Intravenous Fosfomycin for Gram-Negative and Gram-Positive Bacterial Infections: A Systematic Review of the Clinical Evidence
Abstract
1. Introduction
2. Results
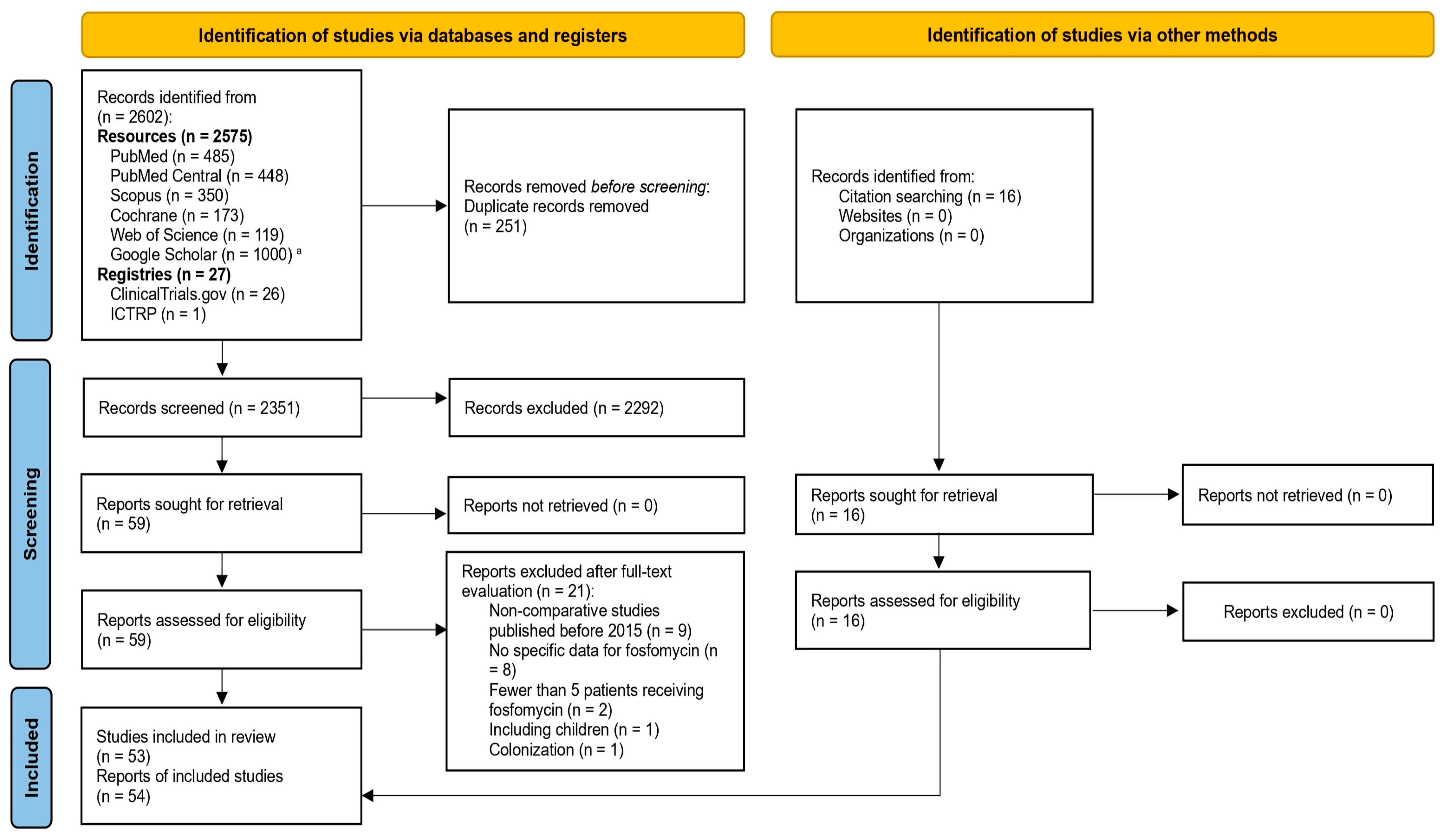
2.1. Literature Search
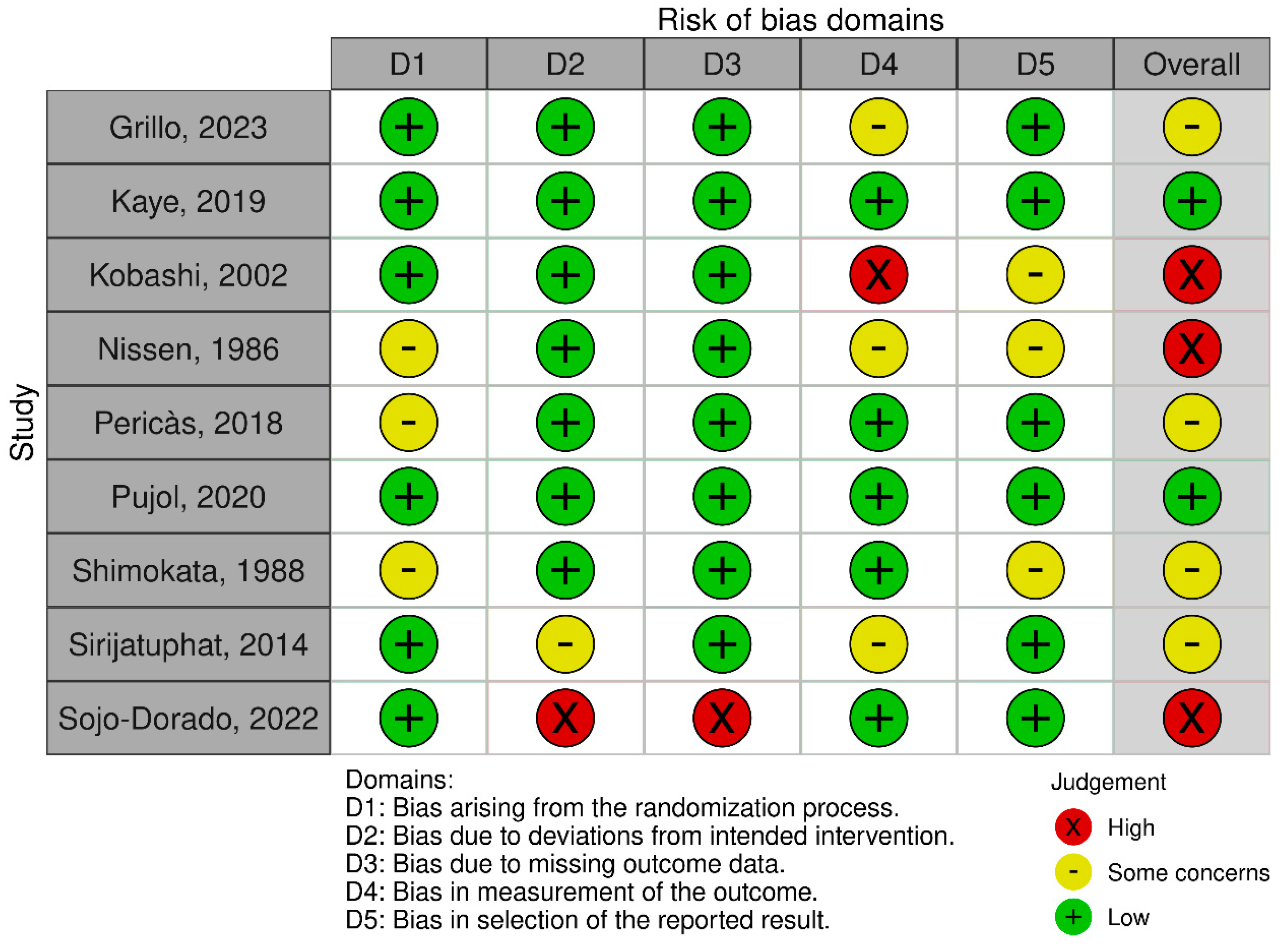
2.2. Evaluation of Risk of Bias
2.3. Tabulation of Extracted Data
3. Discussion
4. Methods
4.1. Adherence to the PRISMA Guidelines
4.2. Eligibility Criteria
4.3. Search Strategy
4.4. Screening Process and Data Extraction
4.5. Risk of Bias Assessment
5. Critical Appraisal of the Available Evidence
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BJI | bone and joint infection |
| CLSI | Clinical and Laboratory Standards Institute |
| CNS | central nervous system |
| CRAB | carbapenem-resistant Acinetobacter baumannii |
| ESBL | extended-spectrum β-lactamase |
| ESC | European Society of Cardiology |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| ICTRP | International Clinical Trials Registry Portal |
| KPC | Klebsiella pneumoniae carbapenemase |
| MBL | metallo-β-lactamase |
| MDR | multidrug resistant |
| MIC | minimal inhibitory concentration |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSSA | methicillin-susceptible Staphylococcus aureus |
| MurA | UDP-N-acetylglucosamine enolpyruvyl transferase |
| PD | pharmacodynamic |
| PDR | pandrug-resistant |
| PK | pharmacokinetic |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | randomized controlled trial |
| RoB 2 | Cochrane risk-of-bias tool for randomized trials |
| Robvis | risk-of-bias visualization |
| ROBINS-I V2 | Risk Of Bias In Non-randomized Studies–of Interventions, Version 2 |
| SOC | standard of care |
| SOFA | sequential organ failure assessment |
| UTI | urinary tract infection |
| VAP | ventilator-associated pneumonia |
| WHO | World Health Organization |
| XDR | extensively drug-resistant |
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. IDR 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Catalano, M.; Romeo, A.M.; Ochoa, L.B. Resistance profiles of the various species of the genus Staphylococcus to 15 clinically-used antimicrobials. Rev. Argent. Microbiol. 1989, 21, 111–119. [Google Scholar] [PubMed]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakas, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef]
- Rodríguez-Gascón, A.; Canut-Blasco, A. Deciphering Pharmacokinetics and Pharmacodynamics of Fosfomycin. Rev. Esp. Quimioter. 2019, 32, 19–24. [Google Scholar]
- Samonis, G.; Maraki, S.; Karageorgopoulos, D.E.; Vouloumanou, E.K.; Falagas, M.E. Synergy of Fosfomycin with Carbapenems, Colistin, Netilmicin, and Tigecycline against Multidrug-Resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa Clinical Isolates. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 695–701. [Google Scholar] [CrossRef]
- Antonello, R.M.; Principe, L.; Maraolo, A.E.; Viaggi, V.; Pol, R.; Fabbiani, M.; Montagnani, F.; Lovecchio, A.; Luzzati, R.; Di Bella, S. Fosfomycin as Partner Drug for Systemic Infection Management. A Systematic Review of Its Synergistic Properties from In Vitro and In Vivo Studies. Antibiotics 2020, 9, 500. [Google Scholar] [CrossRef]
- Nussbaumer-Pröll, A.; Obermüller, M.; Weiss-Tessbach, M.; Eberl, S.; Zeitlinger, M.; Matiba, B.; Mayer, C.; Kussmann, M. Synergistic Activity of Fosfomycin and Flucloxacillin against Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus: In Vitro and in Vivo Assessment. Med. Microbiol. Immunol. 2025, 214, 32. [Google Scholar] [CrossRef]
- Yildiz, I.E.; Mercantepe, T.; Bahceci, I.; Arpa, M.; Batcik, S.; Yildiz, Y.; Tumkaya, L. Investigation of the Effects of Fosfomycin in Kidney Damage Caused by CLP-Induced Sepsis. Life 2025, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Al-Aloul, M.; Nazareth, D.; Walshaw, M. The Renoprotective Effect of Concomitant Fosfomycin in the Treatment of Pulmonary Exacerbations in Cystic Fibrosis. Clin. Kidney J. 2019, 12, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, C.; Ito, K.; Komiya, I.; Horie, T. Protective Effect of Fosfomycin on Gentamicin-Induced Lipid Peroxidation of Rat Renal Tissue. Chem. Biol. Interact. 2004, 148, 139–147. [Google Scholar] [CrossRef]
- Kaye, K.S.; Gales, A.C.; Dubourg, G. Old Antibiotics for Multidrug-Resistant Pathogens: From in Vitro Activity to Clinical Outcomes. Int. J. Antimicrob. Agents 2017, 49, 542–548. [Google Scholar] [CrossRef]
- Önal, U.; Tüzemen, Ü.; Küçükdemirci Kaya, P.; İşçimen, R.; Kelebek Girgin, N.; Özakın, C.; Kahveci, F.; Akalın, H. A Comparative Study of Ceftazidime/Avibactam-Based and Fosfomycin plus Meropenem-Based Regimens for Managing Infections Caused by Carbapenem-Resistant Klebsiella pneumoniae in Critically Ill Patients. J. Chemother. 2025, 37, 1–9. [Google Scholar] [CrossRef]
- Belati, A.; Diella, L.; Bavaro, D.F.; De Santis, L.; Cotugno, S.; De Gennaro, N.; Brindicci, G.; Maggiore, M.E.; Indraccolo, F.; Di Gennaro, F.; et al. Intravenous Fosfomycin as Adjunctive Therapy for Gram-Negative Bacteria Bloodstream Infections: A Propensity Score Adjusted Retrospective Cohort Study. Int. J. Antimicrob. Agents 2024, 64, 107247. [Google Scholar] [CrossRef]
- Fois, M.; De Vito, A.; Cherchi, F.; Ricci, E.; Pontolillo, M.; Falasca, K.; Corti, N.; Comelli, A.; Bandera, A.; Molteni, C.; et al. Efficacy and Safety of Ceftazidime–Avibactam Alone versus Ceftazidime–Avibactam Plus Fosfomycin for the Treatment of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: A Multicentric Retrospective Study from the SUSANA Cohort. Antibiotics 2024, 13, 616. [Google Scholar] [CrossRef]
- Katip, W.; Rayanakorn, A.; Oberdorfer, P.; Taruangsri, P.; Nampuan, T.; Okonogi, S. Comparative Effectiveness and Mortality of Colistin Monotherapy versus Colistin-Fosfomycin Combination Therapy for the Treatment of Carbapenem-Resistant Enterobacteriaceae (CRE) Infections: A Propensity Score Analysis. J. Infect. Public. Health 2024, 17, 727–734. [Google Scholar] [CrossRef]
- Oliva, A.; Curtolo, A.; Falletta, A.; Sacco, F.; Lancellotti, F.; Carnevalini, M.; Ceccarelli, G.; Roma, G.; Bufi, M.; Magni, G.; et al. Efficacy of Fosfomycin-Containing Regimens in Treating Severe Infections Caused by KPC-Producing Klebsiella Pneumoniae and Carbapenem-Resistant Acinetobacter Baumannii in Critically Ill Patients. Int. J. Antimicrob. Agents 2024, 64, 107365. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Karamouzos, V.; Eleftheriotis, G.; Lagadinou, M.; Bartzavali, C.; Kolonitsiou, F.; Paliogianni, F.; Fligou, F.; Marangos, M. Efficacy of Fosfomycin-Containing Regimens for Treatment of Bacteremia Due to Pan-Drug Resistant Acinetobacter Baumannii in Critically Ill Patients: A Case Series Study. Pathogens 2023, 12, 286. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Volpicelli, L.; Di Bari, S.; Curtolo, A.; Borrazzo, C.; Cogliati Dezza, F.; Cona, A.; Agrenzano, S.; Mularoni, A.; Trancassini, M.; et al. Effect of Ceftazidime/Avibactam plus Fosfomycin Combination on 30 Day Mortality in Patients with Bloodstream Infections Caused by KPC-Producing Klebsiella pneumoniae: Results from a Multicentre Retrospective Study. JAC-Antimicrob. Resist. 2022, 4, dlac121. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Bassetti, M.; Bellelli, V.; Bianchi, L.; Marincola Cattaneo, F.; Mazzocchetti, S.; Paciacconi, E.; Cottini, F.; Schiattarella, A.; Tufaro, G.; et al. Efficacy of a Fosfomycin-Containing Regimen for Treatment of Severe Pneumonia Caused by Multidrug-Resistant Acinetobacter Baumannii: A Prospective, Observational Study. Infect. Dis. Ther. 2021, 10, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Sirijatuphat, R.; Thamlikitkul, V. Preliminary Study of Colistin versus Colistin plus Fosfomycin for Treatment of Carbapenem-Resistant Acinetobacter Baumannii Infections. Antimicrob. Agents Chemother. 2014, 58, 5598–5601. [Google Scholar] [CrossRef]
- Kobashi, Y.; Oba, H.; Okimoto, J.; Tada, A.; Kawahara, S.; Ishida, N.; Futaki, Y.; Matsushima, T. Clinical analysis of combined consecutive therapy with fosfomycin and sulbactam/cefoperazone for patients with pneumonia. Jpn. J. Chemother. 2002, 50, 429–434. [Google Scholar] [CrossRef]
- Shimokata, K.; Torikai, K.; Kato, M.; Sakai, S.; Nomura, S.; Ito, T.; Saka, H.; Chida, Y.; Torii, Y.; Ito, T.; et al. A study on the efficacy of Cefotaxime monotherapy versus Cefotaxime combined with Fosfomycin therapy for respiratory tract infections. Jpn. J. Antibiot. 1988, 41, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.R.; Jacobsen, J.; Ravn, T.J.; Wahlgreen, C.; Auning-Hansen, H. Fosfomycin-Ampicillin versus Gentamicin-Ampicillin in the Treatment of Critically Ill Patients with Pneumonia. Infection 1986, 14, 246–249. [Google Scholar] [CrossRef]
- Grillo, S.; Pujol, M.; Miró, J.M.; López-Contreras, J.; Euba, G.; Gasch, O.; Boix-Palop, L.; Garcia-País, M.J.; Pérez-Rodríguez, M.T.; Gomez-Zorrilla, S.; et al. Cloxacillin plus Fosfomycin versus Cloxacillin Alone for Methicillin-Susceptible Staphylococcus aureus Bacteremia: A Randomized Trial. Nat. Med. 2023, 29, 2518–2525. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Chuang, Y.-C.; Yang, J.-L.; Lin, C.-Y.; Huang, S.-H.; Wang, J.-T.; Chen, Y.-C.; Chang, S.-C. The Combination of Daptomycin with Fosfomycin Is More Effective than Daptomycin Alone in Reducing Mortality of Vancomycin-Resistant Enterococcal Bloodstream Infections: A Retrospective, Comparative Cohort Study. Infect. Dis. Ther. 2023, 12, 589–606. [Google Scholar] [CrossRef]
- Pujol, M.; Miró, J.-M.; Shaw, E.; Aguado, J.-M.; San-Juan, R.; Puig-Asensio, M.; Pigrau, C.; Calbo, E.; Montejo, M.; Rodriguez-Álvarez, R.; et al. Daptomycin Plus Fosfomycin Versus Daptomycin Alone for Methicillin-Resistant Staphylococcus aureus Bacteremia and Endocarditis: A Randomized Clinical Trial. Clin. Infect. Dis. 2021, 72, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Rieg, S.; Ernst, A.; Peyerl-Hoffmann, G.; Joost, I.; Camp, J.; Hellmich, M.; Kern, W.V.; Kaasch, A.J.; Seifert, H. Combination Therapy with Rifampicin or Fosfomycin in Patients with Staphylococcus aureus Bloodstream Infection at High Risk for Complications or Relapse: Results of a Large Prospective Observational Cohort. J. Antimicrob. Chemother. 2020, 75, 2282–2290. [Google Scholar] [CrossRef]
- Pericàs, J.M.; Moreno, A.; Almela, M.; García-de-la-Mària, C.; Marco, F.; Muñoz, P.; Peña, C.; De Alarcón, A.; Del Río, A.; Eworo, A.; et al. Efficacy and Safety of Fosfomycin plus Imipenem versus Vancomycin for Complicated Bacteraemia and Endocarditis Due to Methicillin-Resistant Staphylococcus aureus: A Randomized Clinical Trial. Clin. Microbiol. Infect. 2018, 24, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.; Reynaud, A.; Derriennic, M.; Courtieu, A.L. Comparaison entre fosfomycine-p6nicilline M et. p6nicilline M-Gentamicine. La Rev. De Médecine Interne 1987, 8, 109–114. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G. Fosfomycin as a Potential Therapeutic Option in Nonsevere Infections Caused by Metallo-β-Lactamase–Producing Enterobacterales: Need for Evidence. Clin. Infect. Dis. 2025, 80, 237–238. [Google Scholar] [CrossRef]
- Falcone, M.; Giordano, C.; Leonildi, A.; Galfo, V.; Lepore, A.; Suardi, L.R.; Riccardi, N.; Barnini, S.; Tiseo, G. Clinical Features and Outcomes of Infections Caused by Metallo-β-Lactamase–Producing Enterobacterales: A 3-Year Prospective Study from an Endemic Area. Clin. Infect. Dis. 2024, 78, 1111–1119. [Google Scholar] [CrossRef]
- Moreno-Mellado, E.; Aslan, A.T.; Akova, M.; León, E.; Merchante, N.; Vinuesa, D.; Moral-Escudero, E.; Sadyrbaeva-Dolgova, S.; López-Cárdenas, S.; Cano-Yuste, Á.; et al. Effectiveness and Tolerability of Intravenous Fosfomycin in Treating Complicated Urinary Tract Infections Caused by Escherichia coli: A Prospective Cohort Study from the FOSFOMIC Project. Clin. Microbiol. Infect. 2025, 31, 839–846. [Google Scholar] [CrossRef]
- Sojo-Dorado, J.; López-Hernández, I.; Rosso-Fernandez, C.; Morales, I.M.; Palacios-Baena, Z.R.; Hernández-Torres, A.; Merino De Lucas, E.; Escolà-Vergé, L.; Bereciartua, E.; García-Vázquez, E.; et al. Effectiveness of Fosfomycin for the Treatment of Multidrug-Resistant Escherichia coli Bacteremic Urinary Tract Infections: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2137277. [Google Scholar] [CrossRef]
- Kaye, K.S.; Rice, L.B.; Dane, A.L.; Stus, V.; Sagan, O.; Fedosiuk, E.; Das, A.F.; Skarinsky, D.; Eckburg, P.B.; Ellis-Grosse, E.J. Fosfomycin for Injection (ZTI-01) Versus Piperacillin-Tazobactam for the Treatment of Complicated Urinary Tract Infection Including Acute Pyelonephritis: ZEUS, A Phase 2/3 Randomized Trial. Clin. Infect. Dis. 2019, 69, 2045–2056. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Papagni, R.; Belati, A.; Diella, L.; De Luca, A.; Brindicci, G.; De Gennaro, N.; Di Gennaro, F.; Romanelli, F.; Stolfa, S.; et al. Cefiderocol Versus Colistin for the Treatment of Carbapenem-Resistant Acinetobacter Baumannii Complex Bloodstream Infections: A Retrospective, Propensity-Score Adjusted, Monocentric Cohort Study. Infect. Dis. Ther. 2023, 12, 2147–2163. [Google Scholar] [CrossRef]
- Calò, F.; Onorato, L.; De Luca, I.; Macera, M.; Monari, C.; Durante-Mangoni, E.; Massa, A.; Gentile, I.; Di Caprio, G.; Pagliano, P.; et al. Outcome of Patients with Carbapenem-Resistant Acinetobacter Baumannii Infections Treated with Cefiderocol: A Multicenter Observational Study. J. Infect. Public Health 2023, 16, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Dalfino, L.; Stufano, M.; Bavaro, D.F.; Diella, L.; Belati, A.; Stolfa, S.; Romanelli, F.; Ronga, L.; Di Mussi, R.; Murgolo, F.; et al. Effectiveness of First-Line Therapy with Old and Novel Antibiotics in Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter Baumannii: A Real Life, Prospective, Observational, Single-Center Study. Antibiotics 2023, 12, 1048. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Leonildi, A.; Della Sala, L.; Vecchione, A.; Barnini, S.; Farcomeni, A.; Menichetti, F. Cefiderocol-Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter Baumannii. Antimicrob. Agents Chemother. 2022, 66, e02142-21. [Google Scholar] [CrossRef]
- Mazzitelli, M.; Gregori, D.; Sasset, L.; Trevenzoli, M.; Scaglione, V.; Lo Menzo, S.; Marinello, S.; Mengato, D.; Venturini, F.; Tiberio, I.; et al. Cefiderocol-Based versus Colistin-Based Regimens for Severe Carbapenem-Resistant Acinetobacter Baumannii Infections: A Propensity Score-Weighted, Retrospective Cohort Study during the First Two Years of the COVID-19 Pandemic. Microorganisms 2023, 11, 984. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Bruni, A.; Gullì, S.; Borrazzo, C.; Quirino, A.; Lionello, R.; Serapide, F.; Garofalo, E.; Serraino, R.; Romeo, F.; et al. Efficacy of Cefiderocol- vs Colistin-Containing Regimen for Treatment of Bacteraemic Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter Baumannii in Patients with COVID-19. Int. J. Antimicrob. Agents 2023, 62, 106825. [Google Scholar] [CrossRef]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-Avibactam Use for Klebsiella Pneumoniae Carbapenemase–Producing K. pneumoniae Infections: A Retrospective Observational Multicenter Study. Clin. Infect. Dis. 2021, 73, 1664–1676. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, J.; Wang, B.; Cai, J.; Wang, L.; Hou, K.; Zhang, Y.; Zhang, L.; Yang, Z.; He, J.; et al. Ceftazidime-Avibactam in Combination with In Vitro Non-Susceptible Antimicrobials Versus Ceftazidime-Avibactam in Monotherapy in Critically Ill Patients with Carbapenem-Resistant Klebsiella Pneumoniae Infection: A Retrospective Cohort Study. Infect. Dis. Ther. 2021, 10, 1699–1713. [Google Scholar] [CrossRef]
- Khawcharoenporn, T.; Chuncharunee, A.; Maluangnon, C.; Taweesakulvashra, T.; Tiamsak, P. Active Monotherapy and Combination Therapy for Extensively Drug-Resistant Pseudomonas Aeruginosa Pneumonia. Int. J. Antimicrob. Agents 2018, 52, 828–834. [Google Scholar] [CrossRef]
- Meschiari, M.; Faltoni, M.; Kaleci, S.; Tassoni, G.; Orlando, G.; Franceschini, E.; Burastero, G.; Bedini, A.; Serio, L.; Biagioni, E.; et al. Intravenous Fosfomycin in Combination Regimens as a Treatment Option for Difficult-to-Treat Infections Due to Multi-Drug-Resistant Gram-Negative Organisms: A Real-Life Experience. Int. J. Antimicrob. Agents 2024, 63, 107134. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Gullì, S.P.; D’Avino, A.; Borrazzo, C.; Carannante, N.; Dezza, F.C.; Covino, S.; Polistina, G.; Fiorentino, G.; Trecarichi, E.M.; et al. Intravenous Fosfomycin for Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter Baumannii: A Multi-Centre Clinical Experience. Int. J. Antimicrob. Agents 2024, 64, 107190. [Google Scholar] [CrossRef] [PubMed]
- Aysert-Yildiz, P.; Özgen-Top, Ö.; Habibi, H.; Dizbay, M. Efficacy and Safety of Intravenous Fosfomycin for the Treatment of Carbapenem-Resistant Klebsiella pneumoniae. J. Chemother. 2023, 35, 471–476. [Google Scholar] [CrossRef]
- Önal, U.; Tüzemen, N.Ü.; Kaya, P.K.; İşçimen, R.; Girgin, N.K.; Özakın, C.; Kahveci, F.Ş.; Akalın, H. Evaluation of the Combination Treatments with Intravenous Fosfomycin for Carbapenem-Resistant Klebsiella Pneumoniae. Rev. Assoc. Med. Bras. 2023, 69, e20230727. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.; Baxter, M.; Wong, M.; Mirzanejad, Y.; Lee, A.; Dhami, R.; Kosar, J.; Werry, D.; Irfan, N.; Tessier, J.-F.; et al. Real-Life Experience with IV Fosfomycin in Canada: Results from the Canadian LEadership on Antimicrobial Real-Life Usage (CLEAR) Registry. J. Glob. Antimicrob. Resist. 2023, 33, 171–176. [Google Scholar] [CrossRef]
- Gatti, M.; Giannella, M.; Rinaldi, M.; Gaibani, P.; Viale, P.; Pea, F. Pharmacokinetic/Pharmacodynamic Analysis of Continuous-Infusion Fosfomycin in Combination with Extended-Infusion Cefiderocol or Continuous-Infusion Ceftazidime-Avibactam in a Case Series of Difficult-to-Treat Resistant Pseudomonas Aeruginosa Bloodstream Infections and/or Hospital-Acquired Pneumonia. Antibiotics 2022, 11, 1739. [Google Scholar] [CrossRef]
- Thampithak, A.; Chaisiri, K.; Siangsuebchart, O.; Phengjaturat, K.; Aonjumras, W.; Hemapanpairoa, J. Prescription Pattern of Intravenous Fosfomycin in a Provincial Hospital in Thailand. Infect. Chemother. 2022, 54, 699. [Google Scholar] [CrossRef]
- Abdallah, T.A.K.; Elajez, R.; Ibrahim, T.B.; Alimam, A.B.; Omrani, A.S. Efficacy and Safety of Intravenous Fosfomycin for the Treatment of Difficult-to-Treat Gram-Negative Bacterial Infections. J. Infect. Public Health 2021, 14, 1620–1622. [Google Scholar] [CrossRef]
- Ballouz, T.; Zeenny, R.M.; Haddad, N.; Rizk, N.; Kanj, S.S. Retrospective Evaluation of Intravenous Fosfomycin in Multi-Drug Resistant Infections at a Tertiary Care Hospital in Lebanon. J. Infect. Dev. Ctries. 2021, 15, 1308–1313. [Google Scholar] [CrossRef]
- Perdigão Neto, L.V.; Oliveira, M.S.; Martins, R.C.R.; Marchi, A.P.; Gaudereto, J.J.; Da Costa, L.A.T.J.; De Lima, L.F.A.; Takeda, C.F.V.; Costa, S.F.; Levin, A.S. Fosfomycin in Severe Infections Due to Genetically Distinct Pan-Drug-Resistant Gram-Negative Microorganisms: Synergy with Meropenem. J. Antimicrob. Chemother. 2019, 74, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Bodmann, K.-F.; Hagel, S.; Oliva, A.; Kluge, S.; Mularoni, A.; Galfo, V.; Falcone, M.; Pletz, M.W.; Lindau, S.; Käding, N.; et al. Real-World Use, Effectiveness, and Safety of Intravenous Fosfomycin: The FORTRESS Study. Infect. Dis. Ther. 2025, 14, 765–791. [Google Scholar] [CrossRef]
- Luciano, F.; Bertolino, L.; Patauner, F.; Boccia, F.; Gallo, R.; Sommese, P.; Peluso, A.M.C.; Infante, O.; Mercadante, S.; Delle Femine, A.; et al. Efficacy and Safety of Fosfomycin Disodium in Patients with Bacterial Infections: A Single-Center, Real-Life Clinical Study. J. Clin. Med. 2025, 14, 4386. [Google Scholar] [CrossRef] [PubMed]
- Zerbato, V.; Sanson, G.; Fusaro, L.; Gerussi, V.; Sincovich, S.; Dellai, F.; Del Fabro, G.; Geremia, N.; Maurel, C.; Giacomazzi, D.; et al. Intravenous Fosfomycin for Difficult-to-Treat Infections: A Real-Life Multicentric Study in Italy. Antibiotics 2025, 14, 401. [Google Scholar] [CrossRef]
- Anastasia, A.; Bonura, S.; Rubino, R.; Giammanco, G.M.; Miccichè, I.; Di Pace, M.R.; Colomba, C.; Cascio, A. The Use of Intravenous Fosfomycin in Clinical Practice: A 5-Year Retrospective Study in a Tertiary Hospital in Italy. Antibiotics 2023, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Zirpe, K.G.; Mehta, Y.; Pandit, R.; Pande, R.; Deshmukh, A.M.; Patil, S.; Bhagat, S.; Barkate, H. A Real-World Study on Prescription Pattern of Fosfomycin in Critical Care Patients. Indian. J. Crit. Care Med. 2021, 25, 1055–1058. [Google Scholar] [CrossRef]
- Putensen, C.; Ellger, B.; Sakka, S.G.; Weyland, A.; Schmidt, K.; Zoller, M.; Weiler, N.; Kindgen-Milles, D.; Jaschinski, U.; Weile, J.; et al. Current Clinical Use of Intravenous Fosfomycin in ICU Patients in Two European Countries. Infection 2019, 47, 827–836. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Tseng, T.-C.; Wang, J.-T.; Lin, C.-Y.; Huang, S.-H.; Chen, Y.-C.; Chang, S.-C. Influence of Daptomycin Dose and Fosfomycin Susceptibility on Outcome of Vancomycin-Resistant Enterococcus faecium Bloodstream Infections Treated with Daptomycin and Fosfomycin Combination. J. Antimicrob. Chemother. 2022, 77, 1436–1443. [Google Scholar] [CrossRef]
- Coronado-Álvarez, N.M.; Parra, D.; Parra-Ruiz, J. Clinical Efficacy of Fosfomycin Combinations against a Variety of Gram-Positive Cocci. Enfermedades Infecc. Y Microbiol. Clín. 2019, 37, 4–10. [Google Scholar] [CrossRef]
- Karnmueng, P.; Montakantikul, P.; Paiboonvong, T.; Plongla, R.; Chatsuwan, T.; Chumnumwat, S. Mortality Factors and Antibiotic Options in Carbapenem-resistant Enterobacterales Bloodstream Infections: Insights from a High-prevalence Setting with Co-occurring NDM-1 and OXA-48. Clin. Transl. Sci. 2024, 17, e13855. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.; Gracia-Ahufinger, I.; Carmona-Flores, R.; Guzmán-Puche, J.; León, R.; Pérez-Nadales, E.; Muñoz De La Rosa, M.; Natera, A.M.; Castón, J.J.; Cano, Á.; et al. Efficacy of High Doses of Intravenous Fosfomycin for Treatment of Urinary Tract Infection Caused by KPC Carbapenemase-Producing Klebsiella Pneumoniae: An Observational Study. J. Glob. Antimicrob. Resist. 2025, 41, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Kanchanasurakit, S.; Santimaleeworagun, W.; McPherson, C.E.; Piriyachananusorn, N.; Boonsong, B.; Katwilat, P.; Saokaew, S. Fosfomycin Dosing Regimens Based on Monte Carlo Simulation for Treated Carbapenem-Resistant Enterobacteriaceae Infection. Infect. Chemother. 2020, 52, 516. [Google Scholar] [CrossRef]
- Escrihuela-Vidal, F.; Ong, S.W.X.; Oriol, I.; Grillo, S.; Pujol, M.; Pallarès, N.; Tebé, C.; Liu, K.; Miró, J.M.; Tong, S.Y.C.; et al. Adjunctive Fosfomycin for the Treatment of Staphylococcus aureus Bacteremia: A Pooled Post Hoc Analysis of Individual Participant Data From 2 Randomized Trials. Clin. Infect. Dis. 2025, 80, ciaf387. [Google Scholar] [CrossRef]
- Sojo-Dorado, J.; López-Hernández, I.; Gutiérrez-Gutiérrez, B.; De La Rosa-Riestra, S.; Docobo-Pérez, F.; Hernánez-Torres, A.; Pascual, Á.; Rodriguez-Baño, J.; Ciberinfec-Geiras-Forest Group; De Lucas, E.M.; et al. Fosfomycin in Bacteraemic Urinary Tract Infection Due to Multidrug-Resistant Escherichia coli: Insights of Post Hoc DOOR Analysis of the FOREST Trial. Infect. Dis. 2025, 57, 294–300. [Google Scholar] [CrossRef]
- Marino, A.; Augello, E.; Bellanca, C.M.; Cosentino, F.; Stracquadanio, S.; La Via, L.; Maniaci, A.; Spampinato, S.; Fadda, P.; Cantarella, G.; et al. Antibiotic Therapy Duration for Multidrug-Resistant Gram-Negative Bacterial Infections: An Evidence-Based Review. Int. J. Mol. Sci. 2025, 26, 6905. [Google Scholar] [CrossRef]
- Grillo, S.; Cuervo, G.; Carratala, J.; San-Juan, R.; Aguado, J.M.; Morata, L.; Gomez-Zorrilla, S.; López-Contreras, J.; Gasch, O.; Gomila-Grange, A.; et al. Multicentre, Randomised, Open-Label, Phase IV-III Study to Evaluate the Efficacy of Cloxacillin plus Fosfomycin versus Cloxacillin Alone in Adult Patients with Methicillin-Susceptible Staphylococcus aureus Bacteraemia: Study Protocol for the SAFO Trial. BMJ Open 2021, 11, e051208. [Google Scholar] [CrossRef] [PubMed]
- Florent, A.; Chichmanian, R.-M.; Cua, E.; Pulcini, C. Adverse Events Associated with Intravenous Fosfomycin. Int. J. Antimicrob. Agents 2011, 37, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Grabein, B.; Graninger, W.; Rodríguez Baño, J.; Dinh, A.; Liesenfeld, D.B. Intravenous Fosfomycin-Back to the Future. Systematic Review and Meta-Analysis of the Clinical Literature. Clin. Microbiol. Infect. 2017, 23, 363–372. [Google Scholar] [CrossRef]
- Tsegka, K.G.; Voulgaris, G.L.; Kyriakidou, M.; Falagas, M.E. Intravenous Fosfomycin for the Treatment of Patients with Central Nervous System Infections: Evaluation of the Published Evidence. Expert. Rev. Anti Infect. Ther. 2020, 18, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.A.; Patel, N.; O’Donnell, J.N.; Lodise, T.P. Combination Therapy with IV Fosfomycin for Adult Patients with Serious Gram-Negative Infections: A Review of the Literature. J. Antimicrob. Chemother. 2024, 79, 2421–2459. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, S.; Giannitsioti, E.; Mayer, C. Emerging Concepts for the Treatment of Biofilm-Associated Bone and Joint Infections with IV Fosfomycin: A Literature Review. Microorganisms 2025, 13, 963. [Google Scholar] [CrossRef]
- Tsegka, K.G.; Voulgaris, G.L.; Kyriakidou, M.; Kapaskelis, A.; Falagas, M.E. Intravenous Fosfomycin for the Treatment of Patients with Bone and Joint Infections: A Review. Expert. Rev. Anti Infect. Ther. 2022, 20, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kastoris, A.C.; Kapaskelis, A.M.; Karageorgopoulos, D.E. Fosfomycin for the Treatment of Multidrug-Resistant, Including Extended-Spectrum Beta-Lactamase Producing, Enterobacteriaceae Infections: A Systematic Review. Lancet Infect. Dis. 2010, 10, 43–50. [Google Scholar] [CrossRef]
- Albur, M.S.; Noel, A.; Bowker, K.; MacGowan, A. The Combination of Colistin and Fosfomycin Is Synergistic against NDM-1-Producing Enterobacteriaceae in in Vitro Pharmacokinetic/Pharmacodynamic Model Experiments. Int. J. Antimicrob. Agents 2015, 46, 560–567. [Google Scholar] [CrossRef]
- Kastoris, A.C.; Rafailidis, P.I.; Vouloumanou, E.K.; Gkegkes, I.D.; Falagas, M.E. Synergy of Fosfomycin with Other Antibiotics for Gram-Positive and Gram-Negative Bacteria. Eur. J. Clin. Pharmacol. 2010, 66, 359–368. [Google Scholar] [CrossRef]
- Farooq, A.; Martens, M.; Kroemer, N.; Pfaffendorf, C.; Decousser, J.-W.; Nordmann, P.; Wicha, S.G. Pharmacokinetic/Pharmacodynamic Analysis of Meropenem and Fosfomycin Combinations in in Vitro Time-Kill and Hollow-Fibre Infection Models against Multidrug-Resistant and Carbapenemase-Producing Klebsiella Pneumoniae. J. Antimicrob. Chemother. 2025, 80, 701–712. [Google Scholar] [CrossRef]
- Roussos, N.; Karageorgopoulos, D.E.; Samonis, G.; Falagas, M.E. Clinical Significance of the Pharmacokinetic and Pharmacodynamic Characteristics of Fosfomycin for the Treatment of Patients with Systemic Infections. Int. J. Antimicrob. Agents 2009, 34, 506–515. [Google Scholar] [CrossRef]
- Wangchinda, W.; Pogue, J.M.; Thamlikitkul, V.; Leelawattanachai, P.; Koomanachai, P.; Pai, M.P. Population Pharmacokinetic/Pharmacodynamic Target Attainment Analysis of IV Fosfomycin for the Treatment of MDR Gram-Negative Bacterial Infections. J. Antimicrob. Chemother. 2024, 79, 1372–1379. [Google Scholar] [CrossRef]
- Falagas, M.E.; Maraki, S.; Karageorgopoulos, D.E.; Kastoris, A.C.; Mavromanolakis, E.; Samonis, G. Antimicrobial Susceptibility of Multidrug-Resistant (MDR) and Extensively Drug-Resistant (XDR) Enterobacteriaceae Isolates to Fosfomycin. Int. J. Antimicrob. Agents 2010, 35, 240–243. [Google Scholar] [CrossRef]
- Alrowais, H.; McElheny, C.L.; Spychala, C.N.; Sastry, S.; Guo, Q.; Butt, A.A.; Doi, Y. Fosfomycin Resistance in Escherichia coli, Pennsylvania, USA. Emerg. Infect. Dis. 2015, 21, 2045–2047. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Wu, Y.; Lou, N.; Jia, H.; Liu, N.; Zhang, J.; Xie, X.; Ruan, Z. Global Prevalence of Fosfomycin Resistance Genes fosA and fosB in Multidrug-Resistant Bacteria. Int. J. Antimicrob. Agents 2024, 64, 107272. [Google Scholar] [CrossRef]
- Falagas, M.E.; Athanasaki, F.; Voulgaris, G.L.; Triarides, N.A.; Vardakas, K.Z. Resistance to Fosfomycin: Mechanisms, Frequency and Clinical Consequences. Int. J. Antimicrob. Agents 2019, 53, 22–28. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, Y.; Sun, L.; Yu, Y.; Chen, Y. Mining Staphylococcus aureus Genomic Data for Identifying Fosfomycin Resistance Genes. Lancet Microbe 2024, 5, 626. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Risk of Bias Tools—ROBINS-I V2 Tool. Available online: https://www.riskofbias.info/welcome/robins-i-v2 (accessed on 19 December 2024).
- Pintado, V.; Ruiz-Garbajosa, P.; Aguilera-Alonso, D.; Baquero-Artigao, F.; Bou, G.; Cantón, R.; Grau, S.; Gutiérrez-Gutiérrez, B.; Larrosa, N.; Machuca, I.; et al. Executive Summary of the Consensus Document of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) on the Diagnosis and Antimicrobial Treatment of Infections Due to Carbapenem-Resistant Gram-Negative Bacteria. Enfermedades Infecc. Y Microbiol. Clin. (Engl. Ed.) 2023, 41, 360–370. [Google Scholar] [CrossRef]
- Bodmann, K.-F.; Grabein, B.; Kresken, M. S2k Guideline “Calculated Parenteral Initial Treatment of Bacterial Infections in Adults—Update 2018”, 2nd Updated Version: Foreword. GMS Infect. Dis. 2020, 8, Doc20. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ajmone Marsan, N.; De Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.; et al. 2023 ESC Guidelines for the Management of Endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli (Endorsed by European Society of Intensive Care Medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Kuehl, R.; Morata, L.; Boeing, C.; Subirana, I.; Seifert, H.; Rieg, S.; Kern, W.V.; Kim, H.B.; Kim, E.S.; Liao, C.-H.; et al. Defining Persistent Staphylococcus aureus Bacteraemia: Secondary Analysis of a Prospective Cohort Study. Lancet Infect. Dis. 2020, 20, 1409–1417. [Google Scholar] [CrossRef]
- Minejima, E.; Mai, N.; Bui, N.; Mert, M.; Mack, W.J.; She, R.C.; Nieberg, P.; Spellberg, B.; Wong-Beringer, A. Defining the Breakpoint Duration of Staphylococcus aureus Bacteremia Predictive of Poor Outcomes. Clin. Infect. Dis. 2020, 70, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.D.; Lo, C.K.L.; Komorowski, A.S.; Suresh, M.; Guo, K.; Garg, A.; Tandon, P.; Senecal, J.; Del Corpo, O.; Stefanova, I.; et al. How Generalizable Are Randomized Controlled Trials (RCTs) in Staphylococcus aureus Bacteremia? A Description of the Mortality Gap Between RCTs and Observational Studies. Clin. Infect. Dis. 2022, 75, 1449–1452. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Shibata, Y.; Hirai, J.; Ohashi, W.; Sakanashi, D.; Kato, H.; Hagihara, M.; Suematsu, H.; Mikamo, H. A Gap of Patients with Infective Endocarditis between Clinical Trials and the Real World. J. Clin. Med. 2023, 12, 1566. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Martin, T.; Curtis, S.; Faries, D.; Robinson, S.; Johnston, J. A Literature Review on the Representativeness of Randomized Controlled Trial Samples and Implications for the External Validity of Trial Results. Trials 2015, 16, 495. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vouloumanou, E.K.; Sgouros, K.; Athanasiou, S.; Peppas, G.; Siempos, I.I. Patients Included in Randomised Controlled Trials Do Not Represent Those Seen in Clinical Practice: Focus on Antimicrobial Agents. Int. J. Antimicrob. Agents 2010, 36, 1–13. [Google Scholar] [CrossRef]
- University of Melbourne Staphylococcus Aureus Network Adaptive Platform Trial. Available online: https://clinicaltrials.gov/study/NCT05137119 (accessed on 24 September 2025).
- Huttner, A. Ceftazidime Plus Fosfomycin Versus Ceftazidime Alone for Severe Gram-Negative Infections: A Triple-Blind, Placebo-Controlled Point-of-Care Randomized Clinical Trial. Available online: https://clinicaltrials.gov/study/NCT07063095 (accessed on 25 September 2025).
- Yin, M. TREAT-GNB [CR-GNB]. Available online: https://clinicaltrials.gov/study/NCT07004049 (accessed on 25 September 2025).
- Šitum, I. Therapeutic Strategies for Carbapenem-Resistant Acinetobacter Baumannii Infections: Study Protocol. Available online: https://clinicaltrials.gov/study/NCT06440304 (accessed on 25 September 2025).
- Salee, P. Efficacy of Colistin Monotherapy Versus Colistin Combined with Fosfomycin Against Carbapenem-Resistant Acinetobacter Baumannii Infections. Available online: https://clinicaltrials.gov/study/NCT06570850 (accessed on 25 September 2025).
- Piconi, S. Use of Fosfomycin in the Treatment of Bacterial Infections: " Real-Life" Study at the ASST of Lecco. Available online: https://clinicaltrials.gov/study/NCT06814899 (accessed on 25 September 2025).
- NeoSep1: A Study to Determine the Ranking of Existing and New Antibiotics Combinations to Treat Newborn Babies Who Are in Hospital with Severe Sepsis. Available online: https://www.isrctn.com/ISRCTN48721236 (accessed on 25 September 2025).
- Trampuz, A. Efficacy and Safety of Intravenous Fosfomycin in Prosthetic Joint Infection (PJI) Caused by Staphylococci, Streptococci, Enterococci and Gram-Negative Bacilli, Including Mixed Infections and Culture Negative PJI’s (“PROOF-Study”). Available online: https://clinicaltrials.gov/study/NCT05211011 (accessed on 25 September 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

| Author, Year | Type of Study | Fosfomycin N | Companion to Fosfomycin n | Comparator N | Comparator n | Population Characteristics Total or Fosfomycin Group vs. Comparator Group Mean ± SD or Median (IQR) n/N (%) | Infection Type(s) Total or Fosfomycin Group vs. Comparator Group n/N (%) | Pathogen(s) Total or Fosfomycin Group vs. Comparator Group n/N (%) | Fosfomycin Dosage g/d | Mortality Fosfomycin Group vs. Comparator Group n/N (%) | Clinical Cure Fosfomycin Group vs. Comparator Group n/N (%) | Microbiological Cure Fosfomycin Group vs. Comparator Group n/N (%) | Adverse Events Fosfomycin Group vs. Comparator Group n/N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belati, 2024 [14] | Retrospective cohort | 98 | 98 (targeted therapy) 46 carbapenem or carbapenem/BLIs 35 cephalosporin or cephalosporin/BLIs 14 other BL/BLIs 3 other drugs 7/98 additional fluoroquinolones or combination with >2 drugs | 265 | 265 (targeted therapy) 100 carbapenem or carbapenem/BLIs 100 cephalosporin or cephalosporin/BLIs 56 other BL/BLIs 9 other drugs 174/265 monotherapy 91/265 combination 46 aminoglycosides 16 tigecycline 2 colistin 26 fluoroquinolones or combinations with >2 drugs | Age 68 (IQR 57–78) y; males 211/363 (58); ward: medical 223/363 (61), surgical 86/363 (24), ICU 54/363 (15); Pitt score ≥ 4 points 115/363 (32); comorbidities: COPD 28/98 (29) vs. 44/265 (17), p = 0.01; obesity 14/98 (14) vs. 17/265 (6), p = 0.02; acute kidney failure 41/98 (42) vs. 81/265 (31), p = 0.04; deep site-associated bloodstream infection 40/98 (41) vs. 74/265 (28), p = 0.02 | BSI 363/363 (100) | K. pneumoniae 151/363 (42), E. coli 102/363 (28), P. aeruginosa 63/363 (17), S. maltophilia/B. cepacia/Achromobacter xylosoxidans 42/363 (12), E. cloacae complex/K. aerogenes/C. freundii 38/363 (10) | Median (IQR): 16 (16–18) | All-cause 14-day 9/98 (9) vs. 53/265 (20), p = 0.02; all-cause 30-day 19/98 (19) vs. 71/265 (27), p = 0.15; protective effect of fosfomycin combinations [multivariate analysis: aHR (95% CI) 0.51 (0.28–0.92), p = 0.03; IPTW-adjusted multivariable analysis: aHR (95% CI) 0.53 (0.31–0.91), p = 0.02] | 80/98 (82) vs. 188/265 (71) | 63/70 (90) vs. 147/186 (79), p = 0.04 | 12/98 (12) vs. 11/265 (5), p = 0.02 b |
| Fois, 2024 [15] | Retrospective cohort | 41 | Ceftazidime/avibactam | 34 | Ceftazidime/avibactam | Age 65 (IQR 57–73) y; males 61/75 (81); ICU 47/75 (63); CCI 4 (IQR 2–6) | HAP/VAP 75/75 (100) | K. pneumoniae 31/63 (49), P. aeruginosa 28/63 (44), 1/63 (2) of each: E. coli, K. aerogenes, K. pneumoniae/P. aeruginosa, K. pneumoniae/P. aeruginosa/E. coli; 43/63 (68) carbapenem-resistant; 63 patients had isolates detected | NR | 28-day 11/41 (27) vs. 8/34 (24); unadjusted Cox-regression analysis for 28-day: HR (95% CI) 1.14 (0.46–2.83), p = 0.78; adjusted Cox-regression analysis for 28-day: HR (95% CI) 0.32 (0.07–1.39), p = 0.13 | NR | NR | Urticaria 1 vs. 0, AKI 0 vs. 1, multiorgan failure 0 vs. 1 |
| Katip, 2024 [16] | Retrospective cohort | 153 | Colistin | 67 | Colistin | Males 90/153 (59) vs. 41/67 (61); APACHE II 9.9 ± 6.7 vs. 8.2 ± 6.4 | UTI 79/153 (52) vs. 38/67 (57), pneumonia 46/153 (30) vs. 13/67 (19), bacteremia 12/153 (8) vs. 4/67 (6), others 15/153 (10) vs. 13/67 (19) | E. coli 57/153 (37) vs. 26/67 (39), K. pneumoniae 57/153 (37) vs. 22/67 (33), E. cloacae 39/153 (25) vs. 19/67 (28) | 8 (divided into 2 daily doses) | 30-day 40/153 (26) vs. 12/67 (18), p = 0.19; EOT 42/153 (28) vs. 13/67 (19), p = 0.21 | 110/153 (72) vs. 57/67 (85), p = 0.04; propensity score analysis: aOR (95% CI) 1.48 (0.61–3.59), p = 0.38 | 147/153 (96) vs. 62/67 (93), p = 0.27; propensity score analysis: aOR (95% CI) 0.66 (0.18–2.38), p = 0.53 | NR |
| Oliva, 2024 [17] | Retrospective cohort | 37 | 20 ceftazidime/avibactam, 7 cefiderocol 3 meropenem, 3 meropenem/vaborbactam, 1 of each: cefiderocol + tigecycline, cefiderocol + ampicillin/sulbactam, colistin, or colistin + ampicillin/sulbactam | 41 | 15 cefiderocol + ampicillin/sulbactam, 7 ceftazidime/avibactam + meropenem, 7 colistin + ampicillin/sulbactam, 7 colistin + meropenem 2 cefiderocol + tigecycline, 1 of each: ceftazidime/avibactam + colistin, colistin + tigecycline, colistin + ampicillin/sulbactam + tigecycline | Age 67 (IQR 53–74) y; males 33/78 (33); CCI 4 (IQR 2–5), SAPS II 33 (IQR 26–40) | VAP 30/78 (39), BSI 20/78 (26), CLABSI 15/78 (19), CNS 5/78 (6), HAP 5/78 (6), UTI 1/78 (1), SSTI 1/78 (1), IAI 1/78 (1) | CRAB 44/78 (56), KPC-producing K. pneumoniae 34/78 (44) | Median (IQR): 24 (16–24) (divided into 3–4 doses; 3 h infusion) | 7-day mortality 0/37 (0) vs. 6/41 (15), p = 0.03; 14-day mortality 1/37 (3) vs. 9/41 (22), p = 0.02; 30-day mortality 5/37 (14) vs. 14/41 (35), p = 0.04 c | 33/37 (89) vs. 27/41 (66), p = 0.02; early clinical improvement 29/37 (78) vs. 21/41 (51), p = 0.02 | 28/32 (88) vs. 23/37 (62), p = 0.03 | AKI 3/37 (8) vs. 5/41 (12), p = 0.7; transient increase in transaminases 1/37 (3) vs. 0/41 (0) |
| Assimakopoulos, 2023 [18] | Retrospective cohort | 8 | 1 of each: colistin + co-trimoxazole, colistin, colistin + ampicillin/sulbactam + amikacin, colistin + tigecycline, tigecycline + ampicillin/sulbactam + amikacin, colistin + tigecycline, colistin + meropenem + co-trimoxazole + gentamicin, colistin + tigecycline + amikacin + co-trimoxazole | 12 | 2 colistin + tigecycline, 1 of each: colistin + meropenem, colistin + tigecycline + ampicillin/sulbactam + co-trimoxazole + amikacin, colistin + meropenem + tigecycline + ampicillin/sulbactam, colistin + amikacin, colistin + piperacillin/tazobactam, meropenem + gentamicin, colistin + meropenem, meropenem + ampicillin/sulbactam + tigecycline, colistin + tigecycline + ampicillin/sulbactam, colistin + meropenem | Age 62 ± 14 y; males 9/20 (45); ICU 20/20 (100) | BSI 20/20 (100) | PDR-A. baumannii 20/20 (100) | 24 (divided in 3 daily doses; 3 h infusion) | All-cause 28-day 1/8 (13) vs. 9/12 (75), p = 0.005 | NR | 8/8 (100) vs. 6/12 (50) | NR |
| Oliva, 2022 [19] | Retrospective cohort | 61 | Ceftazidime/avibactam | 61 | 40 meropenem + ceftazidime/avibactam, 8 ceftazidime/avibactam + gentamicin, tigecycline or colistin d | Age 68 (IQR 57–78) y; males 84/122 (69); hospitalized 122/122 (100); CCI 6 (IQR 5–9), Pitt score 2 (1–4) | UTI 36/122 (30), BSI 34/122 (28), LRTI 22/122 (18) [VAP 14/22 (64)], IAI 26/122 (21), CLABSI 4/122 (3) | KPC-K. pneumoniae 122/122 (100) | Median (IQR): 16 (12–24) | 7-day 3/61 (5) vs. 3/61 (5), p = 1.0; 14-day 6/61 (10) vs. 5/61 (8), p = 0.75; 30-day 9/61 (15) vs. 11/61 (18), p = 0.81; higher mortality in ceftazidime/avibactam group compared to fosfomycin + ceftazidime/avibactam group, p = 0.05 | 46/61 (75) vs. 37/61 (61), p = 0.12 | 72 h 33/43 (77) vs. 35/37 (95), p = 0.03; 7-day 30/34 (88) vs. 28/29 (97), p = 0.22; 14-day 25/26 (96) vs. 17/19 (90), p = 0.38 e | Secondary infection 17/61 (28) vs. 25/61 (41), p = 0.18; death associated with secondary infection 1/61 (2) vs. 7/61 (12), p = 0.02 |
| Russo, 2021 [20] | Prospective cohort | 44 | 11 colistin, 8 carbapenem + tigecycline, 7 rifampicin, 7 colistin + tigecycline, 6 tigecycline, 3 carbapenem, 2 aminoglycosides | 136 | Various combinations with colistin, tigecycline, aminoglycoside, rifampicin, ampicillin/sulbactam, cotrimoxazole, vancomycin, or carbapenem | Age 66 ± 16 vs. 64 ± 16 y; males 122/180 (68); ward: ICU (79), medical (19), surgical (2); CCI 6.3 ± 1.6 vs. 5.6 ± 1.8, SAPS II 43.9 ± 13.2 vs. 44.1 ± 15.3; comorbidities COPD 27/44 (61) vs. 49/136 (36), p = 0.005; previous MDR infections during hospital stay 4/44 (9) vs. 50/136 (37), p < 0.001 | HAP 180/180 (100) | MDR-A. baumannii 180/180 (100) [out of 112 strains assessed: 31% resistant to fosfomycin; 98% XDR; 2% PDR] | 12–24 (divided into 3–4 daily doses) | 30-day 7/44 (16) vs. 94/136 (69), p < 0.001; fosfomycin combinations associated with 30-day survival: HR (95% CI) 0.04 (0.01–0.13), p < 0.001 | NR | NR | Cardiovascular events after infection onset 14/44 (32) vs. 55/136 (40), p = 0.37 |
| Önal, 2025 [13] | Retrospective cohort | 41 | Meropenem-based regimens; 29 combined treatment approach [17 polymyxin B/colistin, 12 aminoglycosides (amikacin or gentamicin), other] | 30 | Ceftazidime/avibactam-based regimens; 19 combined treatment approach [10 polymyxin B/colistin, 10 aminoglycosides (amikacin or gentamicin), 1 meropenem, 3 tigecycline, 5 cotrimoxazole] | Age 59 ± 3 y; males 44/71 (62) | BSI 24/41 (59) vs. 17/30 (57) | Acinetobacter spp. 11/41 (27) vs. 9/30 (30), P. aeruginosa 4/41 (10) vs. 5/30 (17), Staphylococci 6/41 (15) vs. 3/30 (10), other 2/41 (5) vs. 4/30 (13) a | 12–16 (divided into 2–4 doses; individual doses of a maximum of 8 g) | 14-day 17/41 (42) vs. 10/30 (33), p = 0.49; 30-day 25/41 (61) vs. 15/30 (50), p = 0.36 | NR | NR | NR |
| Sirijatuphat, 2014 [21] | RCT | 47 | Colistin + carbapenem 4/47 (9), piperacillin/tazobactam 1/47 (2), vancomycin 1/47 (2), or other (levofloxacin, metronidazole, amphotericin B) 2/47 (4) | 47 | Colistin + carbapenem 8/47 (17), piperacillin/tazobactam 1/47 (2), vancomycin 2/47 (4), or other (levofloxacin, metronidazole, amphotericin B) 3/47 (6) | Age 67 ± 17 vs. 69 ± 16 y; males 20/47 (43) vs. 24/47 (51); APACHE II 23.0 ± 6.4 vs. 21.9 ± 7.9 | Pneumonia 37/47 (79) vs. 35/47 (75), primary BSI 2/47 (4) vs. 3/47 (6), UTI 3/47 (6) vs. 2/47 (4), SSTI 2/47 (4) vs. 1/47 (2), IAI/GI 2/47 (4) vs. 4/47 (9), CNS 0/47 (0) vs. 1/47 (2), others 1/47 (2) vs. 1/47 (2) | CRAB 94/94 (100); coinfection with: P. aeruginosa 3/47 (6), K. pneumoniae 2/47 (4), MRSA 2/47 (4), other 1/47 (2) vs. K. pneumoniae 6/47 (13), MRSA 2/47 (4), P. aeruginosa 1/47 (2), other 1/47 (2) | 8 | All-cause 28-day (47) vs. (57), p = 0.41; infection-related 28-day (21) vs. (28), p = 0.63 | After 72 h: (71) vs. (66), p = 0.66; EOT (60) vs. (55), p = 0.84 | 72 h (91) vs. (58), p = 0.001; EOT (100) vs. (81), p = 0.01 | AKI (53) vs. (60), p = 0.68; abnormal liver function test (13) vs. (13), p = 1.0 |
| Kobashi, 2002 [22] | RCT | 18 | Sulbactam-cefoperazole | 17 | Sulbactam-cefoperazole | Age 73 (range 32–92) vs. 69 (range 36–86) y; males 15/18 (83) vs. 12/17 (71) | Pneumonia 35/35 (100) | 2/18 (11) of each: S. pneumoniae, MSSA, MRSA, P. aeruginosa, 1/18 (6) of each: K. pneumoniae, K. oxytoca, S. milleri, A. baumannii vs. K. pneumoniae 3/17 (18), S. pneumoniae 2/17 (12), MSSA 2/17 (12), 1/17 (6) of each: P. aeruginosa, H. influenzae, E. cloacae, K. oxytoca, S. marcescens | 4 (divided into 2 daily doses; 30 min infusion) | NR | 17/18 (94) vs. 15/17 (88) | 5/10 (50) vs. 5/8 (63) | 1/18 (6) vs. 0/17 (0); severe gastrointestinal symptoms, leading to discontinuation of treatment |
| Shimokata, 1988 [23] | RCT | 41 | Cefotaxime | 32 | Cefotaxime | Males 29/41 (71) vs. 22/32 (69) | Pneumonia 29/41 (71) vs. 25/32 (78), secondary infection of chronic respiratory distress 6/41 (15) vs. 4/32 (13), bronchitis 3/41 (7) vs. 1/32 (3), pleurisy 0/41 (0) vs. 1/32 (3), pyothorax 0/41 (0) vs. 1/32 (3), suppurative lung disease 3/41 (7) vs. 0/32 (0) | S. agalactiae 2/41 (5), H. influenzae 2/41 (5), K. pneumoniae 2/41 (5), 1/41 (2) of each: S. aureus, S. viridans, Klebsiella spp., P. aeruginosa, S. aureus + S. viridans, S. epidermidis + H. parainfluenza, H. influenzae + K. pneumoniae vs. 1/32 (3) of each: S. agalactiae, S. viridans, S. pneumoniae, E. aerogenes, P. aeruginosa, H. influenzae + K. pneumoniae, Acinetobacter spp. + Pseudomonas spp. | 2–4 | NR | Total improvement 31/41 (76) vs. 26/32 (81); in severe disease 7/9 (78) vs. 1/2 (50), in moderate disease 17/25 (68) vs. 17/22 (77), in mild disease 7/7 (100) vs. 8/8 (100) | Total improvement 31/41 (76) vs. 26/32 (81); in severe disease 7/9 (78) vs. 1/2 (50), in moderate disease 17/25 (68) vs. 17/22 (77), in mild disease 7/7 (100) vs. 8/8 (100) | 5/41 (12) (3 elevated AST/ALT, 1 fever, 1 vascular pain, 1 elevated BUN) vs. 4/32 (13) (1 fever, 1 thrombocytopenia, 1 elevated AST/ALT, 1 elevated ALT) |
| Nissen, 1986 [24] | Randomized trial | 17 | Ampicillin | 15 | Ampicillin + gentamicin | Age 57 ± 19 vs. 58 ± 21 y; males 9/17 (53) vs. 9/15 (60) | Pneumonia 3/17 (18), chronic bronchitis with respiratory insufficiency 3/17 (18), cardiovascular resuscitation after acute myocardial infarction with cardiac arrest 1/17 (6), post-operative pneumonia 1/17 (6), respiratory insufficiency 1/17 (6) vs. cardiovascular resuscitation after acute myocardial infarction with cardiac arrest 2/15 (13), pneumonia 3/15 (20), chronic bronchitis with respiratory insufficiency 2/15 (13), post-operative pneumonia 1/15 (7) | E. coIi 5/17 (29), coagulase-positive staphylococci 3/17 (18), 2/17 (12) of each: pneumococci, α-hemolytic streptococci, B. catarrhalis, K. pneumoniae, P. aeruginosa, 1/17 (6) of each: β-hemolytic streptococcus Group A, H. influenzae, E. cloacae vs. coagulase-positive staphylococci 6/15 (40), E. coIi 5/15 (33), Pneumococcus 4/15 (27), α-hemolytic streptococci 2/15 (13), B. catarrhalis 2/15 (13), 1/15 (7) of each: coagulase-negative staphylococci, β-hemolytic streptococcus Group C, H. influenzae, P. aeruginosa | 12 (divided into 3 daily doses; 30 min infusion) | NR | 10/17 (59) vs. 7/15 (47) | 18/21 (88) vs. 18/20 (90) | Phlebitis 2/3 (67) (out of 3 who received fosfomycin from a peripheral vein), mild transient elevation in AST 1, pain 1 (that disappeared with a slower administration rate) vs. none |
| Grillo, 2023 [25] | Randomized clinical trial (phase III-IV) | 101 | Cloxacillin | 106 | Cloxacillin | Age 64 (IQR 55–72) vs. 68 (IQR 54–77) y; males 69 (66) vs. 81 (74); CCI 4.0 ± 3.1 vs. 4.7 ± 3.5, Pitt score 0.3 ± 0.6 vs. 0.3 ± 0.6 | BSI 207/207 (100) | MSSA 207/207 (100) | 12 (divided into 4 daily doses) | All-cause 7-day 2/101 (2) vs. 0 (0), P = 0.15; all-cause at EOT 10/101 (10) vs. 11/106 (10), p = 0.91; all-cause at TOC 10/101 (10) vs. 14/106 (13), p = 0.46 | 7-day 81/101 (80) vs. 81/106 (76), p = 0.51 | 7-day 81/101 (80) vs. 81/106 (76), p = 0.51; persistent bacteremia at day 3 4/94 (4) vs. 17/99 (17), p = 0.005 | 42/104 (40) vs. 48/110 (44), p = 0.73; hypokalemia 18 (17) vs. 11 (10), p = 0.17; |
| Tseng, 2023 [26] | Retrospective cohort | 48 | Daptomycin | 176 | Daptomycin | Age 67 (IQR 59–78) y; males 132/224 (58.9); CCI 3 (IQR 2–5.5) vs. 4 (IQR 2–5.5), Pitt score 1 (IQR 0–3) vs. 2 (IQR 1–4) | BSI 224/224 (100) | Vancomycin-resistant E. faecium 224/224 (100) | 12 (6–21) | In-hospital 23/48 (48) vs. 136/176 (77), p < 0.001; 14-day 18/48 (38) vs. 89/176 (51), p = 0.11; 28-day 21/48 (44) vs. 109/176 (62), p = 0.02 | 26/48 (54) vs. 64/176 (36) | 25/33 (76) vs. 60/120 (50) f | Elevated CK 2/48 (4) vs. 19/176 (11), thrombocytopenia 6/48 (17) vs. 29/176 (28), hypernatremia 5/48 (10) vs. 5/176 (3), hypokalemia 16/48 (33) vs. 27/176 (15), salt overload with clinical manifestations 3/48 (6) vs. 6/176 (3), AKI 6/48 (13) vs. 41/176 (23), discontinuation due to ADE 0/48 (0) vs. 1/176 (1) |
| Pujol, 2021 [27] | Randomized clinical trial (phase III) | 74 | Daptomycin | 81 | Daptomycin | Age 74 (IQR 61–81) vs. 72 (IQR 62–80) y; males 104/155 (67); CCI 3 (IQR 2–5) vs. 4 (IQR 2–5.8), Pitt score 1.15 ± 1.7 vs. 1.22 ± 2.0 | BSI 155/155 (100), left-sided endocarditis 18/155 (12); recurrent bacteremia 0/74 (0) vs. 4/81 (4), complicated bacteremia 12/74 (16) vs. 26/81 (32), p = 0.02 | MRSA 155/155 (100) | 12 (60 min infusion) | Overall at day-7 3/74 (4) vs. 6/81 (7); overall at TOC 18/74 (24) vs. 22/81 (27) | 6 weeks after EOT 40/74 (54) vs. 34/81 (42); RR (95% CI) 1.29 (0.93–1.8) | 74/74 (100) vs. 72/81 (89) | Leading to treatment discontinuation 13/77 (17) vs. 4/83 (5), p = 0.013 g |
| Rieg, 2020 [28] | Prospective cohort | 58 | Combination therapy (not specified) | 242 | Rifampicin | Median age 67 y; patients with implanted foreign devices, native valve endocarditis, or osteoarticular infections; CCI 3 (IQR 1–5) vs. 3 (IQR 2–5) | BSI 300/300 (100) | MSSA 514/1156 (44), MRSA 64/1156 (6); among all studied patients | 15 | 90-day implanted foreign devices: rifampicin HR (95% CI) 0.75, (0.46–1.25), p = 0.27; fosfomycin HR (95% CI) 0.72 (0.32–1.62), p = 0.43; osteoarticular infections: rifampicin HR (95% CI) 0.71 (0.33–1.49), p = 0.97; fosfomycin HR (95% CI) 0.68 (0.24–1.91), p = 0.71; endocarditis: rifampicin HR (95% CI) 1.16 (0.49–2.75), p = 0.73; fosfomycin HR (95% CI) 0.78 (0.22–2.81), p = 0.71 | NR | NR | NR |
| Pericàs, 2018 [29] | RCT | 8 | Imipenem | 7 | Vancomycin | Age 84 (67–86) vs. 76 (71–80); males 4 (50) vs. 5 (71) | Infectious endocarditis 4(50) vs. 4 (57), complicated bacteremia 4 (50) vs. 3 (43) | MRSA 15/15 (100) | 8 (divided into 4 daily doses) | In-hospital 4/8 (50) vs. 0/7 (0); at 12 weeks after study drug completion 4/8 (50) vs. 1/7 (14) | At the end of study 4/8 (50) vs. 3/7 (42) | 7-day 8/8 (100) vs. 6/7 (86) | 1/8 (13) vs. 2/7 (29); 1 salt overload vs. 1 acute renal failure and leucopenia, 1 acute renal failure |
| Baron, 1987 [30] | Prospective observational | 17 | Anti-staphylococcal penicillin | 18 | Gentamicin + anti-staphylococcal penicillin | NR | BSI 15/17 (88) vs. 17/18 (94); localized infection 2/17 (12) vs. 1/18 (6) | MSSA 35/35 (100) | [Mean: 237 mg/kg/d; 60 min infusion] | 1/17 (6) vs. 1/18 (6) | 16/17 (94) vs. 14/18 (78) | NR | 1/17 (6) vs. 7/18 (39), p < 0.001 (hypokalemia 3, renal damage 1 vs. renal damage 2, superinfection 2, renal impairment (hemodynamic origin) 1, relapse 1) |
| Author, Year | Type of Study | N Fosfomycin | N: n Comparator | Population Characteristics Total or Fosfomycin Group vs. Comparator Group Median (IQR or Range) or Mean ± SD n/N (%) | Infection(s) Total or Fosfomycin Group vs. Comparator Group n/N (%) | Pathogen(s) Total or Fosfomycin Group vs. Comparator Group n/N (%) | Fosfomycin Dosage g/d | Mortality Fosfomycin Group vs. Comparator Group n/N (%) | Clinical Cure Fosfomycin Group vs. Comparator Group n/N (%) | Microbiological Cure Fosfomycin Group vs. Comparator Group n/N (%) | Adverse Events Fosfomycin Group vs. Comparator Group n/N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Falcone, 2024 [32]; Falcone and Tiseo 2025 [31] | Prospective cohort | 22:15 fosfomycin monotherapy a | 215 ceftazidime/avibactam + aztreonam | Age 71 (IQR 60–79) y; males 273/343 (69); ICU 144/343 (42); SOFA score 3 (IQR 1–7) | BSI 3/22 (14) vs. 139/215 (65), HAP/VAP 4/22 (18) vs. 34/215 (16), UTI 13/22 (59) vs. 28/215 (13), IAI 1/22 (5) vs. 9/215 (4), SSTI 1/22 (5) vs. 5/215 (2) | 237/237 (100) MBL-Enterobacterales | 12–24 (divided into 3–4 doses) | 30-day 4/22 (18) vs. 48/215 (22), p = 0.79 | NR | NR | NR |
| Moreno-Mellado, 2025 [33] | Prospective, multicenter, matched-cohort | 155 | 155: 77 ceftriaxone, 25 fosfomycin, 19 piperacillin/tazobactam, 11 ertapenem, 9 amoxicillin/clavulanic acid, 5 meropenem, 5 ciprofloxacin, 4 others | Age 62 (IQR 46–73) vs. 65 (IQR 49–76) y; males 52/155 (34) vs. 52/155 (34); medical ward 107/155 (69) vs. 108/155 (70); ICU 39/155 (25) vs. 35/155 (23); emergency department 8 (5) vs. 10 (7); surgical ward 1/155 (1) vs. 2/155 (1); CCI 1 (IQR 0–3) vs. 1 (IQR 0–3) | Pyelonephritis 94/155 (61) vs. 92/155 (59), cUTI with bacteremia 69/155 (45) vs. 70/155 (45), cystitis 31/155 (20) vs. 27/155 (17), not localizable UTI 8/155 (5) vs. 15/155 (10), renal abscess 3/155 (2) vs. 4/155 (3), cUTI associated with a device 14/155 (9) vs. 13/155 (8), cUTI with hydronephrosis 10/155 (7) vs. 5/155 (3), cUTI with other features 5/155 (3) vs. 4/155 (3) | E. coli 310 (100) | 16 (124 pts); 12 (23 pts); 8 (8 pts) | 30-day 3/155 (2) vs. 9/155 (6), p = 0.08 | 145/155 (94) vs. 140/155 (90), p = 0.30 | NR | Severe 3/155 (2) vs. 1/155 (1), p = 0.34; non-severe 36/155 (23) vs. 12/155 (8), p < 0.001 |
| Sojo-Dorado, 2022 [34] | RCT | 70 b | 73: 31 ceftriaxone, 42 meropenem c | Age 69 (IQR 62–81) vs. 73 (IQR 62–84) y; males 36 (51) vs. 34 (47) | Community-acquired infection 33 (47) vs. 39 (53), health care–associated infection 25 (36) vs. 23 (32), nosocomial infection 12 (17) vs. 11 (15) | E. coli | 16 (over 60 min) | 30-day 2/61 (3) 2/71 (3), p = 0.44 | 59/61 (97) vs. 64/71 (90), p = 0.05 d | 48/58 (83) vs. 59/69 (86), p = 0.33 e | 44/70 (63) vs. 41/73 (56), p = 0.41; serious 13/70 (19) vs. 10/73 (14), p = 0.42 |
| Kaye, 2019 [35] | RCT | 184 | 178 piperacillin/tazobactam | Age 49.9 ± 20.9 vs. 51.3 ± 20.7 y; males 65/184 (35) vs. 67/178 (38); CCI 1 (IQR 0–3) vs. 2 (IQR 1–3), Pitt score 1 (IQR 0–1.25) vs. 1 (IQR 0–2) | Acute pyelonephritis 100/184 (54) vs. 96/178 (54), cUTI 84/184 (46) vs. 82/178 (46) | E. coli 133/184 (72) vs. 133/178 (75), K. pneumoniae 27/184 (15) vs. 25/178 (14), Enterobacterales 10/184 (5) vs. 9/178 (5), E. cloacae species complex 9/184 (5) vs. 3/178 (2), P. aeruginosa 8/184 (4) vs. 9/178 (5), P. mirabilis 9/184 (5) vs. 5/178 (3), E. faecalis 3/184 (2) vs. 7/178 (4), K. oxytoca 3/184 (2) vs. 2/178 (1), C. amalonaticus/farmeri 1/184 (1) vs. 0/178 (0), R. ornithinolytica 1/184 (1) vs. 1/178 (1), S. marcescens 1/184 (1) vs. 1/178 (1), M. morganii 0/184 (0) vs. 1/178 (1), A. baumannii-calcoaceticus species complex 2/184 (1) vs. 0/178 (0), S. aureus 1/184 (1) vs. 0/178 (0), S. saprophyticus 0/184 (0) vs. 1/178 (1) | 18 (over 60 min) | NR | TOC (day 19–21) 121/184 (66) vs. 100/178 (56) | TOC (day 19–21) 167 (91) vs. 163 (92) | 99/233 (43) vs. 74/231 (32) f |
| Author, Year | Type of Study | N Combination (n Receiving Fosfomycin) | N Comparator (n Receiving Fosfomycin) | Infection type(s) Total or Combination vs. Comparator n/N (%) | Pathogen(s) Total n/N (%) | Mortality Combination vs. Comparator n/N (%) | Clinical Cure Combination vs. Comparator n/N (%) | Microbiological Cure Combination vs. Comparator n/N (%) |
|---|---|---|---|---|---|---|---|---|
| Bavaro, 2023 [36] | Retrospective cohort | 43 cefiderocol (20) | 75 colistin (22) | Primary BSI or UTI 16/43 (37) vs. 21/75 (28), CVC-related 11/43 (26) vs. 25/75 (33), IAI 3/43 (7) vs. 16/75 (21), pneumonia 7/43 (16) vs. 5/75 (7), SSTI 6/43 (14) vs. 6/75 (8), endovascular infection 0/43 (0) vs. 1/75 (1), osteoarticular infection 0/43 (0) vs. 1/75 (1); CCI 6 (IQR 4–8) vs. 6 (IQR 4–7), Pitt score > 4 10/43 (23) vs. 20/75 (27) | CRAB 118/118 (100) | 30-day all-cause 17/43 (40) vs. 44/75 (59), p = 0.045; 30-day infection related 13/43 (30) vs. 42/75 (56), p = 0.007; 90-day all-cause 19/43 (42) vs. 48/75 (64), p = 0.032 | 26/43 (46) vs. 31/75 (54) | NR |
| Calò, 2023 [37] | Retrospective/prospective observational | 11 cefiderocol (4) | 29 cefiderocol monotherapy | Primary or CVC-related BSI 5/11 (46) vs. 13/29 (45), pneumonia 5/11 (46) vs. 11/29 (38), SSTI 1/11 (9) vs. 1/29 (3), UTI 0/11 (0) vs. 1/29 (3), bone infection 0/11 (0) vs. 2/29 (7), IAI 0/11 (0) vs. 1/29 (3) a; CCI 3 (IQR 4) vs. 3 (IQR 3.75), Pitt score 2 (IQR 5) vs. 2 (IQR 4.5), SOFA 5.5 (IQR 6.25) vs. 6 (IQR 1) | CRAB 40/40 (100) | 5/11 (46) vs. 14/29 (48), p = 0.87 | 7-day 4/11 (36) vs. 17/29 (59); EOT 6/11 (55) vs. 21/29 (72) | 7-day 10/11 (91) vs. 22/29 (76); EOT 11/11 (100) vs. 25/29 (86) |
| Falcone, 2024 [32]; Falcone and Tiseo 2025 [31] | Prospective observational | 37 other active antibiotics (OAA) (22) [15 fosfomycin monotherapy] 26 colistin (20) 33 cefiderocol (15) | 215 ceftazidime-avibactam/aztreonam (23) | BSI 199/343 (58), HAP/VAP 60/343 (17), UTI 60/343 (17), IAI 13/343 (4), SSTI 11/343 (3); SOFA score 3 (IQR 1–7) | MBL-producing Enterobacterales (344/344) [NDM-producing Enterobacterales: K. pneumoniae 326, E. coli 2; VIM-producing Enterobacterales: K. pneumoniae 5, E. cloacae 4, K. aerogenes 2, C. freundii 2, E. bugandensis 1, Providentia stuartii 1, E. coli 1] | 30-day 3/37 (13.5) (OAA), 11/33 (33) (cefiderocol), 13/26 (50) (colistin) vs. 48/215 (22) b | NR | NR |
| Dalfino, 2023 [38] | Prospective observational | 40 cefiderocol + inhaled colistin (21) | 50 colistin (0) | VAP 90/90 (100); CCI 5 (IQR 2–6) vs. 7 (IQR 2–8) | CRAB 90/90 (100) | 14-day 4/40 (10) vs. 19/50 (38), p = 0.03 | 30/40 (75) vs. 26/50 (52), p = 0.02 | 25/35 (70) vs. 16/40 (40) p = 0.003 c |
| Mazzitelli, 2023 [40] | Retrospective cohort | 60 cefiderocol (8) | 51 colistin (3) | BSI 34/60 (57) vs. 19/51 (37), pneumonia 26/60 (43) vs. 32/51 (63); APACHE 10 (IQR 7–13) vs. 10 (IQR 7.8–13.2); SOFA 2 (IQR 1–4) vs. 3.5 (IQR 2–5) | CRAB 111/111 (100) | 30-day all-cause 26/60 (43) vs. 22/51 (43), p = 0.13 | 44/60 (73) vs. 34/51 (67), p = 0.44 | 26/60 (43) vs. 21/51 (41), p = 0.82 |
| Russo, 2023 [41] | Retrospective cohort | 19 cefiderocol (14) | 54 colistin (5) | VAP + concomitant positive blood cultures 73/73 (100); CCI 2.6 (IQR 1.25–3.75) vs. 2.9 (IQR 1–4); SOFA 9 (IQR 9–10) vs. 10 (9–11) | CRAB 73/73 (100) | 14-day 1/19 (5) vs. 41/54 (76), p < 0.001; 30-day 6/19 (32) vs. 53/54 (98), p < 0.001 d | NR | NR |
| Falcone, 2022 [39] | Retrospective cohort | 47 cefiderocol (8) | 77 colistin (5) | BSI 27/47 (57) vs. 52/77 (68), VAP 12/47 (26) vs. 23/77 (30), other 8/47 (17) vs. 2/77 (3); APACHE II 18 (IQR 9–25) vs. 16 (IQR 11–22), CCI 3 (IQR 1–5) vs. 3 (IQR 1–5); SOFA 9 (IQR 6–11) vs. 9 (IQR 4–11) | CRAB 124/124 (100) | 30-day 16/47 (34) vs. 43/77 (56), p = 0.02 | NR | 38/46 (83) vs. 69/74 (93) e |
| Tumbarello, 2021 [42] | Retrospective cohort | 412 ceftazidime/avibactam (92) | 165 ceftazidime/avibactam monotherapy | BSI 391/577, (68) cUTI 71/577 (12), LRTI 59/577 (10), IAI 35/577 (6), others 21/577 (4); CCI ≥ 3 339/412 (82) vs. 150/165 (91) | KPC-producing K. pneumoniae 577/577 (100) | 30-day 103/412 (25) vs. 43/165 (26), p = 0.79 | NR | NR |
| Zheng, 2021 [43] | Retrospective cohort | 41 ceftazidime/avibactam (6) | 21 ceftazidime/avibactam monotherapy | BSI 7/41 (17) vs. 2/21 (10), RTI 14/41 (34) vs. 11/21 (52), IAI 9/41 (22) vs. 3/21 (14), UTI 7/41 (17) vs. 4/21 (19), others 4/41 (10) vs. 1/21 (5); APACHE II 18 (IQR 14–20.5) vs. 17 (IQR 16–19), CCI 4 (IQR 3–5) 4 vs. (IQR 3.5–6) | Carbapenem-resistant K. pneumoniae 62/62 (100) | 30-day 10/41 (24) vs. 11/21 (52) f | NR | 30-day 25/41 (61) vs. 9/21 (43) |
| Khawcharoenporn, 2018 [44] | Retrospective cohort | 40 active combined two-drug therapy (22) | 74 active monotherapy (12) 22 inactive therapy (2) | HAP/VAP 40/40 (100) vs. 74/74 (100) (active monotherapy), 22/22 (100) (inactive therapy); APACHE II 15 IQR (11–18) vs. 17 (IQR 13–24) (active monotherapy), 16 (IQR 12–26) (inactive therapy) | XDR P. aeruginosa | 28-day 36/40 (90) vs. 38/74 (51) (active monotherapy), 0/22 (0) (inactive therapy) g | NR | EOT 36/40 (90) vs. 40/74 (54) (active monotherapy), 0/22 (0) (inactive therapy) |
| Author, Year | Type of Study | N | Population Characteristics Mean ± SD or Median (IQR or Range) n (%) | Infection(s) n (%) | Pathogen(s) n (%) | Resistance n (%) | Fosfomycin IV Dosage g/d | Companion to IV Fosfomycin n (%) | Mortality n (%) | Clinical Cure n (%) | Microbiological Cure n (%) | Adverse Events n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meschiari, 2024 [45] | Retrospective cohort | 70 | Age 69 (IQR 61–73) y; males 57 (81.4); ward: ICU 18 (25.7), rehabilitation or post-ICU 23 (32.9), medical 8 (11.4), COVID-19-ICU 6 (8.6), surgical 3 (4.3); CCI 4 (IQR 3–6); SOFA 8 (IQR 6–14); comorbidities: DM 25 (35.7), CHF/CHD 22 (31.4), COVID-19 21 (30.0), renal disease 12 (17.1), solid tumor 12 (17.1), chronic lung disease/COPD 7 (10.0), solid organ transplant 6 (8.6), liver cirrhosis 5 (7.1), hematologic malignancy 2 (2.9) | VAP 16 (22.9), HAP 14 (20.0), VAP/HAP 30 (42.9), osteomyelitis/PJI 12 (17.1), IAI 8 (11.4), cSSTI 8 (11.4), primary BSI 1 (1.4), CLABSI 3 (4.3), cUTI/prostatitis 5 (7.1), meningitis/CNS infection 3 (4.3) | E. coli 10 (14.3), K. pneumoniae 16 (22.9), P. aeruginosa 40 (57.1), K. aerogenes 1 (1.4), P. mirabilis 1 (1.4), S. marcescens 1 (1.4), C. freundii 1 (1.4) | ESBL 10 (14.3), AmpC 15 (21.4), carbapenem-resistant 38 (54.3), carbapenem-resistant (non-carbapenemase producers) 22 (31.4), KPC 10 (14.3), VIM 3 (4.3), NDM 3 (4.3), ceftazidime/avibactam resistant 22 (31.4), ceftolozane/tazobactam resistant 20 (27.8) | 24 [39 (55.7) pts], 18 [5 (7.1) pts], 16 [26 (37.1) pts]; different dose regimens, based on the severity of the patient’s clinical condition, site of infection, and in vitro sensitivity | Ceftazidime/avibactam 21 (30), meropenem 14 (20), cefiderocol 8 (11.4), piperacillin/tazobactam 6 (8.6), ceftolozane/tazobactam 5 (7.1), tigecycline 4 (5.7), imipenem 2 (2.9), meropenem/vaborbactam 2 (2.9), amikacin 1 (1.4), colistin 1 (1.4) | 30-day 11 (15.7), 90-day 22 (31.4) | 39 (55.7) | 33 (47.1) | Skin reactions 2 (2.9), gastrointestinal reactions 3 (4.3), development of fosfomycin resistance 7 (10) |
| Russo, 2024 [46] | Retrospective cohort | 102 | Age 62 (IQR 58.3–71.8); males 78 (76.5); ICU 92 (90.2); CCI 3 (IQR 1–4.75), SOFA 10 (IQR 10–11); comorbidities: CVD 52 (51), DM 48 (47.1), solid tumor 18 (17.6), COPD 10 (9.8), HF 6 (5.9), CKD 4 (3.9), hematological malignancies 4 (3.9) | VAP 60 (58.8), primary BSI 22 (21.6), CVC related 16 (15.6), IAI 2 (2), UTI 1 (1), SSTI 1 (1) | A. baumannii 102/102 (100) | CRAB 102/102 (100) | Loading dose of 8 g followed by 12–24 (divided into doses every 6–8 h) | Cefiderocol 54 (52.9), colistin 48 (47.1), ampicillin/sulbactam 18 (17.6) | 30-day 48 (47.1), 15-day 32 (31.4) | 44 (43.1) | NR | NR |
| Aysert-Yildiz, 2023 [47] | Retrospective cohort | 94 | Age 69 (IQR 60–76) y; males 55 (57.9); ICU 52 (54.7); CCI 5 (IQR 4–8); comorbidities: sepsis/septic shock 49 (52.1), solid tumor 41 (43.2), CVD 39 (41.1), DM 39 (41.1), chronic neurological disease 34 (35.8), CKD 24 (25.3), COPD 15 (15.8), chronic hepatic disease 8 (8.4), rheumatic diseases 6 (6.3), hematologic malignancy 2 (2.1) | UTI 27 (28.4), HAP/VAP 25 (26.3), BSI 19 (20.0), IAI 10 (10.5), SSTI 9 (9.5), PJI 2 (2.1), empyema 3 (3.2) | K. pneumoniae 94/94 (100) | Resistant to: quinolones 94/94 (100), cotrimoxazole 78/94 (82.9), aminoglycosides 55/94 (58.5), colistin 52/85 (61.2), tigecycline 34/41 (82.9), fosfomycin 10/28 (35.7), ceftazidime/avibactam 7/21 (33.3) | 12–24 (divided into 2–3 daily doses); 20–24 in patients with sepsis/septic shock | Most frequently combined with meropenem, polymyxins, or tigecycline; combination with other antimicrobials 87 (92.5); combination with ≥2 antimicrobials 42 (44.7); meropenem-containing regimens 55 (58.5), polymyxin-containing regimens 44 (46.8), tigecycline-containing regimens 20 (21.3) a | 30-day 31 (33) | 70/93 (75.3) b | 55/86 (63.8) c | 46 (48.9); leading to discontinuation of therapy 3 (3.2); hypokalemia 35 (37.2), hypernatremia 21 (22.3), elevated LFTs 10 (10.6), hypomagnesemia 8 (8.5), thrombocytopenia 8 (8.5), diarrhea 5 (5.3), eosinophilia 2 (2.1), neutropenia 1 (1.1) |
| Önal, 2023 [48] | Retrospective cohort | 62 | Males 23 (37.1); ward: 62 (100); comorbidities: HTN 32 (51.6), DM 28 (45.2), immunosuppression 20 (32.3), malignancy 19 (30.6) | BSI 33 (53.2), VAP 29 (46.8) | K. pneumoniae 62 (100) | Carbapenem-resistant 62 (100) K. pneumoniae | Patients without 30-day mortality: mean ± SD daily dose 12.08 ± 0.69; patients with 30-day mortality: mean ± SD daily dose 12.37 ± 0.92 | Meropenem + colistin/polymyxin B 14 (22.6), meropenem + amikacin/gentamicin 9 (14.5), meropenem 12 (19.4), colistin/polymyxin B 7 (11.3), amikacin/gentamicin 3 (4.8), colistin/polymyxin B + amikacin/gentamicin 2 (3.2), two others d 10 (16.1), others d 5 (8.1), | 14-day all-cause 22 (36.5); 30-day all-cause 34 (54.8) | NR | NR | At 30 days: hypernatremia 34 (54.8), hypokalemia 34 (54.8) |
| Gatti, 2022 [50] | Retrospective case series | 6 | Age 57.7 ± 21.7 y; males 3 (50); ward: ICU 4 (66.7), infectious disease unit 1 (16.7), hematology + ICU 1 (16.7) | BSI + VAP 2 (33.3), VAP 2 (33.3), BSI 1 (16.7), HAP 1 (16.7) | P. aeruginosa 6 (100) | DTR P. aeruginosa 6 (100): ceftazidime/avibactam-resistant 4 (66.7), ceftolozane/tazobactam-resistant 2 (33.3) | 16–24 (continuous infusion; 8 g loading dose) | Cefiderocol 3 (50), ceftazidime/avibactam 2 (33.3), cefiderocol 1 (16.7) | Total 30-day 2 (33.3) | NR | 5 (83.3) | 0 (0) |
| Thampithak, 2022 [51] | Retrospective descriptive | 254 | Age 59.6 ± 17.7 y; males 142 (53.6); ward: critical care 26 (9.8), general internal medicine 163 (61.5), critical surgery 8 (3.0), surgical 68 (25.7); comorbidities: CKD 147 (55.5), HTN 62 (23.4), DM 53 (20.0), CVD 39 (14.7), solid tumor 24 (9.1), hematologic malignancy 22 (8.3) (these values refer to a total of 265 patients who received IV fosfomycin; 11 received it as surgical prophylaxis and 254 received it for the treatment of infections) | RTI 118 (46.5), UTI 53 (20.9), SSTI 29 (11.4), BSI 24 (9.4), IAI 7 (2.8), CNS infection 1 (0.4), BJI 1 (0.4), unknown source 21 (8.3) | Enterobacterales 125 (47.2), A. baumannii 116 (43.8), P. aeruginosa 24 (9.1) | Carbapenem-resistant 141 (87.6) | Based on the infection site: RTI 2–16, UTI 2–12, SSTI 1–12, BSI 2- 12, IAI 2–12, febrile neutropenia 8–12, CNS infection 8, BJI 4 | Colistin 132, aminoglycosides 41, tigecycline 2, carbapenem 8, levofloxacin 4, ampicillin/sulbactam 2, ceftazidime 2, colistin–ampicillin/sulbactam 17, colistin–carbapenem 12, colistin–aminoglycosides 3, colistin–levofloxacin 7, colistin–piperacillin/tazobactam 1, colistin–tigecycline 1, colistin–cotrimoxazole 1, aminoglycosides–tigecycline 1, aminoglycosides–meropenem 2, meropenem–levofloxacin 2, meropenem–piperacillin/tazobactam 1, aminoglycosides–cotrimoxazole 1 e | 14-day all-cause 119 (45) f | NR | NR | NR |
| Abdallah, 2021 [52] | Retrospective cohort | 30 | Age 63.5 (IQR 46–73) y; males 19 (63.3); CCI 6 (IQR 3.8–9); comorbidities: recent hospitalization 23 (76.6), DM 19 (63.3), CKD 16 (53.3), history of recurrent UTIs 14 (46.6), malignancy 11 (36.7), recent surgery 10 (33.3), transplant recipient 5 (16.7), CHD 2 (6.6), chronic lung disease 2 (6.6) | UTI 17 (56.7), BSI 4 (13.3), SSTI 4 (13.3), IAI 2 (6.7), RTI 2 (6.7), CSF infections 1 (3.3) | K. pneumoniae 17 (56.7), E. coli 7 (23.3), other 6 (20) | DTR Gram-negative bacteria 30 (100) | 12–24 g (divided into 2–4 daily doses) | Meropenem 8 (26.6), tigecycline 8 (26.6), aminoglycoside 5 (15.7), colistin 3 (10), fluoroquinolone 3 (10) g | Total 30-day 7 (23.3) | 22 (73.3) | 20 (66.7) | Most frequent adverse events: hypokalemia 13 (43.3), hypernatremia 7 (23.3), resistance to fosfomycin within 90 days of initiation of fosfomycin therapy 5 (16.7) |
| Ballouz, 2021 [53] | Retrospective cohort | 28 | Age 56.43 ± 17.66 y; males 20 (71); comorbidities: hematologic 9 (32), HTN 9 (32), dyslipidemia 7 (25), DM 5 (18), acute myeloid leukemia 4 (14), coronary artery disease 3 (11), colorectal cancer 2 (7), diffuse large B-cell lymphoma 2 (7), acute lymphoblastic leukemia 1 (4), bladder cancer 1 (4), chronic lung disease 1 (4), CKD 1 (4), COPD 1 (4), Hodgkin lymphoma 1 (4), squamous cell carcinoma of the mandible 1 (4), T-cell lymphomas 1 (4) | BSI 18 (64), UTI 5 (16), RTI 3 (10), febrile neutropenia 3 (10) | E. coli 11 (40), A. baumannii 9 (32), P. aeruginosa 4 (14), Enterobacter spp. 2 (7), K. pneumoniae 2 (7) | A. baumannii (MDR 22, XDR 78), E. coli (MDR 50, XDR 50), P. aeruginosa (MDR 50, XDR 50), K. pneumoniae (MDR 64, XDR 27), Enterobacter spp. (MDR 25, XDR 50), | 12–16 (divided in 2–4 daily doses); higher daily doses (up to 24 g) were used in severe infections | Fosfomycin was administered in combination with other antibiotics 26 (mainly tigecycline 8, amikacin 3, based on susceptibility data) h | Overall in-hospital 7 (26) | EOT 14 (45); out of 31 episodes that were treated | At EOT 8/11 (73; out of the 11 cultures available at the EOT | Hypokalemia (14), hypernatremia (6), diarrhea 5), out of which 4 were due to C. difficile; development of resistance to fosfomycin (2) |
| Perdigão Neto, 2019 [54] | Prospective case series | 13 | Age 52 ± 24 y; males 8 (61.5); ICU 9 (69.2); comorbidities: recent surgery 8 (61.5), DM 7 (53.8), HTN 7 (53.8), immunosuppression 7 (53.8), dyslipidemia 2 (15.4), Chagas’ disease 1 (7.7), OA 1 (7.7) | CLABSI 6 (46.2), BSI-IAI 3 (23.1), BSI-UTI 1 (7.7), BSI-VAP 1 (7.7), SSI 1 (7.7), VAP 1 (7.7) | K. pneumoniae 9 (69.2), S. marcescens 3 (23.1), P. aeruginosa 1 (7.7) | KPC-2 10 (76.9), CTX-M 9 (69.2), SHV 9 (69.2), TEM 8 (61.5), OXA 5 (38.5), NDM-1 1 (7.7), PAO 1 (7.7) | 16 (divided in 4 daily doses) | Meropenem 10 (76.9), amikacin 3 (23.1), tigecycline 2 (15.4), colistin 2 (15.4), ertapenem 1 (7.7); some received more than one agent | 14-day 5 (38.5), 28-day 7 (53.8) | 8 (61.5) | NR | 8 (61.5); some patients had more than one (hypokalemia 8, nausea 3, vomiting 2, diarrhea 2, hypertension 2) |
| Bodmann, 2025 [55] | Prospective non-interventional | 716 | Age 62.8 ± 14.75 y; males 454 (63.4); ICU 370 (51.7); comorbidities: cardiovascular 476 (66.5), renal 253 (35.3), endocrinologic 252 (35.2), electrolyte disorders 226 (31.6), respiratory 210 (29.3), immunocompromised 163 (22.8), oncologic 128 (17.9), immunosuppressive 120 (16.8), hepatic 109 (15.2), orthopedic 100 (14.0), traumatic injury/fractures 60 (8.4), other 273 (38.1) | BSI 169 (23.6), cUTI 129 (18), BJI 124 (17.3), HAP/VAP 79 (11), cSSTI 65 (9.1), BM/CNS 56 (7.8), IE 46 (6.4) | S. aureus 225 (31.4), Klebsiella spp. 123 (17.2), E. coli 102 (14.2), CoNS 93 (13), other Enterobacterales 78 (10.9), P. aeruginosa 60 (8.4), Enterococcus spp. 58 (8.1), Streptococcus spp. 32 (4.5), other Gram-negative pathogens 15 (2.1), other Gram-positive pathogens 12 (1.7), anaerobes 8 (1.1), Acinetobacter spp. 7 (1) | MDR 248 (34.6) [methicillin resistance 53 (7.4), vancomycin-resistant 13 (1.8), carbapenem-resistant 79 (11), ESBL-producing 62 (8.7), other mechanism of resistance 75 (10.5)]; some isolates had more than one mechanism of resistance | Median daily targeted dose of 15 (with targeted doses up to 24 g); median daily targeted dose: 15 in Germany and the UK, 16 in Italy, 20 in Greece, 24 in Austria | BL/BLIs 181 (25.3), carbapenems 157 (21.9), penicillins 109 (15.2), 3rd/4th/5th/next-generation cephalosporins 80 (11.2), 1st/2nd-genenration cephalosporins: 79 (11), daptomycin 56 (7.8), fluoroquinolones 37 (5.2), aminoglycoside 28 (3.9), linezolid 23 (3.2), colistin 16 (2.2) | Overall in-hospital 76 (10.6); none was related to treatment with fosfomycin | 539 (75.3) [clinical response 597 (83.4)] | 590 (82.4) | 417 (58); serious 59 (8) [hypokalemia 189 (26.4), hypernatremia 109 (15.2)] |
| Luciano, 2025 [56] | Retrospective cohort | 56 | Age 62.4 (IQR 30–75) y; males 38 (68); ward: internal medicine 27 (48), ICU 17 (30), cardio-thoracic surgery unit 6 (11), respiratory disease unit 4 (7), cardiovascular disease unit 2 (4); CCI 4 (IQR 3–6); comorbidities: renal disease 17 (30.4), DM 16 (28.6), heart disease 15 (26.8), HTN 13 (23.2) | RTI 23 (41.1), BSI 11 (19.6), bone infections 9 (16.1), UTI 9 (16.1), surgical site infections 4 (7.1) | P. aeruginosa 14 (17.3), E. coli 12 (14.8), K. pneumoniae 11 (13.6), S. aureus 7 (8.6), A. baumannii 5 (6.2), E. faecium 4 (4.9), S. epidermidis 4 (4.9), S. hominis 4 (4.9), E. cloacae 3 (3.7), E. faecalis 2 (2.5), K. aerogenes 2 (2.5), S. haemolyticus 2 (2.5), S. maltophilia 2 (2.5), C. striatum 1 (1.2), E. raffinosus 1 (1.2), H. influenzae 1 (1.2), H. alvei 1 (1.2), K. oxtytoca 1 (1.2), M. catarrhalis 1 (1.2), P. putida 1 (1.2), S. simulans 1 (1.2), S. paucimobilis 1 (1.2) | MDR 36 (64.3) [methicillin-resistant 14 (25), ESBL 8 (14.3), DTR 4 (7.1), KPC 4 (7.1), XDR 4 (7.1), AmpC 1 (1.8)], AmpR 1 (1.8)] | NR | Meropenem 16 (28.6), colistin 11 (19.6), ceftazidime/avibactam 9 (16.1), daptomycin 5 (8.9) cefiderocol 5 (8.9), ceftolozane/tazobactam 4 (7.1), ceftobiprole 4 (7.1), linezolid 4 (7.1), tigecycline 4 (7.1), piperacillin/tazobactam 3 (5.4), amikacin 2 (3.6), gentamicin 2 (3.6), amoxicillin/clavulanate 2 (3.6), cotrimoxazole 2 (3.6), teicoplanin 2 (3.6), vancomycin 2 (3.6), meropenem/vaborbactam 2 (3.6), other (1 of each: ertapenem, cefepime, metronidazole, ceftaroline, ampicillin/sulbactam, cefazolin) 6 (10.7) | All-cause at EOT 14 (25) | 22 (39.3) | 25 (69.4); follow-up cultures were performed in 36 patients | Electrolyte imbalance 16 (29.6); neutrophil count reduction: median 8340 to 5730, p = 0.01; increase in serum Na+ concentration: median 138 to 140 mEq/L, p < 0.01 |
| Zerbato, 2025 [57] | Retrospective cohort | 393 | Age 69 (IQR 59–76) y; males 268 (68.2); ward: ICU 178 (45.3), medical 91 (23.2), surgical 83 (21.1), infectious disease 41 (10.4) | Pneumonia 113 (34.3), BSI 71 (21.7), UTI 70 (21.1), other i | E. coli 56 (23), P. aeruginosa 55 (22.6), K. pneumoniae 43 (17.7), S. aureus 32 (13.2), E. faecium 12 (4.9), S. epidermidis 12 (4.9), A. baumannii 3 (1.2), S. enterica 1 (0.4) (among the 296 total) | ESBL-producers 32 (10.8), AmpC β-lactamase producers 14 (4.7), KPC-producers 11 (3.7), methicillin-resistant S. epidermidis 9 (3), MRSA 8 (2.7), OXA-48-producers 7 (2.4), VRE 7 (2.4), XDR isolates 6 (2), MBL producers 2 (0.7), | 2–24 (continuous infusion in 192 patients) | Piperacillin/tazobactam 82 (20.9), new β-lactamase inhibitor combination (ceftazidime/avibactam, ceftolozane/tazobactam, or meropenem/vaborbactam) 71 (18.1), carbapenem 70 (17.8), daptomycin 42 (10.6), tigecycline 31 (8.0), linezolid 27 (6.8), vancomycin 22 (5.6), anti-pseudomonal cephalosporin 17 (4.3), aminoglycoside 17 (4.3), third generation cephalosporin 15 (3.8), ceftazidime 10 (2.5), colistin 8 (2.0), others 66 (16.8) | 30-day 85 (21.6), 60-day 105 (26.7), 90-day 115 (29.3) | NR | NR | C. difficile infection 8 (2) |
| Anastasia, 2023 [58] | Retrospective cohort | 343 | Age 68 ± 13.9 (19–95) y; males 216 (62.9); high-ICU 57/343 (16.6); comorbidities: CVD 57 (16.6), lung diseases 57 (16.6), DM 48 (13.9), solid neoplasm 31 (9.1), hematological diseases 29 (8.4), kidney failure 29 (8.4), SARS-CoV-2 18 (5.2), HIV/AIDS 11 (3.2), other 59 (17.2) | UTI/pyelonephritis 69 (20.1), IE 13 (3.8), SSTI 49 (14.3), CNSI 10 (2.9), osteomyelitis 37 (10.8), BSI 52 (15.2), intrathoracic infections 6 (1.7), IAI 37 (10.8), pneumonia 63 (18.4), other 7 (2) | K. pneumoniae 193 (56.2), P. aeruginosa 42 (12.2), A. baumannii 36 (10.2), Enterococcus spp. 28 (8.2), S. aureus 16 (4.2), other 28 (8) | Resistant K. pneumoniae: cotrimoxazole 164/193 (85.0), carbapenems 159/193 (82.4), amikacin 182/193 (94.3), ceftazolane/tazobactam 78/84 (92.9), colistin 47/193 (24.4), fosfomycin 15/193 (7.8), ceftazidime/avibactam 10/100 (10), meropenem/vaborbactam 0/22 (0); resistant A. baumannii: fluoroquinolones 36/36 (100), cotrimoxazole 31/36 (86.1), aminoglycosides 29/36 (80.6), colistin 2/36 (5.6) | 16–24 (divided into 3–4 daily doses) | Ceftazidime/avibactam 122 (35.5), meropenem 57 (16.6), colistin 49 (14.1), daptomycin 39 (11.4), vancomycin 28 (8.2) j | 90/343 (26.2) | 226/343 (65.8) | NR | 20 (5.8); nausea 7 (2), isolated hypernatremia 4 (1.2), isolated hypokalemia 4 (1.2), diarrhea 3 (0.9), hypernatremia + hypokalemia 1 (0.3), rash 1 (0.3) |
| Zhanel, 2023 [49] | Registry-based cohort | 51 | Ward: ICU 35 (68.6), non-ICU 16 (31.4) | BSI/sepsis 14 (27.5), VAP 10 (19.6), HAP 8 (15.7), cIAI 6 (11.8), cUTI 6 (11.8), BJI 2 (3.3), CABP (3.3), endocarditis 2 (3.3), CNSI 1 (2) | Klebsiella spp. 16, E. coli 12, P. aeruginosa 12, Enterobacter spp. 4, E. faecium 4, Citrobacter spp. 2, S. aureus 1, unknown 1; 2 patients had mixed infections (E. coli and Klebsiella spp. and ESBL Klebsiella spp. + P. aeruginosa) | CRE 21 (CRE Klebsiella 11, CRE E. coli 10); VRE 3; ESBL 3 (ESBL Klebsiella 2, ESBL-containing mixed infection 1) | 2–24 (divided into 1–4 daily doses); 3 patients received 2 g post-dialysis, and 1 patient received an unknown dose | Carbapenem 13, carbapenem + tigecycline 9, aminoglycoside 8, colistin or polymyxin b 4, carbapenem + colistin 3, aminoglycoside + carbapenem 2, carbapenem + fluoroquinolone 1, carbapenem + inhaled colistin 1, carbapenem + piperacillin/tazobactam 1, cefazolin + daptomycin + rifampin 1, ceftazidime + intrathecal colistin 1, daptomycin 1, doxycycline 1, fluoroquinolone + piperacillin/tazobactam 1, imipenem/relebactam 1, tigecycline 1, vancomycin 1 | 14 (27.5) | 13 (25.5) [2 patients had an unknown outcome] | 19 (31.1); presumed eradication 15 (29.4) [5 patients had an unknown outcome] | Hypokalaemia 7 (13.7), gstrointestinal 2 (3.92), hypernatraemia 2 (3.92) |
| Zirpe, 2021 [59] | Retrospective cohort | 309 | Age 60.59 ± 15.90 y; males 193 (62.5) | BSI 140 (45.3), UTI 53 (13.9), pneumonia 49 (15.9), SS 44 (14.2), SSTI 10 (3.2), IE 4 (1.3), meningitis 2 (0.7), osteomyelitis 2 (0.7), IAI 3 (1), gynecological infection 1 (0.3) | K. pneumoniae 149 (48.2), E. coli 49 (15.9), P. aeruginosa 27 (8.7), Staphylococcus spp.12 (3.9), E. aerogenes 3 (0.9), P. mirabilis 3 (0.9), S. marcescens 2 (0.6), mixed 47 (15.2), no growth 17 (5.5) | Suspected CRE infection 1 (0.3) | 3- >24 | NR | Overall 139 (45) | 50 (16.2) | NR | Hypokalemia 190 (61.5), hypernatremia 75 (24.3) |
| Putensen, 2019 [60] | Prospective non-interventional | 209 | Age 59.1 ± 16.4 y; males 132 (63.2); ICU 194 (92.8); comorbidities: diabetes mellitus, smoking, immunosuppression/corticosteroids, chronic renal insufficiency, liver cirrhosis, intensive antibiotic pretreatment within the last month | CNS 45 (21.5), pneumonia (CAP, HAP, VAP) 32 (15.3), BJI 23 (11), IAI 23 (11), sepsis/BSI 22 (10.5), endocarditis (all with sepsis/BSI) 9 (4.3), cUTI 8 (3.8); APACHE II (in 71 patients) 23 ± 8, APACHE III (in 19 patients) 104 ± 18 | S. aureus 58 (22.3), S. epidermidis/CoNS 37 (14.2), E. coli 32 (12.3), Enterococcus spp. 28 (10.8), Klebsiella spp. 20 (7.7), Enterobacter/Citrobacter spp. 17 (6.5), P. aeruginosa 12 (4.6), aerobes 9 (3.5), Streptococcus spp. 9 3.5), Proteus spp. 6 (2.3), Serratia spp. 4 (1.5), other Gram-negative pathogens 19 (7.3), other Gram-positive pathogens 9 (3.6) | At least one MDR pathogen: 51 (24.4); ESBL 4 (1.9); S. aureus infections: MSSA 21 (75), MRSA 7 (25) | Total dose range 3–24 [15 g or less per day (166 pts), more than 15 g (41 pts)] | Carbapenem 102 (48.8), glycopeptide 66 (31.6), 3rd- or 4th-generation cephalosporin 58 (27.8), penicillins/BLI 30 (14.4), metronidazole 26 (12.4), quinolone 23 (11.0), penicillin 22 (10.5), 1st or 2nd generation cephalosporin 13 (6.2), linezolid 13 (6.2), aminoglycoside 13 (6.2), macrolide 12 (5.7), sulfonamide 11 (5.3), daptomycin 10 (4.8), colistin 7 (3.3), rifampicin 7 (3.3), tigecycline 6 (2.9), other 9 (4.3) | All-cause 15 (7.2) | 148 (81.3) [clinical per protocol (cPP) population, n = 182] | 63 (70.0) [microbiological per protocol (mPP) population, n = 90] | 70 (30.1), non-serious 36 (14.8), serious 3 (1.4); hypernatremia 31 (14.8), hypokalemia 13 (6.2), diarrhea 3 (1.4), nausea 2 (1.0), transaminases increased 1 (0.5), pyrexia 1 (0.5), allergic reaction 1 (0.5), hyponatremia 1 (0.5), hyperkalemia 1 (0.5), not specified 1 (0.5) |
| Chuang, 2022 [61] | Prospective cohort | 106 | Age 67.8 (IQR 54.6–79.9) y; males 66 (62.3); CCI 3 (IQR 2–5); comorbidities: immunosuppressive use 57 (53.8), steroid use 30 (28.3), DM 26 (24.5), CKD 25 (23.6), CHD 10 (9.43), leukemia 17 (16.0), liver cirrhosis 7 (6.6) | CLABSI 70 (66), UTI 34 (32.1), primary BSI 20 (18.9), IAI 11 (10.4) | E. faecium 106 (100) | Vancomycin-resistant E. faecium | 16 (8–22.5) | Daptomycin 106 (100) | Overall 62 (58.5), 28-day 43 (40.6) | 59 (55.7) | 80 (75.5) | Elevated CK 11 (10.4), new onset of thrombocytopenia 18 (17), hypernatremia: 15 (14.2), hypokalemia: 49 (46.2) |
| Coronado-Álvarez, 2019 [62] | Retrospective cohort | 75 | NR | BSI 75 (100), CRBSI 16 (21.3), IE 29 (38.7), PJI 3 (4) | MRSA 45 (60), MSSA 22 (29.3), E. faecium 8 (10.7) | Methicillin-resistant 45 (60) | NR | daptomycin 30, oxacillin 22, vancomycin 15, linezolid 8 | NR | 61 (81.3) | 61 (81.3) | Minor 9 (phlebitis or minor hypernatremia; severe: 1 k |
| Karnmueng, 2024 [63] | Retrospective cohort | 134 (23 received fosfomycin) | Age 63.6 (mean, SD 19.2) y, males 80 (60), ICU 52 (39); APACHE II 20.03 ± 6.5, SOFA 7.04 ± 4.6, Pitt score 2.60 ± 2.2; comorbidities: solid malignancies 47 (35), diabetes mellitus 43 (32), chronic kidney disease 23 (17); CCI 5.38 (mean, SD 3.1), APACHE II 20.72 (mean, SD 6.5), SOFA score 7.04 (mean, SD 4.6), Pitt bacteremia score 2.6 (mean, 2.2 SD), INCREMENT-CPE score 9.86 (mean, 3.8 SD) | BSI catheter-related 32 (24), BSI primary 28 (21), pneumonia 28 (21) | K. pneumoniae 115 (86), E. coli 16 (12), E. cloacae 3 (2) | CPE 60 (45) [NDM-1 + OXA-48 31 (52), OXA-48 19 (32), NDM-1 9 (15)] | NR | NR | Overall all-cause 14-day mortality 47 (35) l | NR | NR | NR |
| Author, Year | Type of Study | N | Population Characteristics Mean ± SD or Median (IQR or Range) or n (%) | Infection(s) n (%) | Pathogen(s) n (%) | Resistance n (%) | Fosfomycin IV Dosage g/d | Mortality n (%) | Clinical Cure n (%) | Microbiological Cure n (%) | Adverse Events n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rodríguez-Gómez, 2025 [64] | Retrospective cohort | 47 | Age 80 (74–86) y; males 21 (9.9); CCI 6 (IQR 4–9); comorbidities: DM 29 (61.7), CRF 20 (42.6), CHF 18 (38.3), hemodialysis 2 (4.3), solid organ transplantation 2 (4.3) | UTI 47 (100) | K. pneumoniae 47 (100) | KPC carbapenemase-producing K. pneumoniae 47 (100) | 16 (divided into 4 daily doses) | 30-day all-cause 12 (25.5) | 21-day 33 (70.2) | 14-day 22 (73.3) (with commercial microdilution; out of 30 patients with post-treatment urine culture obtained) | Hypokalemia 7 (14.9), hyponatremia 6 (12.8) |
| Falcone, 2024 [32]; Falcone, 2025 and Tiseo [31] | Prospective cohort | 15 | NR | UTI 12 (80), BSI 1 (6.7), SSTI 1 (6.7), HAP 1 (6.7) | Enterobacterales | MBL-producing 15 (100) | NR | 3 (20) | NR | NR | NR |
| Aysert-Yildiz, 2023 [47] | Retrospective cohort | 7 | NR | UTI 6 (86), SSTI 1 (14) | K. pneumoniae 7 (100) | Carbapenem resistant 7 (100) | 12–24 (divided into 2–3 daily doses; infused over at least 30–60 min) | 0 (0) | 7 (100) | NR | NR |
| Zhanel, 2023 [49] | Registry-based cohort | 8 | Ward: ICU 4 (50), non-ICU 4 (50) | cUTI 6 (75), VABP 1 (13), BSI/sepsis 1 (13); 1 patient with cUTI had concomitant BSI/sepsis | E. coli 2 (25), Klebsiella spp. 2 (25), P. aeruginosa 2 (25), Citrobacter spp. 1 (12.5), Enterobacter spp. 1 (12.5) | NR | 4–24 (divided into 2–3 daily doses; all were administered over a period of 15 min–1 h) a | 1 (12.5) | 5 (62.5); improvement 1, unknown 1) | 5 (62.5); (persistence 2, unknown 1) | 1 (12.5) elevated liver enzymes |
| Kanchanasurakit, 2020 [65] | Prospective pilot | 8 | Age 66.92 ± 7.26 y; males 3 (37.5); comorbidities: type 2 DM 5 (62.5), HTN 4 (50), AF 2 (25), BPH 1 (12.5), COPD 1 (12.5), Ischemic stroke 1 (12.5), Schizophrenia 1 (12.5), stage 3a CKD 1 (12.5), stage 3b CKD 1 (12.5) | UTI with sepsis/septic shock 8 (100) | K. pneumoniae 6 (75), E. coli 2 (25) | Carbapenem resistant 8 (100) | 6–16 (divided into 3–4 daily doses; loading dose 2–4 g; 4–6 h infusion) b | 0 (0) | NR | 7 (87.5) | Hypokalemia 3 (37.5), hypernatremia (12.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falagas, M.E.; Kontogiannis, D.S.; Romanos, L.T.; Ragias, D.; Agoranou, M.E.; Kakoullis, S.A. Intravenous Fosfomycin for Gram-Negative and Gram-Positive Bacterial Infections: A Systematic Review of the Clinical Evidence. Antibiotics 2025, 14, 1193. https://doi.org/10.3390/antibiotics14121193
Falagas ME, Kontogiannis DS, Romanos LT, Ragias D, Agoranou ME, Kakoullis SA. Intravenous Fosfomycin for Gram-Negative and Gram-Positive Bacterial Infections: A Systematic Review of the Clinical Evidence. Antibiotics. 2025; 14(12):1193. https://doi.org/10.3390/antibiotics14121193
Chicago/Turabian StyleFalagas, Matthew E., Dimitrios S. Kontogiannis, Laura T. Romanos, Dimitrios Ragias, Maria Eleni Agoranou, and Stylianos A. Kakoullis. 2025. "Intravenous Fosfomycin for Gram-Negative and Gram-Positive Bacterial Infections: A Systematic Review of the Clinical Evidence" Antibiotics 14, no. 12: 1193. https://doi.org/10.3390/antibiotics14121193
APA StyleFalagas, M. E., Kontogiannis, D. S., Romanos, L. T., Ragias, D., Agoranou, M. E., & Kakoullis, S. A. (2025). Intravenous Fosfomycin for Gram-Negative and Gram-Positive Bacterial Infections: A Systematic Review of the Clinical Evidence. Antibiotics, 14(12), 1193. https://doi.org/10.3390/antibiotics14121193




