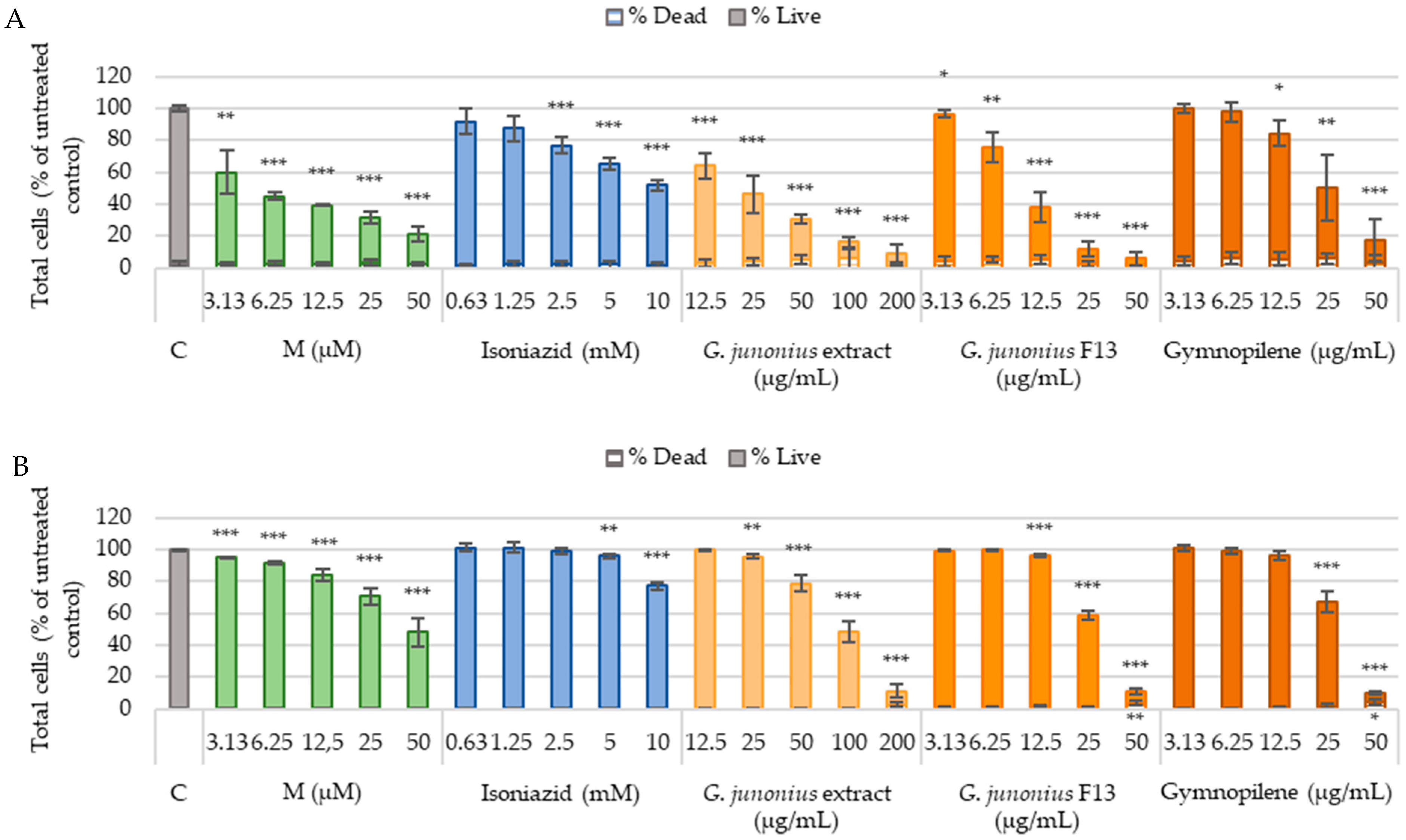
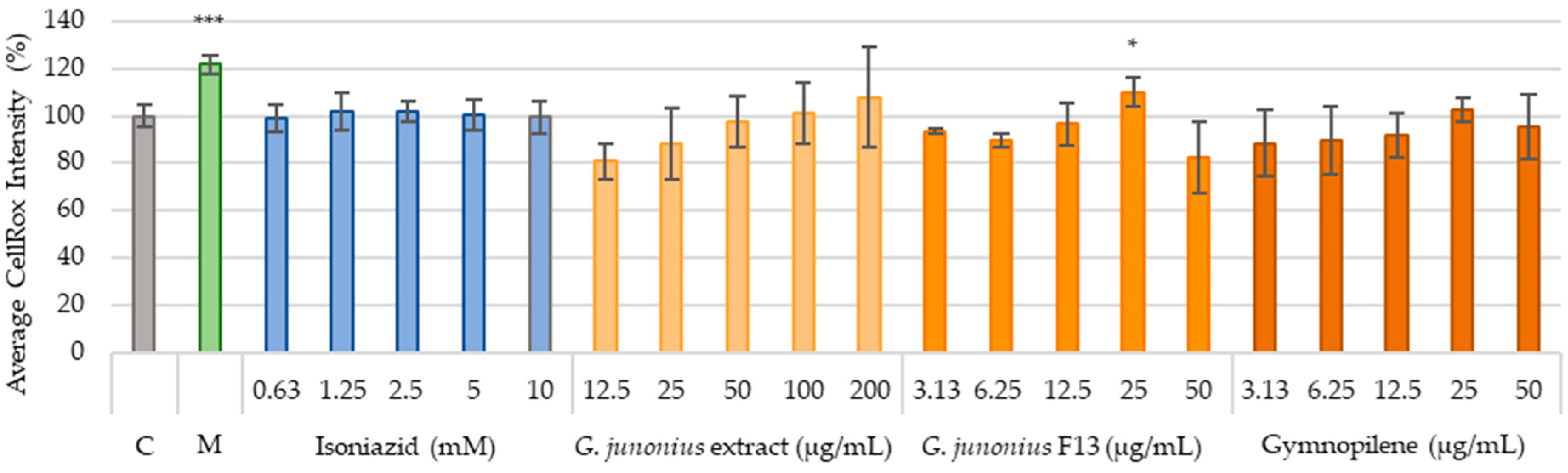
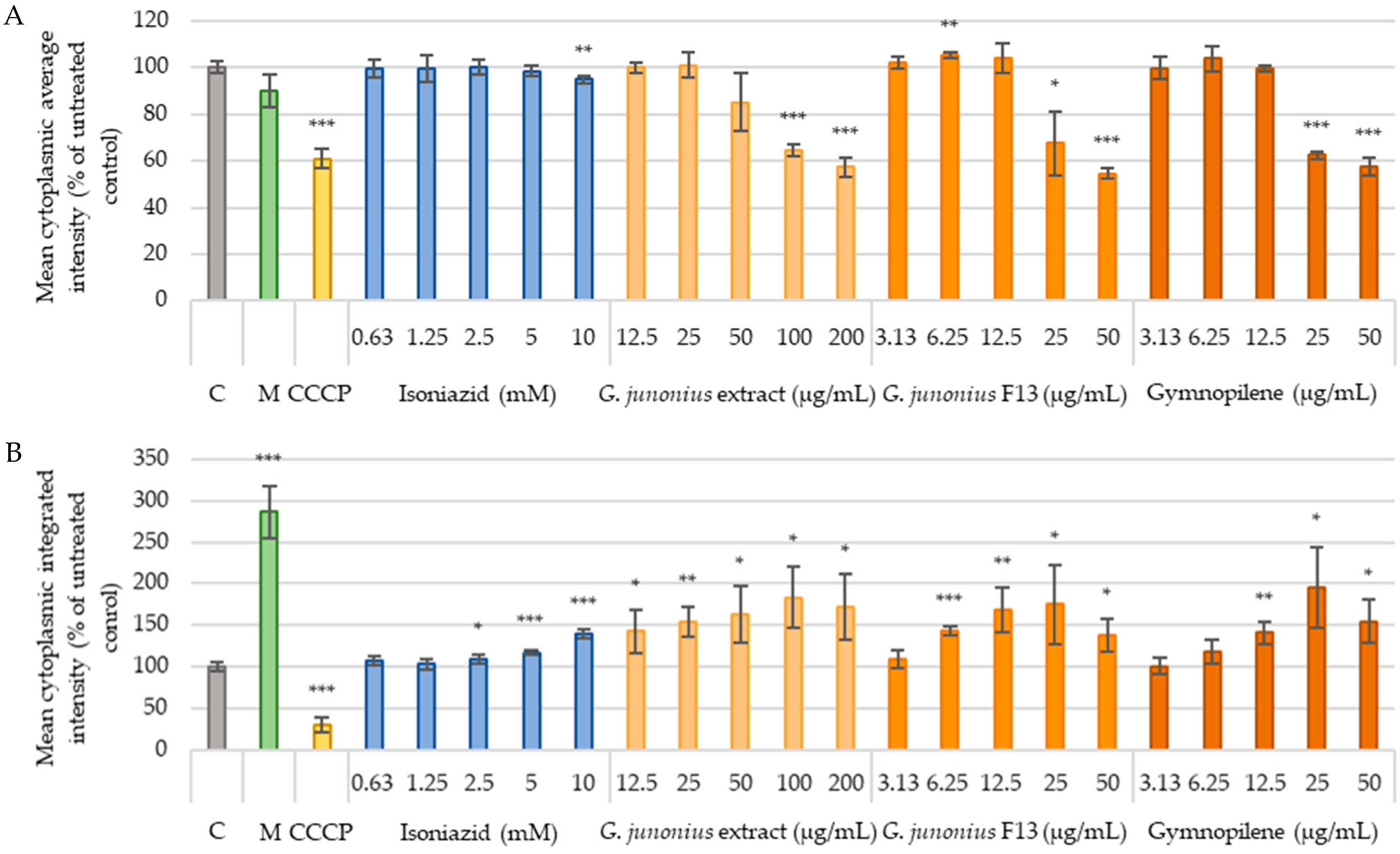
2.6. Cytotoxicity Against Human Liver Cells (C3A) and African Green Monkey Kidney Cells (Vero)
2.7. Hepatotoxicity Assessment
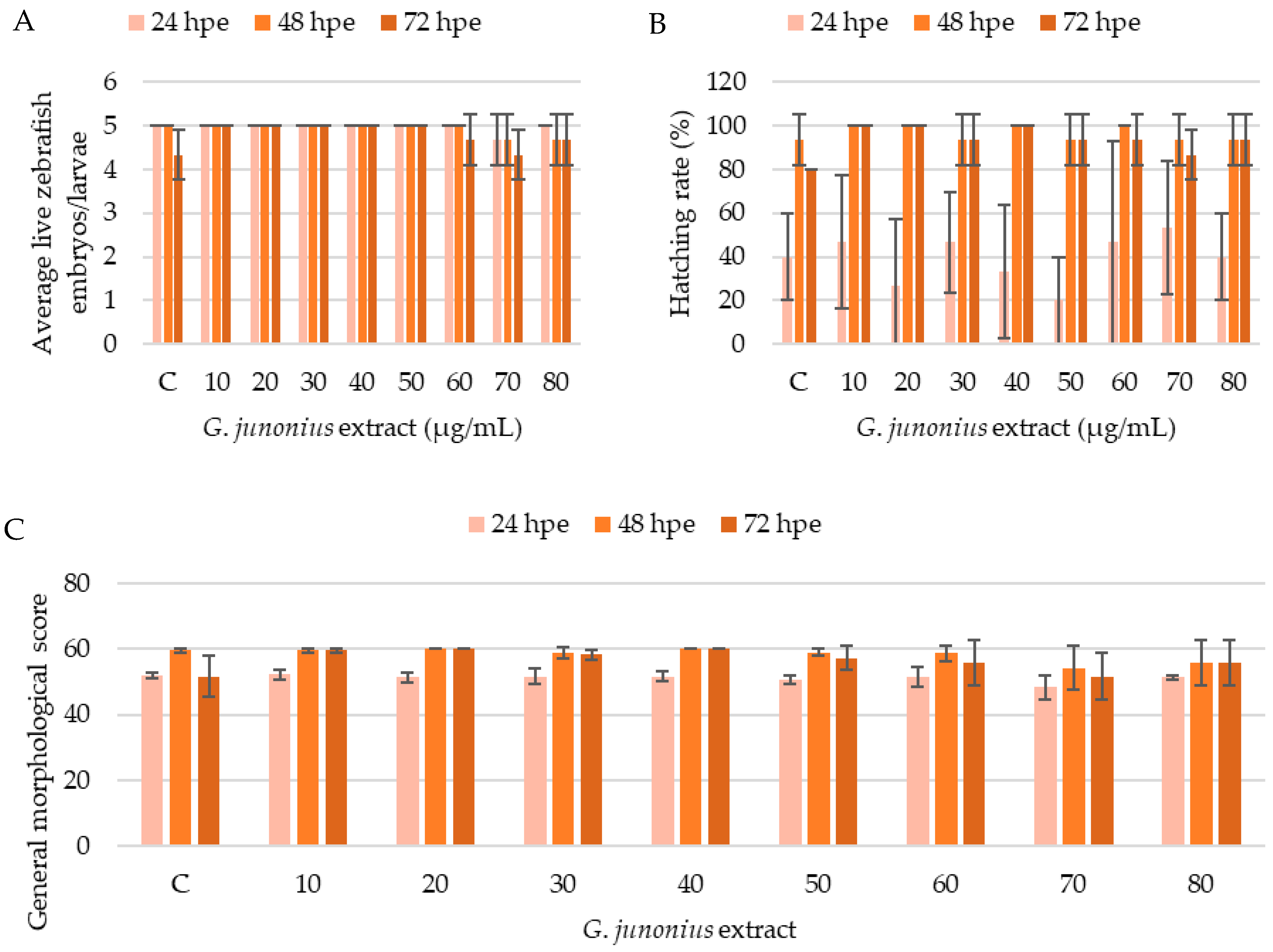
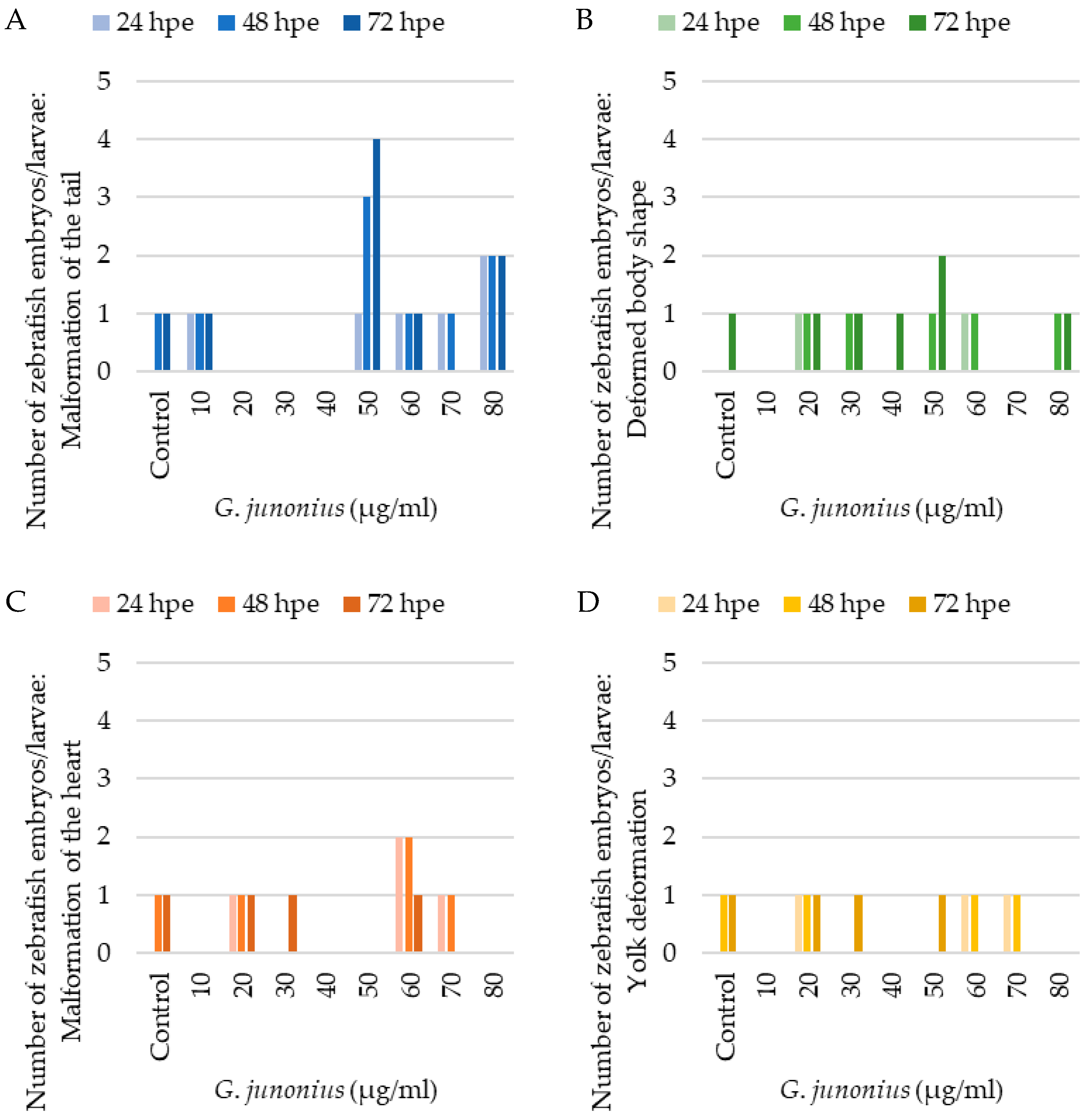
2.8. Zebrafish Toxicity Screening
3. Discussion
4. Materials and Methods
4.1. Macrofungal Collection, Identification, and Extract Preparation
4.2. Bioactivity-Guided Fractionation of Ethanol Extract
4.2.1. Silica Gel Chromatography
4.2.2. The Determination of the Antimycobacterial Activity of the Crude Extract and Fractions Using REMA
4.2.3. Isolation of Active Antimycobacterial Compound from G. junonius Fraction Using Preparative TLC
4.2.4. HPLC-HRESI-MS Acquisition
4.2.5. NMR Structural Elucidation
4.3. TEM
4.3.1. M. tuberculosis Growth Conditions and Treatment for Electron Microscopy
4.3.2. TEM Sample Processing
4.4. In Vitro Toxicity Screening
4.4.1. Cell Culture Conditions and Cell Maintenance
4.4.2. Image Acquisition and Data Analysis
4.4.3. In Vitro Cytotoxicity
4.4.4. Hepatotoxicity
Production of ROS
Mitochondrial Content and Mitochondrial Membrane Potential
4.4.5. Statistical Analysis
4.5. Zebrafish Embryo/Larvae Toxicity Screening
4.5.1. Zebrafish Maintenance (Care), Breeding, and Embryo Collection
4.5.2. Embryo Exposure to Extract
4.5.3. Evaluation of Morphology and Embryotoxicity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boukes, G.J.; Koekemoer, T.C.; van de Venter, M.; Govender, S. Cytotoxicity of thirteen South African macrofungal species against five cancer cell lines. S. Afr. J. Bot. 2017, 113, 62–67. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024.
- Joshi, J.M. Tuberculosis chemotherapy in the 21st century: Back to the basics. Lung India 2011, 28, 193–200. [Google Scholar] [CrossRef]
- Bhat, Z.S.; Rather, M.A.; Maqbool, M.; Ahmad, Z. Drug targets exploited in Mycobacterium tuberculosis: Pitfalls and promises on the horizon. Biomed. Pharmacother. 2018, 103, 1733–1747. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, S.S.R.; Gunosewoyo, H. Tuberculosis: Pathogenesis, current treatment regimens and new drug targets. Int. J. Mol. Sci. 2023, 24, 5202. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Ishizaki, Y.; Takahashi, Y. New antituberculous drugs derived from natural products: Current perspectives and issues in antituberculous drug development. J. Antibiot. 2018, 71, 15–25. [Google Scholar] [CrossRef]
- Intorasoot, S.; Intorasoot, A.; Tawteamwong, A.; Butr-Indr, B.; Phunpae, P.; Tharinjaroen, C.S.; Wattananandkul, U.; Sangboonruang, S.; Khantipongse, J. In Vitro Antimycobacterial Activity of Human Lactoferrin-Derived Peptide, D-hLF 1-11, against Susceptible and Drug-Resistant Mycobacterium tuberculosis and Its Synergistic Effect with Rifampicin. Antibiotics 2022, 11, 1785. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, L.; Li, W.; Yang, Y.; Xu, L.; Ding, Z. Anti-Mycobacterium tuberculosis Active Metabolites from an Endophytic Streptomyces sp. YIM65484. Rec. Nat. Prod. 2015, 9, 196–200. [Google Scholar]
- Kumar, A.; Chettiar, S.; Parish, T. Current challenges in drug discovery for tuberculosis. Expert Opin. Drug Discov. 2017, 12, 1–4. [Google Scholar] [CrossRef]
- Isaka, M.; Sappan, M.; Suvannakad, R.; Boonpratuang, T.; Thummarukcharoen, T. Drimane sesquiterpenoids from cultures of the basidiomycete Gymnopilus sp. BCC 19384. Phytochem. Lett. 2020, 35, 141–146. [Google Scholar] [CrossRef]
- Caldas, L.A.; Santos, P.D.; Carbonero, E.R.; Ionta, M.; Miyazawa, M.; Caixeta, E.S.; Fregnan, A.M.; Nóbrega, B.B.; Di Medeiros, M.C.B.; Menolli, N.; et al. Immunomodulatory effect of polysaccharides from the mushroom-forming basidiomycete Gymnopilus imperialis (Agaricomycetes, Basidiomycota). Pharmaceuticals 2022, 15, 1179. [Google Scholar] [CrossRef]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.H.; Kim, S.H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharm. Res. 2020, 43, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Branch, M. Sasol Mushrooms of Southern Africa: A First Field Guide; Penguin Random House South Africa: Johannesburg, South Africa, 2001. [Google Scholar]
- Gryzenhout, M. Pocket Guide to Mushrooms of South Africa; Penguin Random House South Africa: Johannesburg, South Africa, 2010. [Google Scholar]
- Ragupathi, V.; Stephen, A.; Arivoli, D.; Kumaresan, S. Antibacterial activiity, In vitro antioxidant potential and GC-MS characterization of methanolic extract of Gymnopilus junonius, a wild mushroom from Southern Western Ghats, India. European J. Biomed. Pharm. Sci. 2018, 5, 650–657. [Google Scholar]
- Kim, K.H.; Choi, S.U.; Lee, K.R. Gymnopilin K: A new cytotoxic gymnopilin from Gymnopilus spectabilis. J. Antibiot. 2012, 65, 135–137. [Google Scholar] [CrossRef]
- Lee, L.-K.; Cho, S.-M.; Seok, S.-J.; Yun, B.-S. Chemical constituents of Gymnopilus spectabilis and their antioxidant activity. Mycobiology 2008, 36, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Boukes, G.J.; Didloff, J.; van de Venter, M.; Govender, S. Antitubercular and HIV-1 Enzyme Inhibitory Activities of South African Macrofungi; The 43rd FEBS Congress: Prague, Czech Republic, 2018; p. 284. [Google Scholar]
- Didloff, J.; Boukes, G.J.; van de Venter, M.; Viljoen, B.; Lee, M.; Blom, C.; Dwyer, R.A.; Govender, S. Investigation of the inhibition of respiratory bacterial pathogens and HIV-1 enzymes by twenty-one South African mushroom species. S. Afr. J. Bot. 2024, 166, 375–385. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Mambe, F.T.; Na-Iya, J.; Fotso, G.W.; Ashu, F.; Ngameni, B.; Ngadjui, B.T.; Beng, V.P.; Kuete, V. Antibacterial and antibiotic modifying potential of crude extracts, fractions, and compounds from Acacia polyacantha Willd. against MDR Gram-negative bacteria. Evid. Based Complement. Alternat. Med. 2019, 2019, 7507549. [Google Scholar] [CrossRef] [PubMed]
- Aro, A.O.; Dzoyem, J.P.; Awouafack, M.D.; Selepe, M.A.; Eloff, J.N.; McGaw, L.J. Fractions and isolated compounds from Oxyanthus speciosus subsp. stenocarpus (Rubiaceae) have promising antimycobacterial and intracellular activity. BMC Complement. Altern. Med. 2019, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.A.; He, Z. Minor constituents of Gymnopilus spectabilis. J. Nat. Prod. 1991, 54, 184–189. [Google Scholar] [CrossRef]
- Rampa, K.M.; Van De Venter, M.; Koekemoer, T.C.; Swanepoel, B.; Venables, L.; Hattingh, A.C.; Viljoen, A.M.; Kamatou, G.P. Exploring four South African Croton species for potential anti-inflammatory properties: In vitro activity and toxicity risk assessment. J. Ethnopharmacol. 2022, 282, 114596. [Google Scholar] [CrossRef]
- Hermsen, S.A.; van den Brandhof, E.J.; van der Ven, L.T.; Piersma, A.H. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies. Toxicol. Vitr. 2011, 25, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Beekhuijzen, M.; de Koning, C.; Flores-Guillén, M.-E.; de Vries-Buitenweg, S.; Tobor-Kaplon, M.; van de Waart, B.; Emmen, H. From cutting edge to guideline: A first step in harmonization of the zebrafish embryotoxicity test (ZET) by describing the most optimal test conditions and morphology scoring system. Reprod. Toxicol. 2015, 56, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Ferreira, I.C.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The antibacterial activity of Coriolus versicolor methanol extract and its effect on ultrastructural changes of Staphylococcus aureus and Salmonella Enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar] [CrossRef]
- Maya, M.R.; Ramanaiah, I.; Venkatakrishna, K.; Rameshkumar, K.; Veeramanikandan, V.; Eyini, M.; Balaji, P. Investigation of bioactive compounds of Capsicum Frutescence and Annona Muricata by chromatographic techniques. J. Drug Deliv. Ther. 2019, 9, 485–495. [Google Scholar] [CrossRef]
- Nafi, N.E.M.; Zin, N.B.M.; Pauzi, N.; Khadar, A.S.A.; Anisava, A.R.; Badiazaman, A.A.M.; Mohd, K.S. Cytotoxicity, antioxidant and phytochemical screening of propolis extracts from four different Malaysian stingless bee species. Mal. J. Fund. Appl. Sci. 2019, 15, 307–312. [Google Scholar] [CrossRef]
- Jack, I.R.; Ekong, R.E.; Ndukwe, G.I. Phytochemical constituents of dichloromethane fraction and essential oil of Napoleonaea imperialis rind. J. Pharmacogn. Phytochem. 2020, 9, 59–66. [Google Scholar] [CrossRef]
- Lo, M.-M.; Benfodda, Z.; Dunyach-Rémy, C.; Bénimélis, D.; Roulard, R.; Fontaine, J.-X.; Mathiron, D.; Quéro, A.; Molinié, R.; Meffre, P. Isolation and identification of flavones responsible for the antibacterial activities of Tillandsia bergeri extracts. ACS Omega 2022, 7, 35851–35862. [Google Scholar] [CrossRef] [PubMed]
- Fathoni, A.; Hudiyono, S.; Budianto, E.; Cahyana, A.H.; Agusta, A. Metabolite detection and antibacterial activity of fungal endophytic extracts isolated from Brotowali (Tinospora crispa) plants using TLC-bioautography assay. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1011, 012041. [Google Scholar] [CrossRef]
- Dasgupta, A.; Acharya, K. Mushrooms: An emerging resource for therapeutic terpenoids. 3 Biotech 2019, 9, 369. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.Y.; Fayez, S.; Xiao, H.; Xu, B. New insights into antimicrobial and antibiofilm effects of edible mushrooms. Food Res. Int. 2022, 162, 111982. [Google Scholar] [CrossRef] [PubMed]
- Stanikunaite, R.; Radwan, M.M.; Trappe, J.M.; Fronczek, F.; Ross, S.A. Lanostane-type triterpenes from the mushroom Astraeus pteridis with antituberculosis activity. J. Nat. Prod. 2008, 71, 2077–2079. [Google Scholar] [CrossRef] [PubMed]
- Arpha, K.; Phosri, C.; Suwannasai, N.; Mongkolthanaruk, W.; Sodngam, S. Astraodoric acids A-D: New lanostane triterpenes from edible mushroom Astraeus odoratus and their anti-Mycobacterium tuberculosis H37Ra and cytotoxic activity. J. Agric. Food Chem. 2012, 60, 9834–9841. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Chinthanom, P.; Kongthong, S.; Srichomthong, K.; Choeyklin, R. Lanostane triterpenes from cultures of the Basidiomycete Ganoderma orbiforme BCC 22324. Phytochemistry 2013, 87, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, S.; Koike, Y.; Tsuji, E.; Kusano, G.; Seto, H. Isolation and structure of gymnoprenols, a novel type of polyisoprenepolyols from Gymopilus spectabilis. Tetrahedron Lett. 1983, 24, 1731–1734. [Google Scholar] [CrossRef]
- Suh, W.S.; Lee, S.R.; Kim, C.S.; Moon, E.; Kim, S.Y.; Choi, S.U.; Kang, K.S.; Lee, K.R.; Kim, K.H. A new monoacylglycerol from the fruiting bodies of Gymnopilus spectabilis. J. Chem. Res. 2016, 40, 156–159. [Google Scholar] [CrossRef]
- Yamada, H.; Yamaguchi, M.; Chikamatsu, K.; Aono, A.; Mitarai, S. Structome analysis of virulent Mycobacterium tuberculosis, which survives with only 700 ribosomes per 0.1 fl of cytoplasm. PLoS ONE 2015, 10, e0117109. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, A.; Nam, K.-W.; Jang, W.S.; Kim, Y.-H.; Kim, S.-K.; Lee, B.-E.; Song, H.-Y. Antimycobacterial activity of methanolic plant extract of Artemisia capillaris containing ursolic acid and hydroquinone against Mycobacterium tuberculosis. J. Infect. Chemother. 2016, 22, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Carcamo-Noriega, E.N.; Sathyamoorthi, S.; Banerjee, S.; Gnanamani, E.; Mendoza-Trujillo, M.; Mata-Espinosa, D.; Hernández-Pando, R.; Veytia-Bucheli, J.I.; Possani, L.D.; Zare, R.N. 1,4-Benzoquinone antimicrobial agents against Staphylococcus aureus and Mycobacterium tuberculosis derived from scorpion venom. Proc. Natl. Acad. Sci. USA 2019, 116, 12642–12647. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Chinthanom, P.; Srichomthong, K.; Thummarukcharoen, T. Lanostane triterpenoids from fruiting bodies of the bracket fungus Fomitopsis feei. Tetrahedron Lett. 2017, 58, 1758–1761. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Dulay, R.M.; Valdez, B.C.; Li, Y.; Chakrabarti, S.; Dhillon, B.; Kalaw, S.P.; Reyes, R.G.; Cabrera, E.C. Cytotoxicity of Gymnopilus purpureosquamulosus extracts on hematologic malignant cells through activation of the SAPK/JNK signaling pathway. PLoS ONE 2021, 16, e0252541. [Google Scholar] [CrossRef]
- Serras, A.S.; Rodrigues, J.S.; Cipriano, M.; Rodrigues, A.V.; Oliveira, N.G.; Miranda, J.P. A critical perspective on 3D liver models for drug metabolism and toxicology studies. Front. Cell Dev. Biol. 2021, 9, 626805. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Q.; Guo, J.; Yuan, H.; Peng, H.; Cui, L.; Yin, J.; Zhang, L.; Zhao, J.; Li, J.; et al. Non-cytotoxic concentrations of acetaminophen induced mitochondrial biogenesis and antioxidant response in HepG2 cells. Environ. Toxicol. Pharmacol. 2016, 46, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Silveira, E.S.R.d.C.d.; Zhong, C. Mitochondrial biogenesis in response to chromium (VI) toxicity in human liver cells. Int. J. Mol. Sci. 2017, 18, 1877. [Google Scholar] [CrossRef]
- Venables, L.; Koekemoer, T.C.; Van de Venter, M.; Goosen, E.D. Isoalantolactone, a sesquiterpene lactone from Artemisia afra Jacq. ex Willd and its in vitro mechanism of induced cell death in HeLa cells. S. Afr. J. Bot. 2016, 103, 216–221. [Google Scholar] [CrossRef]
- Gini, T.G.; Jeya Jothi, G. Column chromatography and HPLC analysis of phenolic compounds in the fractions of Salvinia molesta mitchell. Egypt J. Basic and Appl. Sci. 2019, 5, 197–203. [Google Scholar] [CrossRef]
- Franzblau, S.G.; Witzig, R.S.; McLaughlin, J.C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M.T.; Cook, M.B.; Quenzer, V.K.; Ferguson, R.M.; et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998, 36, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Case, R.J.; Wang, Y.; Zhang, H.-J.; Tan, G.T.; Van Hung, N.; Cuong, N.M.; Franzblau, S.G.; Soejarto, D.D.; Fong, H.H.; et al. Anti-tuberculosis constituents from the stem bark of Micromelum hirsutum. Planta Med. 2005, 71, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Venable, J.H.; Coggeshall, R. A simplified lead citrate stain for use in electron microscopy J. Cell Biol. 1965, 25, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 1997, 21, A.3B.1–A.3B.2. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, B.; Nitulescu, G.M.; Olaru, O.T.; Venables, L.; van de Venter, M. Anti-Cancer Activity of a 5-Aminopyrazole Derivative Lead Compound (BC-7) and Potential Synergistic Cytotoxicity with Cisplatin against Human Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5559. [Google Scholar] [CrossRef]
- Gaur, H.; Pullaguri, N.; Nema, S.; Purushothaman, S.; Bhargava, Y.; Bhargava, A. ZebraPace: An Open-Source Method for Cardiac-Rhythm Estimation in Untethered Zebrafish Larvae. Zebrafish 2018, 15, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Murugesu, S.; Khatib, A.; Ahmed, Q.U.; Ibrahim, Z.; Uzir, B.F.; Benchoula, K.; Yusoff, N.I.N.; Perumal, V.; Alajmi, M.F.; Salamah, S.; et al. Toxicity study on Clinacanthus nutans leaf hexane fraction using Danio rerio embryos. Toxicol. Rep. 2019, 6, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, S.K.; Rama Krishnan, K.; Ei, T.; Husna Shafie, N.; Arapoc, D.J.; Bahari, H. Evaluation of the Effect of Aqueous Momordica charantia Linn. Extract on Zebrafish Embryo Model through Acute Toxicity Assay Assessment. Evid. Based Complement. Alternat. Med. 2019, 2019, 9152757. [Google Scholar]

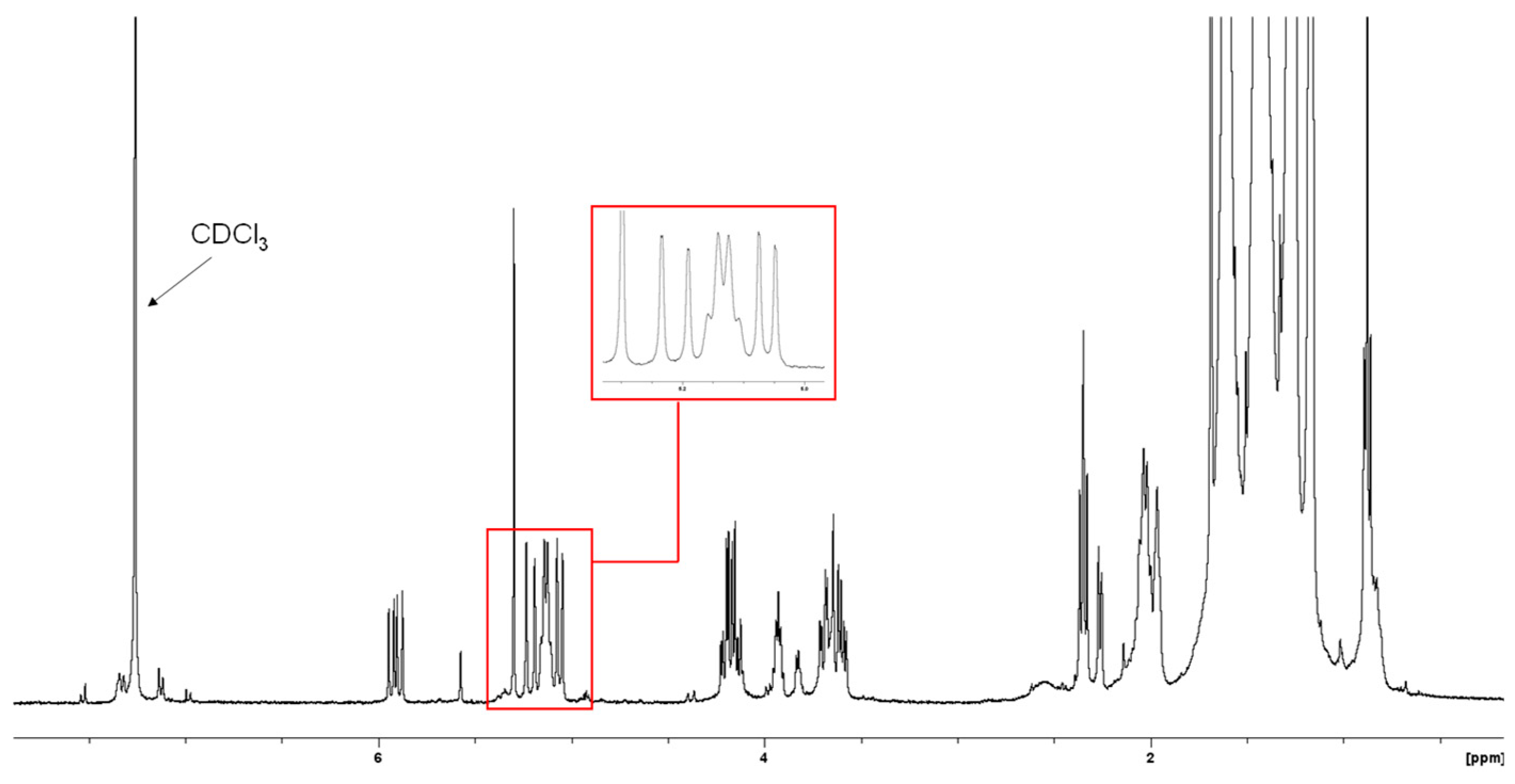
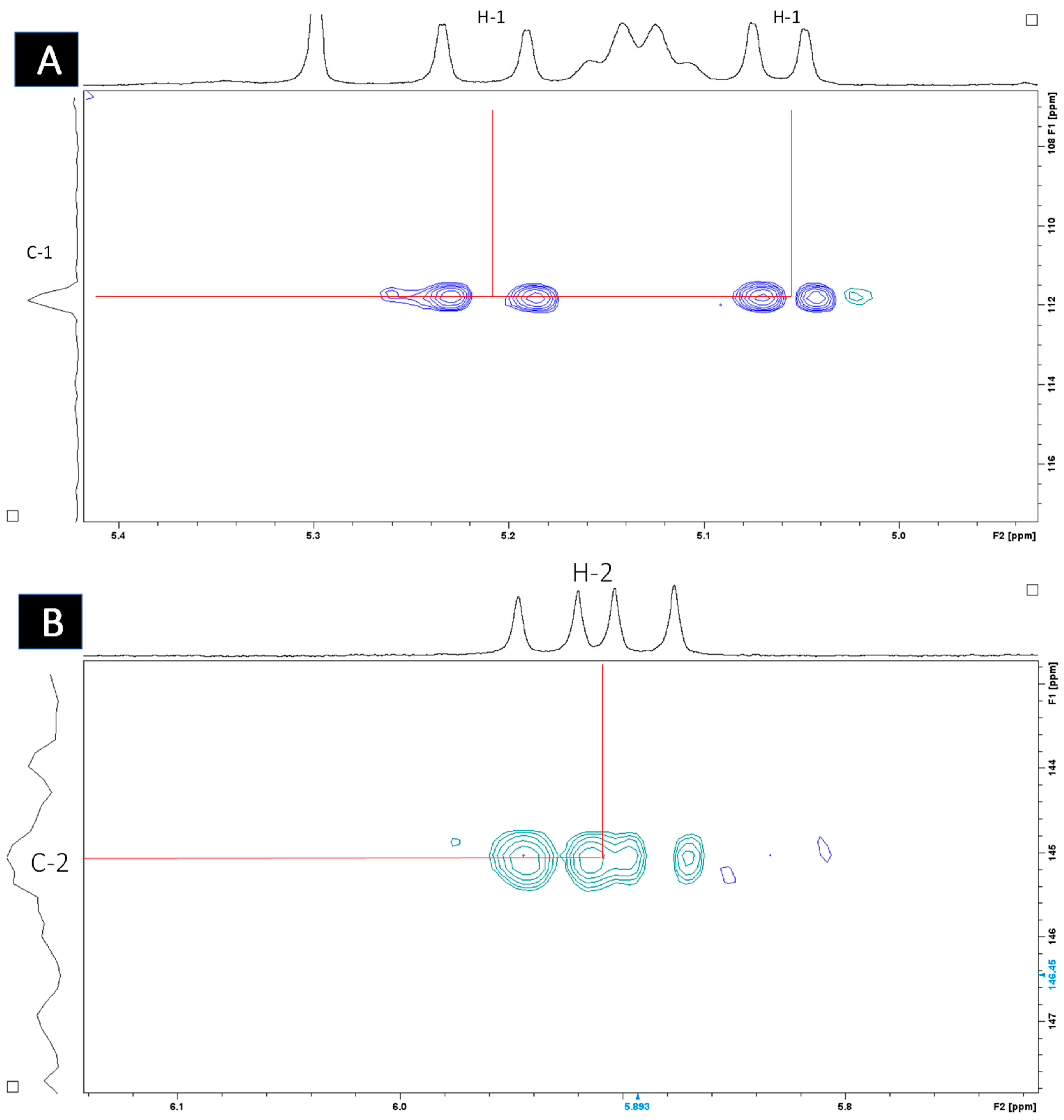
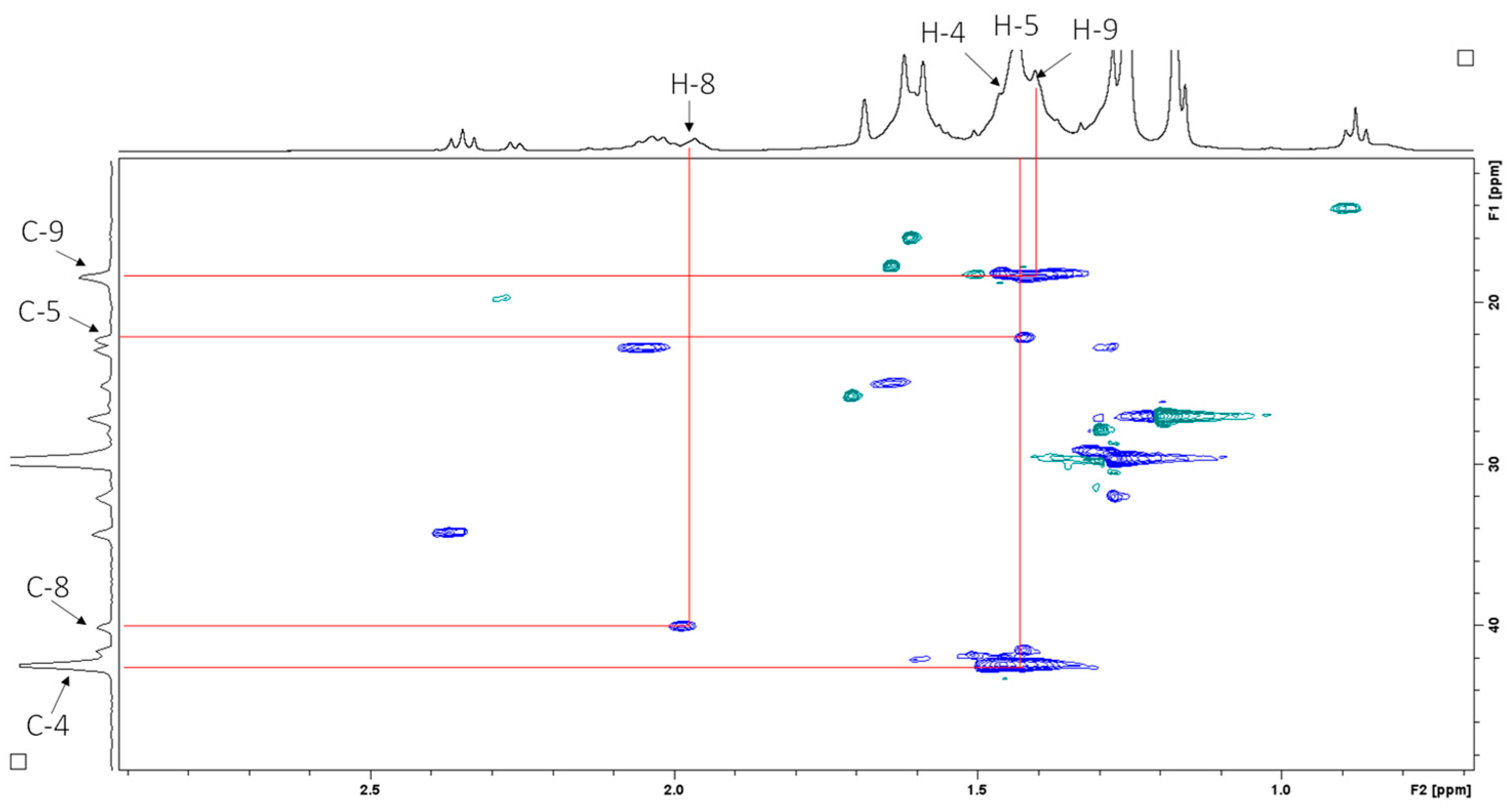
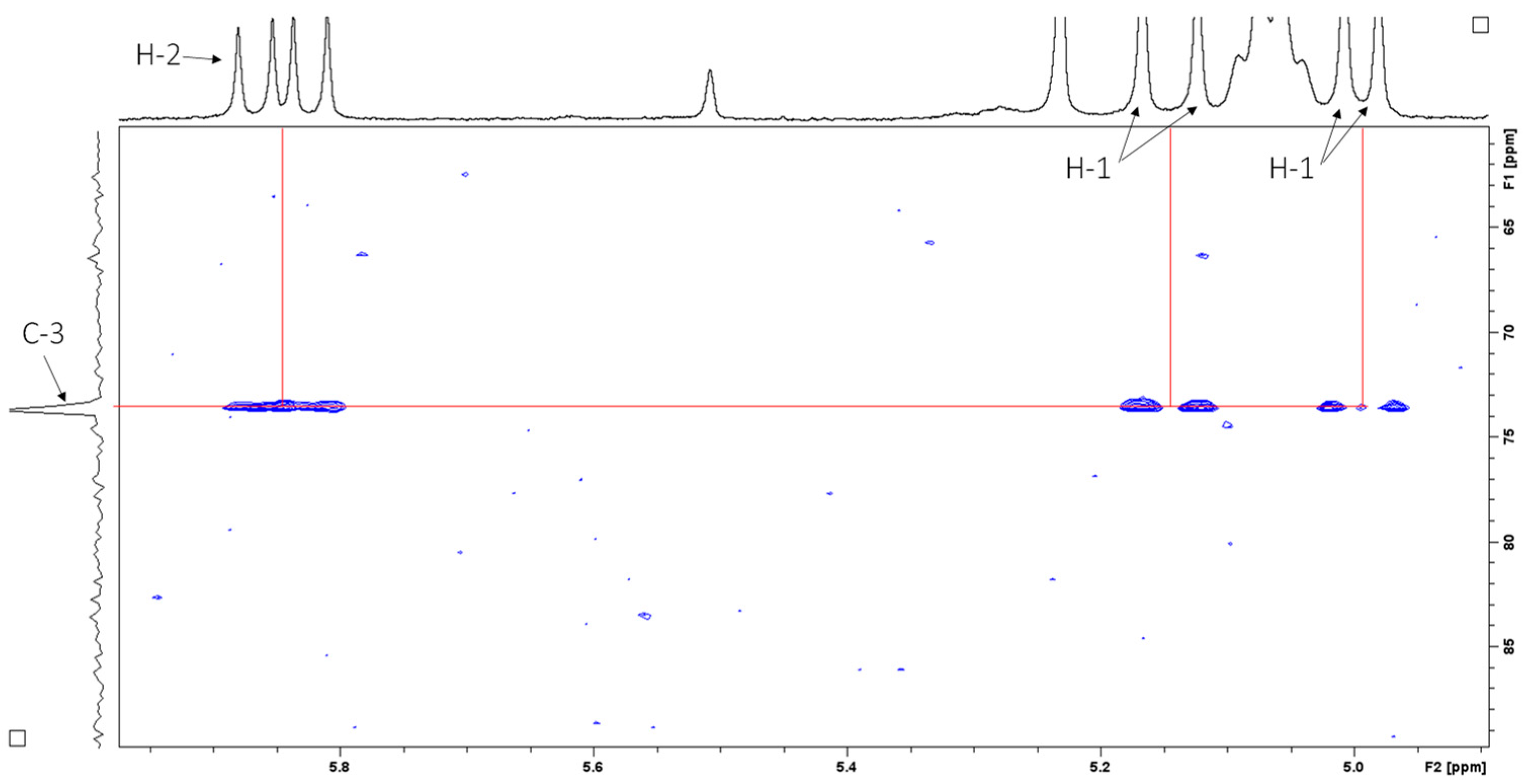
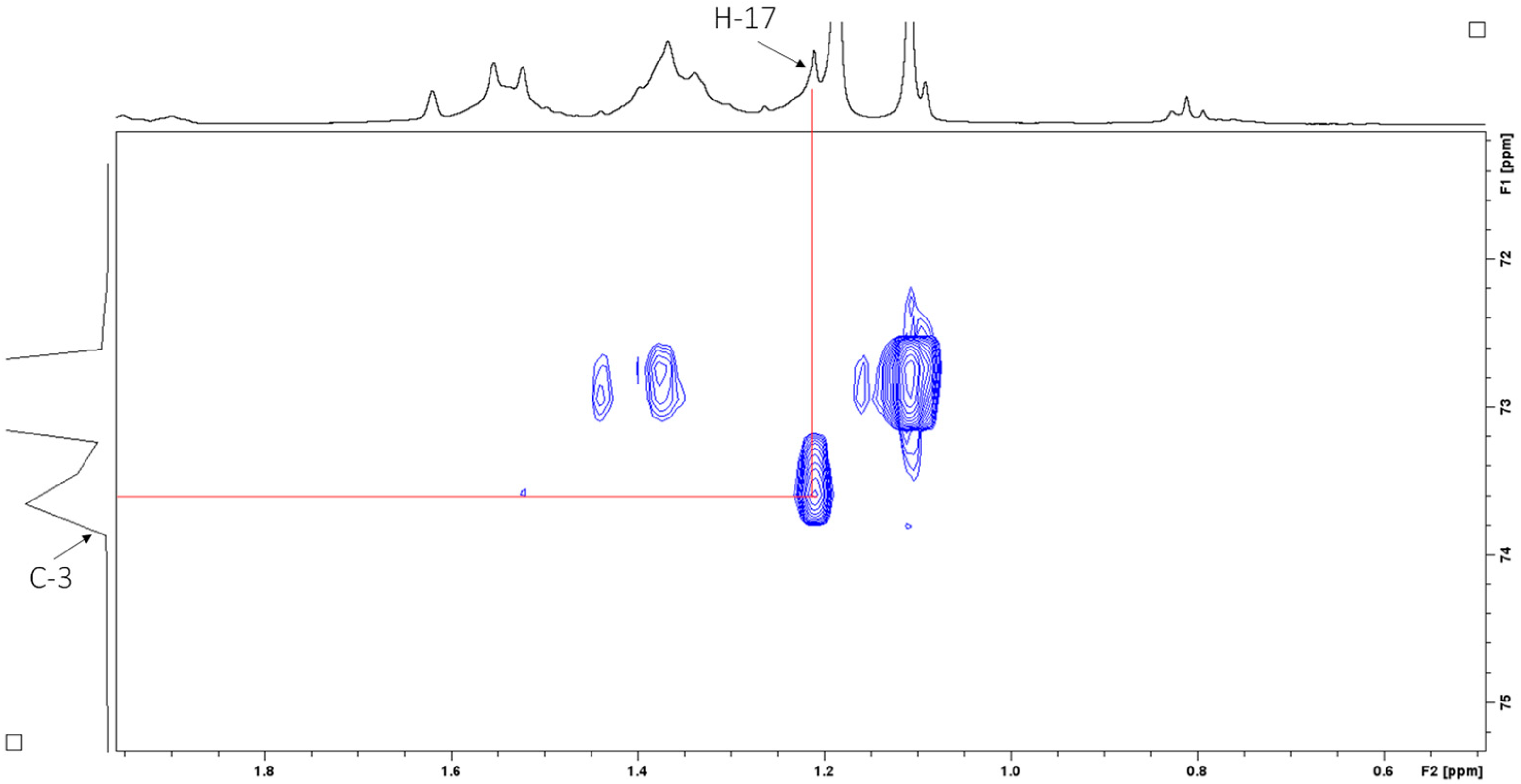
| Carbon No. | 13C (lit), CD3OD@ | 1H (lit), CD3OD | 1H (CDCl3) | 13C (CDCl3) # |
|---|---|---|---|---|
| 1 | 112.0 (CH2) | 5.20, dd, J = 17.4, 1.7 Hz 5.02, dd, 10.7, 1.7 | 5.21 5.06 | 111.6 |
| 2 | 146.3 (CH) | 5.91, dd, J = 17.4, 10.7 Hz, | 5.91 | 145.0 |
| 3 | 73.8 (C) | - | - | - |
| 4 | 42.7 (CH2) | 1.55–1.27, m (44H) | 1.42, br m | 42.2 |
| 5 | 23.2 (CH2) | 2.00, m | 2.03, br m | 22.6 |
| 6 | 125.8 (CH) | 5.10, m | 5.14 | 124.5 |
| 7 | 136.0 (C) | - | ||
| 8 | 41.3 (CH2) | 2.00, m | 1.97 | 39.8 |
| 9 | 19.4 (CH2 × 7) | 1.55–1.27, m (×7) | 1.42, br m | 18.1 |
| 10 | 43.4 (CH2 × 7) | 1.55–1.27, m (×7) | 1.42, br m | 42.2 |
| 11 | 73.4 (C × 7) | - | ||
| 12 | 43.4 (CH2 × 7) | 1.55–1.27, m (×7) | 1.42, br m | 42.2 |
| 13 | 23.7 (CH2) | 2.00, m | 2.03, br m | 22.6 |
| 14 | 125.8 (CH) | 5.10, m | 5.14 | 124.5 |
| 15 | 132.0 | - | ||
| 16 | 25.9 (CH3) | 1.60–1.67, s | 1.68 | 25.7 * |
| 17 | 27.6 (CH3) | 1.24, 3H, s | 1.27 | 27.2 |
| 18 | 15.9 (CH3) | 1.60–1.67, s | 1.61 | 15.8 * |
| 19 | 27.0 (CH3 × 7) | 1.15, br s (×7) | 1.17 | 26.9 |
| 20 | 17.8 (CH3) | 1.60–1.67, s | 1.58 | 17.6 * |
| M. tuberculosis MIC | Vero IC50 | Vero SI (IC50/MIC) | C3A IC50 | C3A SI (IC50/MIC) | |
|---|---|---|---|---|---|
| Crude Extract | 250 µg/mL | 90.86 ± 1.04 | 0.36 | 18.93 ± 1.09 | 0.076 |
| Fraction 13 | 62.5 µg/mL | 27.02 ± 1.01 | 0.43 | 9.16 ± 1.05 | 0.15 |
| Sub-Fraction 13.6 (Gymnopilene) | 31.25 µg/mL | 28.53 ± 1.02 (34.59 µM) | 0.91 | 22.38 ± 1.09 (27.14 µM) | 0.72 |
| Fraction | Hexane (mL) | Ethyl Acetate (mL) | Methanol (mL) |
|---|---|---|---|
| 1 | 10 | 0 | - |
| 2 | 9 | 1 | - |
| 3 | 8 | 2 | - |
| 4 | 7 | 3 | - |
| 5 | 6 | 4 | - |
| 6 | 5 | 5 | - |
| 7 | 4 | 6 | - |
| 8 | 3 | 7 | - |
| 9 | 2 | 8 | - |
| 10 | 1 | 9 | - |
| 11 | 0 | 10 | - |
| 12 | - | 8 | 2 |
| 13 | - | 6 | 4 |
| 14 | - | 4 | 6 |
| 15 | - | 2 | 8 |
| 16–21 | - | 0 | 10 |
| Assay | Fluorescent Dye | Filter Set | Excitation/Emission Wavelength (nm) |
|---|---|---|---|
| Cytotoxicity | Hoechst 33342 PI | DAPI Texas Red | 377/477 562/624 |
| Reactive oxygen species (ROS) | CellRox Orange | TRITC | 543/593 |
| Mitochondrial content and membrane potential | TMRE MTG | TRITC FITC | 543/593 482/536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didloff, J.; Boukes, G.J.; Nyambe, M.N.; Beukes, D.R.; Lerata, M.S.; Vilane, V.; Lee, M.; Govender, S.; van de Venter, M. Exploring Antimycobacterial Potential: Safety Evaluation and Active Compound Isolation from Gymnopilus junonius. Antibiotics 2025, 14, 179. https://doi.org/10.3390/antibiotics14020179
Didloff J, Boukes GJ, Nyambe MN, Beukes DR, Lerata MS, Vilane V, Lee M, Govender S, van de Venter M. Exploring Antimycobacterial Potential: Safety Evaluation and Active Compound Isolation from Gymnopilus junonius. Antibiotics. 2025; 14(2):179. https://doi.org/10.3390/antibiotics14020179
Chicago/Turabian StyleDidloff, Jenske, Gerhardt J. Boukes, Mutenta N. Nyambe, Denzil R. Beukes, Mookho S. Lerata, Velile Vilane, Michael Lee, Sharlene Govender, and Maryna van de Venter. 2025. "Exploring Antimycobacterial Potential: Safety Evaluation and Active Compound Isolation from Gymnopilus junonius" Antibiotics 14, no. 2: 179. https://doi.org/10.3390/antibiotics14020179
APA StyleDidloff, J., Boukes, G. J., Nyambe, M. N., Beukes, D. R., Lerata, M. S., Vilane, V., Lee, M., Govender, S., & van de Venter, M. (2025). Exploring Antimycobacterial Potential: Safety Evaluation and Active Compound Isolation from Gymnopilus junonius. Antibiotics, 14(2), 179. https://doi.org/10.3390/antibiotics14020179






