Nasopharyngeal Carriage, Antimicrobial Resistance, and Serotype Distribution of Streptococcus pneumoniae in Children Under Five in Lebanon: Baseline Data Prior to PCV13 Introduction
Abstract
:1. Introduction
2. Results
2.1. Streptococcus pneumoniae Carriage
2.2. Antibiotic Susceptibility of Streptococcus pneumoniae
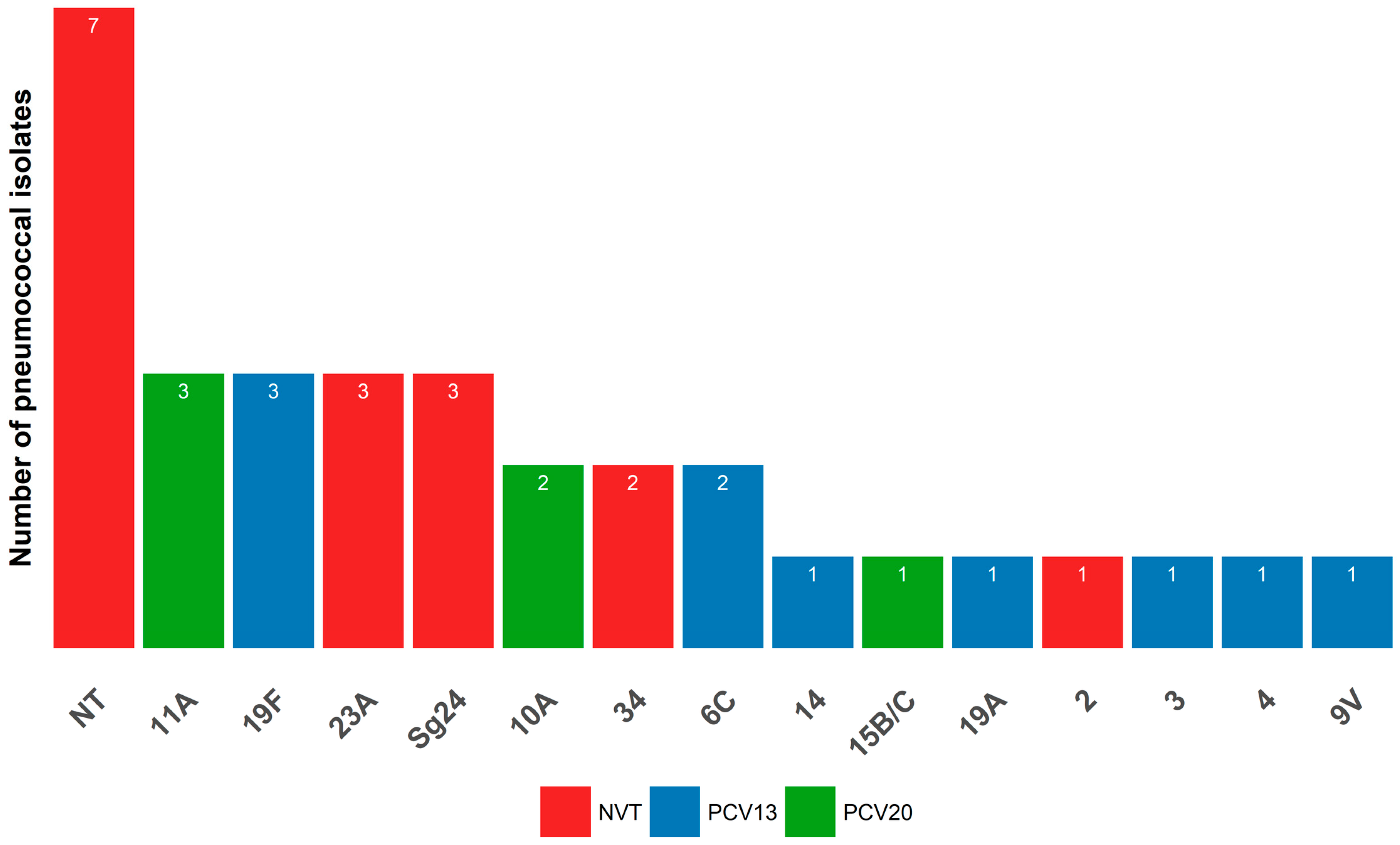
2.3. Molecular Serotyping
3. Discussion
4. Materials and Methods
4.1. Study Groups and Bacterial Isolation
4.2. Antimicrobial Susceptibility Testing
4.3. Molecular Serotyping
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, Colonization and Invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- El Moujaber, G.; Osman, M.; Rafei, R.; Dabboussi, F.; Hamze, M. Molecular Mechanisms and Epidemiology of Resistance in Streptococcus pneumoniae in the Middle East Region. J. Med. Microbiol. 2017, 66, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A.; et al. Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Berical, A.C.; Harris, D.; Dela Cruz, C.S.; Possick, J.D. Pneumococcal Vaccination Strategies. An Update and Perspective. Ann. Am. Thorac. Soc. 2016, 13, 933–944. [Google Scholar] [CrossRef]
- Kang, D.-W.; June Choe, Y.; Lee, J.-Y.; Suk, I.-A.; Kim, Y.-S.; Kim, H.-Y.; Byun, B.-K.; Park, S.-K. Cost-Effectiveness Analysis of the 20-Valent Pneumococcal Conjugate Vaccine for the Pediatric Population in South Korea. Vaccine 2024, 42, 126000. [Google Scholar] [CrossRef]
- Brooks, L.R.K.; Mias, G.I. Streptococcus pneumoniae’s Virulence and Host Immunity: Aging, Diagnostics, and Prevention. Front. Immunol. 2018, 9, 1366. [Google Scholar] [CrossRef]
- Subramanian, K.; Henriques-Normark, B.; Normark, S. Emerging Concepts in the Pathogenesis of the Streptococcus pneumoniae: From Nasopharyngeal Colonizer to Intracellular Pathogen. Cell Microbiol. 2019, 21, e13077. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, S.; Teräsjärvi, J.; Auranen, K.; Schuez-Havupalo, L.; Siira, L.; He, Q.; Waris, M.; Peltola, V. Acquisition and Transmission of Streptococcus pneumoniae Are Facilitated during Rhinovirus Infection in Families with Children. Am. J. Respir. Crit. Care Med. 2017, 196, 1172–1180. [Google Scholar] [CrossRef]
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO Global Priority Pathogens List: A Bibliometric Analysis of Medline-PubMed for Knowledge Mobilization to Infection Prevention and Control Practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef]
- Takano, C.; Kuramochi, Y.; Seki, M.; Kim, D.W.; Omagari, D.; Sasano, M.; Chang, B.; Ohnishi, M.; Kim, E.J.; Fuwa, K.; et al. Molecular Serotype-Specific Identification of Streptococcus pneumoniae Using Loop-Mediated Isothermal Amplification. Sci. Rep. 2019, 9, 19823. [Google Scholar] [CrossRef] [PubMed]
- Reslan, L.; Youssef, N.; Boutros, C.F.; Assaf-Casals, A.; Fayad, D.; Khafaja, S.; Akl, F.; Finianos, M.; Rizk, A.A.; Shaker, R.; et al. The Impact of Vaccination on the Burden of Invasive Pneumococcal Disease from a Nationwide Surveillance Program in Lebanon: An Unexpected Increase in Mortality Driven by Non-Vaccine Serotypes. Expert Rev. Vaccines 2022, 21, 1905–1921. [Google Scholar] [CrossRef]
- Cui, Y.A.; Patel, H.; O’Neil, W.M.; Li, S.; Saddier, P. Pneumococcal Serotype Distribution: A Snapshot of Recent Data in Pediatric and Adult Populations around the World. Hum. Vaccines Immunother. 2017, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dawood, H.N.; Al-Jumaili, A.H.; Radhi, A.H.; Ikram, D.; Al-Jabban, A. Emerging Pneumococcal Serotypes in Iraq: Scope for Improved Vaccine Development. F1000Research 2023, 12, 435. [Google Scholar] [CrossRef]
- Noharet-Koenig, R.; Lasota, K.; Faivre, P.; Langevin, E. Evolution of Pneumococcal Vaccine Recommendations and Criteria for Decision Making in 5 Western European Countries and the United States. MDM Policy Pract. 2023, 8, 23814683231174432. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Levy, C.; Varon, E. The Latest News in France before Distribution of Third-Generation Pneumococcal Conjugate Vaccines. Infect. Dis. Now. 2024, 54, 104937. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, E.; Van Heirstraeten, L.; Willen, L.; Desmet, S.; Wouters, I.; Vermeulen, H.; Lammens, C.; Goossens, H.; Van Damme, P.; Verhaegen, J.; et al. Serotype 19A and 6C Account for One Third of Pneumococcal Carriage among Belgian Day-Care Children Four Years after a Shift to a Lower-Valent PCV. J. Pediatr. Infect. Dis. Soc. 2022, 12, 36–42. [Google Scholar] [CrossRef]
- Reslan, L.; Finianos, M.; Bitar, I.; Moumneh, M.B.; Araj, G.F.; Zaghlout, A.; Boutros, C.; Jisr, T.; Nabulsi, M.; Kara Yaccoub, G.; et al. The Emergence of Invasive Streptococcus pneumoniae Serotype 24F in Lebanon: Complete Genome Sequencing Reveals High Virulence and Antimicrobial Resistance Characteristics. Front. Microbiol. 2021, 12, 637813. [Google Scholar] [CrossRef]
- Moghnieh, R.; Tamim, H.; Awad, L.; Abdallah, D.; Sleiman, R.; Jisr, T.; Al-Helou, M.; Ibrahim, A.; Mugharbil, A.; Droubi, N.; et al. Epidemiology of Invasive and Non-Invasive Pneumococcal Infections in Hospitalised Adult Patients in a Lebanese Medical Centre, 2006–2015. J. Infect. Public Health 2020, 13, 2092–2100. [Google Scholar] [CrossRef]
- Salloum, T.; Tannous, E.; Merheb-Ghoussoub, S.; Ghoussoub, E.; Tokajian, S. Genome Analysis of a MDR Streptococcus pneumoniae 23F Serotype Causing Meningoencephalitis in a 10-Months Refugee Infant. J. Infect. Dev. Ctries. 2018, 12, 196–203. [Google Scholar] [CrossRef]
- Hanna-Wakim, R.; Chehab, H.; Mahfouz, I.; Nassar, F.; Baroud, M.; Shehab, M.; Pimentel, G.; Wasfy, M.; House, B.; Araj, G.; et al. Epidemiologic Characteristics, Serotypes, and Antimicrobial Susceptibilities of Invasive Streptococcus pneumoniae Isolates in a Nationwide Surveillance Study in Lebanon. Vaccine 2012, 30, G11–G17. [Google Scholar] [CrossRef]
- Hurst, J.H.; Shaik-Dasthagirisaheb, Y.B.; Truong, L.; Boiditswe, S.C.; Patel, S.M.; Gilchrist, J.; Maciejewski, J.; Luinstra, K.; Smieja, M.; Steenhoff, A.P.; et al. Serotype Epidemiology and Antibiotic Resistance of Pneumococcal Isolates Colonizing Infants in Botswana (2016–2019). PLoS ONE 2024, 19, e0302400. [Google Scholar] [CrossRef]
- Cooper, D.; Yu, X.; Sidhu, M.; Nahm, M.H.; Fernsten, P.; Jansen, K.U. The 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Elicits Cross-Functional Opsonophagocytic Killing Responses in Humans to Streptococcus pneumoniae Serotypes 6C and 7A. Vaccine 2011, 29, 7207–7211. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Kuttel, M.M.; Ravenscroft, N.; Thompson, A.; Prasad, A.K.; Gangolli, S.; Tan, C.; Cooper, D.; Watson, W.; Liberator, P.; et al. Streptococcus pneumoniae Serotype 15B Polysaccharide Conjugate Elicits a Cross-Functional Immune Response against Serotype 15C but Not 15A. Vaccine 2022, 40, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Adegbola, R.A.; DeAntonio, R.; Hill, P.C.; Roca, A.; Usuf, E.; Hoet, B.; Greenwood, B.M. Carriage of Streptococcus pneumoniae and Other Respiratory Bacterial Pathogens in Low and Lower-Middle Income Countries: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e103293. [Google Scholar] [CrossRef] [PubMed]
- Safari, D.; Daningrat, W.O.D.; Milucky, J.L.; Khoeri, M.M.; Paramaiswari, W.T.; Tafroji, W.; Salsabila, K.; Winarti, Y.; Soebandrio, A.; Hadinegoro, S.R.; et al. Nasopharyngeal Carriage of Streptococcus pneumoniae among Children < 5 Years of Age in Indonesia Prior to Pneumococcal Conjugate Vaccine Introduction. PLoS ONE 2024, 19, e0297041. [Google Scholar] [CrossRef]
- Candeias, C.; Almeida, S.T.; Paulo, A.C.; Simões, A.S.; Ferreira, B.; Cruz, A.R.; Queirós, M.; Touret, T.; Brito-Avô, A.; de Lencastre, H.; et al. Streptococcus pneumoniae Carriage, Serotypes, Genotypes, and Antimicrobial Resistance Trends among Children in Portugal, after Introduction of PCV13 in National Immunization Program: A Cross-Sectional Study. Vaccine 2024, 42, 126219. [Google Scholar] [CrossRef]
- Ricketson, L.J.; Wood, M.L.; Vanderkooi, O.G.; MacDonald, J.C.; Martin, I.E.; Demczuk, W.H.B.; Kellner, J.D. Calgary Streptococcus pneumoniae Epidemiology Research (CASPER) investigators Trends in Asymptomatic Nasopharyngeal Colonization with Streptococcus pneumoniae after Introduction of the 13-Valent Pneumococcal Conjugate Vaccine in Calgary, Canada. Pediatr. Infect. Dis. J. 2014, 33, 724–730. [Google Scholar] [CrossRef]
- International Vaccine Access Center. Introducing the Pneumococcal Conjugate Vaccine (PCV) in Jordan—A compilation of Relevant Information from Countries Within the World Health Organization Eastern Mediterranean Region. 2023. Available online: https://publichealth.jhu.edu/sites/default/files/2024-02/introducing-the-pcv-in-jordan-reportax.pdf (accessed on 4 February 2025).
- El-Nawawy, A.A.; Hafez, S.F.; Meheissen, M.A.; Shahtout, N.M.; Mohammed, E.E. Nasopharyngeal Carriage, Capsular and Molecular Serotyping and Antimicrobial Susceptibility of Streptococcus pneumoniae among Asymptomatic Healthy Children in Egypt. J. Trop. Pediatr. 2015, 61, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, A. Multicenter Study of Pneumococcal Carriage in Children 2 to 4 Years of Age in the Winter Seasons of 2017-2019 in Irbid and Madaba Governorates of Jordan. PLoS ONE 2020, 15, e0237247. [Google Scholar] [CrossRef]
- Nasereddin, A.; Shtayeh, I.; Ramlawi, A.; Salman, N.; Salem, I.; Abdeen, Z. Streptococcus pneumoniae from Palestinian Nasopharyngeal Carriers: Serotype Distribution and Antimicrobial Resistance. PLoS ONE 2013, 8, e82047. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; De Groot, R.; Hermans, P.W.M. Streptococcus pneumoniae Colonisation: The Key to Pneumococcal Disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Ben Ayed, N.; Ktari, S.; Jdidi, J.; Gargouri, O.; Smaoui, F.; Hachicha, H.; Ksibi, B.; Mezghani, S.; Mnif, B.; Mahjoubi, F.; et al. Nasopharyngeal Carriage of Streptococcus pneumoniae in Tunisian Healthy Under-Five Children during a Three-Year Survey Period (2020 to 2022). Vaccines 2024, 12, 393. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, K.; Pietras, A.; Jablonska, J.; Bakiera, A.; Borek, A.; Niedzielska, G.; Grzegorczyk, M.; Grywalska, E.; Korona-Glowniak, I. Impact of Pneumococcal Vaccination on Nasopharyngeal Carriage of Streptococcus pneumoniae and Microbiota Profiles in Preschool Children in South East Poland. Vaccines 2022, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, A.; Khanfar, N.; Albataina, N.; Al Shwayat, R.; Altwal, R.; Abulfeilat, T.; Alawneh, G.; Khurd, M.; Alqadi Altamimi, A. Urban and Rural Disparities in Pneumococcal Carriage and Resistance in Jordanian Children, 2015–2019. Vaccines 2021, 9, 789. [Google Scholar] [CrossRef] [PubMed]
- Ouldali, N.; Cohen, R.; Levy, C.; Gelbert-Baudino, N.; Seror, E.; Corrard, F.; Vie Le Sage, F.; Michot, A.-S.; Romain, O.; Bechet, S.; et al. Pneumococcal Susceptibility to Antibiotics in Carriage: A 17 Year Time Series Analysis of the Adaptive Evolution of Non-Vaccine Emerging Serotypes to a New Selective Pressure Environment. J. Antimicrob. Chemother. 2019, 74, 3077–3086. [Google Scholar] [CrossRef]
- Kobayashi, M.; Conklin, L.M.; Bigogo, G.; Jagero, G.; Hampton, L.; Fleming-Dutra, K.E.; Junghae, M.; Carvalho, M.d.G.; Pimenta, F.; Beall, B.; et al. Pneumococcal Carriage and Antibiotic Susceptibility Patterns from Two Cross-Sectional Colonization Surveys among Children Aged <5 Years Prior to the Introduction of 10-Valent Pneumococcal Conjugate Vaccine–Kenya, 2009–2010. BMC Infect. Dis. 2017, 17, 25. [Google Scholar] [CrossRef]
- Matran, Y.M.; Al-Haddad, A.M.; Kour, A.; Al-Shehabi, H.; Sharma, S.; Suttee, A.; Sharma, S. Streptococcus pneumoniae among the Children of Aden, Yemen: A Cross-Sectional Report of Post-Pneumococcal Conjugate Vaccine. J. Infect. Dev. Ctries. 2024, 18, 579–586. [Google Scholar] [CrossRef]
- Daoud, Z.; Cocozaki, A.; Hakime, N. Antimicrobial Susceptibility Patterns of Haemophilus Influenzae and Streptococcus pneumoniae Isolates in a Beirut General University Hospital between 2000 and 2004. Clin. Microbiol. Infect. 2006, 12, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Moghnieh, R.; Araj, G.F.; Awad, L.; Daoud, Z.; Mokhbat, J.E.; Jisr, T.; Abdallah, D.; Azar, N.; Irani-Hakimeh, N.; Balkis, M.M.; et al. A Compilation of Antimicrobial Susceptibility Data from a Network of 13 Lebanese Hospitals Reflecting the National Situation during 2015–2016. Antimicrob. Resist. Infect. Control 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Jamsheer, A.; Rafay, A.M.; Daoud, Z.; Morrissey, I.; Torumkuney, D. Results from the Survey of Antibiotic Resistance (SOAR) 2011–13 in the Gulf States. J. Antimicrob. Chemother. 2016, 71 (Suppl. S1), i45–i61. [Google Scholar] [CrossRef]
- Daoud, Z.; Kourani, M.; Saab, R.; Nader, M.A.; Hajjar, M. Resistance of Streptococcus pneumoniae Isolated from Lebanese Patients between 2005 and 2009. Rev. Esp. Quimioter. 2011, 24, 84–90. [Google Scholar]
- Uwaydah, M.; Mokhbat, J.E.; Karam-Sarkis, D.; Baroud-Nassif, R.; Rohban, T. Penicillin-Resistant Streptococcus pneumoniae in Lebanon: The First Nationwide Study. Int. J. Antimicrob. Agents 2006, 27, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.C.A.; Neves, F.P.G.; Souza, A.R.V.; Oliveira, L.M.A.; Costa, N.S.; Castro, L.F.S.; Mendonça-Souza, C.R.d.V.; Peralta, J.M.; Teixeira, L.M. Evolution of Penicillin Non-Susceptibility Among Streptococcus pneumoniae Isolates Recovered From Asymptomatic Carriage and Invasive Disease Over 25 Years in Brazil, 1990–2014. Front. Microbiol 2019, 10, 486. [Google Scholar] [CrossRef]
- El Khoury, G.; Ramia, E.; Salameh, P. Misconceptions and Malpractices Toward Antibiotic Use in Childhood Upper Respiratory Tract Infections Among a Cohort of Lebanese Parents. Eval. Health Prof. 2018, 41, 493–511. [Google Scholar] [CrossRef] [PubMed]
- El Ashkar, S.; Osman, M.; Rafei, R.; Mallat, H.; Achkar, M.; Dabboussi, F.; Hamze, M. Molecular Detection of Genes Responsible for Macrolide Resistance among Streptococcus pneumoniae Isolated in North Lebanon. J. Infect. Public Health 2017, 10, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.; Araj, G.F.; Wakim, R.H.; Kanj, S.S.; Kanafani, Z.A.; Sabra, A.; Khairallah, M.-T.; Nassar, F.J.; Shehab, M.; Baroud, M.; et al. Genotypes and Serotype Distribution of Macrolide Resistant Invasive and Non-Invasive Streptococcus pneumoniae Isolates from Lebanon. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 2. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Zhang, H.; Guo, J.; Wei, X.; Xue, M.; Ma, X. Severe problem of macrolides resistance to common pathogens in China. Front. Cell. Infect. Microbiol. 2023, 13, 1181633. [Google Scholar] [CrossRef]
- Desmet, S.; Wouters, I.; Heirstraeten, L.V.; Beutels, P.; Van Damme, P.; Malhotra-Kumar, S.; Maes, P.; Verhaegen, J.; Peetermans, W.E.; Lagrou, K.; et al. In-Depth Analysis of Pneumococcal Serotypes in Belgian Children (2015–2018): Diversity, Invasive Disease Potential, and Antimicrobial Susceptibility in Carriage and Disease. Vaccine 2021, 39, 372–379. [Google Scholar] [CrossRef]
- Mallah, N.; Badro, D.A.; Figueiras, A.; Takkouche, B. Association of Knowledge and Beliefs with the Misuse of Antibiotics in Parents: A Study in Beirut (Lebanon). PLoS ONE 2020, 15, e0232464. [Google Scholar] [CrossRef] [PubMed]
- Al Omari, S.; Al Mir, H.; Wrayde, S.; Merhabi, S.; Dhaybi, I.; Jamal, S.; Chahine, M.; Bayaa, R.; Tourba, F.; Tantawi, H.; et al. First Lebanese Antibiotic Awareness Week Campaign: Knowledge, Attitudes and Practices towards Antibiotics. J. Hosp. Infect. 2019, 101, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Zahreddine, L.; Hallit, S.; Shakaroun, S.; Al-Hajje, A.; Awada, S.; Lahoud, N. Knowledge of Pharmacists and Parents towards Antibiotic Use in Pediatrics: A Cross-Sectional Study in Lebanon. Pharm. Pract. 2018, 16, 1194. [Google Scholar] [CrossRef]
- Menezes, A.P.d.O.; Azevedo, J.; Leite, M.C.; Campos, L.C.; Cunha, M.; Carvalho, M.d.G.S.; Reis, M.G.; Ko, A.I.; Weinberger, D.M.; Ribeiro, G.; et al. Nasopharyngeal Carriage of Streptococcus pneumoniae among Children in an Urban Setting in Brazil Prior to PCV10 Introduction. Vaccine 2016, 34, 791–797. [Google Scholar] [CrossRef]
- Tvedskov, E.S.F.; Hovmand, N.; Benfield, T.; Tinggaard, M. Pneumococcal Carriage among Children in Low and Lower-Middle-Income Countries: A Systematic Review. Int. J. Infect. Dis. 2022, 115, 1–7. [Google Scholar] [CrossRef]
- Daningrat, W.O.D.; Amalia, H.; Ayu, I.M.; Satzke, C.; Safari, D. Carriage of Streptococcus pneumoniae in Children under Five Years of Age Prior to Pneumococcal Vaccine Introduction in Southeast Asia: A Systematic Review and Meta-Analysis (2001–2019). J. Microbiol. Immunol. Infect. 2022, 55, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Swedan, S.F.; Hayajneh, W.A.; Bshara, G.N. Genotyping and Serotyping of Macrolide and Multidrug Resistant Streptococcus pneumoniae Isolated from Carrier Children. Indian J. Med. Microbiol. 2016, 34, 159–165. [Google Scholar] [CrossRef]
- Ziane, H.; Manageiro, V.; Ferreira, E.; Moura, I.B.; Bektache, S.; Tazir, M.; Caniça, M. Serotypes and Antibiotic Susceptibility of Streptococcus pneumoniae Isolates from Invasive Pneumococcal Disease and Asymptomatic Carriage in a Pre-Vaccination Period, in Algeria. Front. Microbiol. 2016, 7, 803. [Google Scholar] [CrossRef]
- Cohen, R.; Levy, C.; Ouldali, N.; Goldrey, M.; Béchet, S.; Bonacorsi, S.; Varon, E. Invasive Disease Potential of Pneumococcal Serotypes in Children After PCV13 Implementation. Clin. Infect. Dis. 2021, 72, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Balsells, E.; Dagan, R.; Yildirim, I.; Gounder, P.P.; Steens, A.; Muñoz-Almagro, C.; Mameli, C.; Kandasamy, R.; Givon Lavi, N.; Daprai, L.; et al. The Relative Invasive Disease Potential of Streptococcus pneumoniae among Children after PCV Introduction: A Systematic Review and Meta-Analysis. J. Infect. 2018, 77, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Mohale, T.; Wolter, N.; Allam, M.; Nzenze, S.A.; Madhi, S.A.; du Plessis, M.; von Gottberg, A. Genomic Differences among Carriage and Invasive Nontypeable Pneumococci Circulating in South Africa. Microb. Genom. 2019, 5, e000299. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-H.; Hsu, M.-H.; Wu, T.-L.; Li, H.-C.; Chen, C.-L.; Janapatla, R.P.; Su, L.-H.; Chiu, C.-H. Non-Typeable Streptococcus pneumoniae Infection in a Medical Center in Taiwan after Wide Use of Pneumococcal Conjugate Vaccine. J. Microbiol. Immunol. Infect. 2020, 53, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Ganaie, F.; Maruhn, K.; Li, C.; Porambo, R.J.; Elverdal, P.L.; Abeygunwardana, C.; van der Linden, M.; Duus, J.Ø.; Sheppard, C.L.; Nahm, M.H. Structural, Genetic, and Serological Elucidation of Streptococcus pneumoniae Serogroup 24 Serotypes: Discovery of a New Serotype, 24C, with a Variable Capsule Structure. J. Clin. Microbiol. 2021, 59, e0054021. [Google Scholar] [CrossRef] [PubMed]
- Balsells, E.; Guillot, L.; Nair, H.; Kyaw, M.H. Serotype Distribution of Streptococcus pneumoniae Causing Invasive Disease in Children in the Post-PCV Era: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0177113. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.; Levy, C.; Ouldali, N.; Bonacorsi, S.; Béchet, S.; Delobbe, J.-F.; Batard, C.; Donikian, I.; Goldrey, M.; Assouline, J.; et al. Dynamics of Antibiotic Resistance of Streptococcus pneumoniae in France: A Pediatric Prospective Nasopharyngeal Carriage Study from 2001 to 2022. Antibiotics 2023, 12, 1020. [Google Scholar] [CrossRef]
- Neves, F.P.G.; Pinto, T.C.A.; Corrêa, M.A.; dos Anjos Barreto, R.; de Souza Gouveia Moreira, L.; Rodrigues, H.G.; Cardoso, C.A.; Barros, R.R.; Teixeira, L.M. Nasopharyngeal Carriage, Serotype Distribution and Antimicrobial Resistance of Streptococcus pneumoniae among Children from Brazil before the Introduction of the 10-Valent Conjugate Vaccine. BMC Infect. Dis. 2013, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Ricketson, L.J.; Lidder, R.; Thorington, R.; Martin, I.; Vanderkooi, O.G.; Sadarangani, M.; Kellner, J.D. PCR and Culture Analysis of Streptococcus pneumoniae Nasopharyngeal Carriage in Healthy Children. Microorganisms 2021, 9, 2116. [Google Scholar] [CrossRef]
- Carvalho, M.d.G.S.; Tondella, M.L.; McCaustland, K.; Weidlich, L.; McGee, L.; Mayer, L.W.; Steigerwalt, A.; Whaley, M.; Facklam, R.R.; Fields, B.; et al. Evaluation and Improvement of Real-Time PCR Assays Targeting lytA, Ply, and psaA Genes for Detection of Pneumococcal DNA. J. Clin. Microbiol. 2007, 45, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.A.; Yee, Y.C.; Goldschmidt, R.; Bush, K.; Sahm, D.F.; Evangelista, A. Infrequent Occurrence of Single Mutations in Topoisomerase IV and DNA Gyrase Genes among US Levofloxacin-Susceptible Clinical Isolates of Streptococcus pneumoniae from Nine Institutions (1999–2003). J. Antimicrob. Chemother. 2006, 57, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Milenkov, M.; Albrich, W.C.; van der Linden, M.P.G.; Bénet, T.; Chou, M.; Sylla, M.; Barreto Costa, P.; Richard, N.; Klugman, K.P.; et al. The Relevance of a Novel Quantitative Assay to Detect up to 40 Major Streptococcus pneumoniae Serotypes Directly in Clinical Nasopharyngeal and Blood Specimens. PLoS ONE 2016, 11, e0151428. [Google Scholar] [CrossRef] [PubMed]
- Kesteman, T.; Ghassani, A.; Hajjar, C.; Picot, V.; Osman, M.; Alnajjar, Z.; Komurian-Pradel, F.; Messaoudi, M.; Pouzol, S.; PEARL Study Group; et al. Investigating Pneumonia Etiology Among Refugees and the Lebanese Population (PEARL): A Study Protocol. Gates Open Res. 2018, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Kassem, I.I.; Dabboussi, F.; Cummings, K.J.; Hamze, M. The Indelible Toll of Enteric Pathogens: Prevalence, Clinical Characterization, and Seasonal Trends in Patients with Acute Community-Acquired Diarrhea in Disenfranchised Communities. PLoS ONE 2023, 18, e0282844. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Pneumococcal Vaccine Recommendations. 2024. Available online: https://www.cdc.gov/pneumococcal/hcp/vaccine-recommendations/index.html (accessed on 4 February 2025).
| Dar Al Zahra’ | Sainte Famille Maronite | Jil Alwa’ed | Total | |
|---|---|---|---|---|
| N and (%) of examined individuals | 60 (57.7) | 12 (11.5) | 32 (30.8) | 104 (100) |
| N and (%) of females | 23 (38.3) | 7 (58.3) | 15 (46.9) | 45 (43.3) |
| N and (%) of males | 37 (61.7) | 5 (41.7) | 17 (53.1) | 59 (56.7) |
| Female/male ratio | 0.62 | 1.40 | 0.88 | 0.76 |
| Mean age ± standard deviation (Min–Max) | 4.02 ± 0.13 (4−5) | 4.67 ± 0.52 (4−5) | 4.35 ± 0.80 (3−5) | 4.16 ± 0.51 (3−5) |
| N and (%) of Streptococcus pneumoniae carriage | 39 (65.0) | 6 (50.0) | 12 (37.5) | 57 (54.8) |
| Descriptive Analysis | Fisher’s Exact Test | Multivariable Logistic Regression Analysis | |||||
|---|---|---|---|---|---|---|---|
| Total | Carriage N (%) | p-Value | adj. OR | 95% CI | p-Value | ||
| Sex | Male 1 | 59 | 32 (54.2) | ||||
| Female | 45 | 25 (55.6) | 1.00 | ||||
| Age | 3 | 6 | 3 (50.0) | ||||
| 4 1 | 69 | 44 (63.8) | |||||
| 5 | 22 | 10 (45.5) | 0.266 | ||||
| Socioeconomic status | High 1 | 44 | 18 (40.9) | ||||
| Low | 60 | 39 (65.0) | 0.018 | 2.68 | 1.21–6.07 | 0.016 | |
| No. of Antibiotics to Which the Isolates Were Resistant Against | No. (%) of Pneumococci Isolates a | Antiobitic Resistance Profile of the Isolates b | No. of Antibiotic Classes | Serotypes c | Macrolide Resistance Genotype |
|---|---|---|---|---|---|
| 0 | 11 | NA | NA | NT d (2), 11A (1), 23A (1), 3 (1), 4 (1), 6C (1) | - |
| 1 | 23 | OXA | 1 | NT (3), 19F (3), 23A (2), 15BC (1), 24 (1), 19A (1), 34 (1) | - |
| 1 | 1 | SXT | 1 | 34 (1) | - |
| 2 | 4 | OXA, SXT | 2 | 10A (2), 11A (2) | - |
| 2 | 1 | OXA, TET | 2 | - | - |
| 2 | 1 | SXT, TET | 2 | - | - |
| 2 | 1 | OXA, ERY | 2 | NT (1) | M phenotype; mefA-mefE |
| 4 | 3 e | OXA, TET, MNO, SXT | 3 | 2 (1), NT (1) | - |
| 4 | 1 e | OXA, ERY, CLN, TET | 4 | - | cMLSb f; ermB |
| 5 | 6 e | OXA, ERY, CLN, TET, MNO | 4 | 14 (1), 6C (1), 24 (1) | cMLSb; ermB |
| 5 | 3 e | OXA, ERY, CLN, TET, SXT | 5 | - | cMLSb; ermB-mefA (2), ermB-mefA-mefE (1) |
| 5 | 1 e | OXA, ERY, TET, MNO, SXT | 4 | 9V (1) | M phenotype; mefA-mefE |
| 6 | 1 e | OXA, ERY, CLN, TET, MNO, TEC | 5 | 24 (1) | cMLSb; ermB |
| Descriptive Analysis | Fisher’s Exact Test | Multivariable Logistic Regression Analysis | |||||
|---|---|---|---|---|---|---|---|
| Total | Resistance to Erythromycin N (%) | p-Value | adj. OR | 95% CI | p-Value | ||
| Sex | Male 1 | 32 | 7 (21.9) | ||||
| Female | 25 | 6 (24.0) | 1.00 | ||||
| Age | 3 | 3 | 1 (33.3) | ||||
| 4 1 | 44 | 8 (18.2) | |||||
| 5 | 10 | 4 (40.0) | 0.255 | ||||
| Socioeconomic status | High 1 | 18 | 8 (44.4) | ||||
| Low | 39 | 5 (11.4) | 0.015 | 0.18 | 0.05–0.67 | 0.012 | |
| Descriptive Analysis | Fisher’s Exact Test | Multivariable Logistic Regression Analysis | |||||
|---|---|---|---|---|---|---|---|
| Total | Multidrug Resistance N (%) | p-Value | adj. OR | 95% CI | p-Value | ||
| Sex | Male 1 | 32 | 9 (28.1) | ||||
| Female | 25 | 6 (24.0) | 0.771 | ||||
| Age | 3 | 3 | 1 (33.3) | ||||
| 4 1 | 44 | 10 (22.7) | |||||
| 5 | 10 | 4 (40.0) | 0.452 | ||||
| Socioeconomic status | High 1 | 18 | 8 (44.4) | ||||
| Low | 39 | 7 (17.9) | 0.052 | 0.27 | 0.08–0.94 | 0.040 | |
| Descriptive Analysis | Fisher’s Exact Test | Multivariable Logistic Regression Analysis | |||||
|---|---|---|---|---|---|---|---|
| Total | Resistance to Oxacillin N (%) | p-Value | adj. OR | 95% CI | p-Value | ||
| Sex | Male 1 | 32 | 26 (81.3) | ||||
| Female | 25 | 18 (72.0) | 0.528 | ||||
| Age | 3 | 3 | 2 (66.7) | ||||
| 4 1 | 44 | 34 (77.3) | |||||
| 5 | 10 | 8 (80.0) | 0.859 | ||||
| Socioeconomic status | High 1 | 18 | 14 (77.8) | ||||
| Low | 39 | 30 (76.9) | 1.00 | ||||
| Serotype | PCV Serotype a | No. of Isolates | Susceptibility to Penicillin | Susceptibility to Erythromycin |
|---|---|---|---|---|
| 19F | PCV13 a | 3 | Reduced (3/3) | S |
| 11A | PCV20 b | 3 | Reduced (2/3) | S |
| 23A | NVT c | 3 | Reduced (2/3) | S |
| Sg24 d | NVT | 3 | Reduced (3/3) | R (2/3, cMLSb e) |
| 10A | PCV20 | 2 | Reduced (2/2) | S |
| 34 | NVT | 2 | Reduced (1/2) | S |
| 6C f | PCV13 | 2 | Reduced (1/2) | R (1/2, cMLSb) |
| 14 | PCV13 | 1 | Reduced | R (cMLSb) |
| 15B/C f | PCV20 | 1 | Reduced | S |
| 19A | PCV13 | 1 | Reduced | S |
| 2 | NVT | 1 | Reduced | R |
| 3 | PCV13 | 1 | S | S |
| 4 | PCV13 | 1 | S | S |
| 9V | PCV13 | 1 | Reduced | R (M phenotype) |
| NT g | - | 7 | Reduced (5/7) | R (1/7, M phenotype) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafei, R.; Zaylaa, M.; Diab, M.; Kassem, I.I.; El Omari, K.; Halimeh, F.B.; El Moujaber, G.; Achour, A.; Ismail, B.; Mallat, H.; et al. Nasopharyngeal Carriage, Antimicrobial Resistance, and Serotype Distribution of Streptococcus pneumoniae in Children Under Five in Lebanon: Baseline Data Prior to PCV13 Introduction. Antibiotics 2025, 14, 168. https://doi.org/10.3390/antibiotics14020168
Rafei R, Zaylaa M, Diab M, Kassem II, El Omari K, Halimeh FB, El Moujaber G, Achour A, Ismail B, Mallat H, et al. Nasopharyngeal Carriage, Antimicrobial Resistance, and Serotype Distribution of Streptococcus pneumoniae in Children Under Five in Lebanon: Baseline Data Prior to PCV13 Introduction. Antibiotics. 2025; 14(2):168. https://doi.org/10.3390/antibiotics14020168
Chicago/Turabian StyleRafei, Rayane, Mazen Zaylaa, Mohamad Diab, Issmat I. Kassem, Khaled El Omari, Fatima B. Halimeh, Grace El Moujaber, Afaf Achour, Bassel Ismail, Hassan Mallat, and et al. 2025. "Nasopharyngeal Carriage, Antimicrobial Resistance, and Serotype Distribution of Streptococcus pneumoniae in Children Under Five in Lebanon: Baseline Data Prior to PCV13 Introduction" Antibiotics 14, no. 2: 168. https://doi.org/10.3390/antibiotics14020168
APA StyleRafei, R., Zaylaa, M., Diab, M., Kassem, I. I., El Omari, K., Halimeh, F. B., El Moujaber, G., Achour, A., Ismail, B., Mallat, H., Hamze, M., Dabboussi, F., & Osman, M. (2025). Nasopharyngeal Carriage, Antimicrobial Resistance, and Serotype Distribution of Streptococcus pneumoniae in Children Under Five in Lebanon: Baseline Data Prior to PCV13 Introduction. Antibiotics, 14(2), 168. https://doi.org/10.3390/antibiotics14020168








