Current Advances in Developing New Antimicrobial Agents Against Non-Tuberculous Mycobacterium
Abstract
1. Introduction
1.1. Types of NTM
1.1.1. Mycobacterium avium Complex (MAC)
1.1.2. Mycobacterium abscessus
1.1.3. Mycobacterium kansasii
1.1.4. Mycobacterium smegmatis
1.2. Challenges in Developing New Antibacterials for NTM
2. Epidemiology and Prevalence of NTM
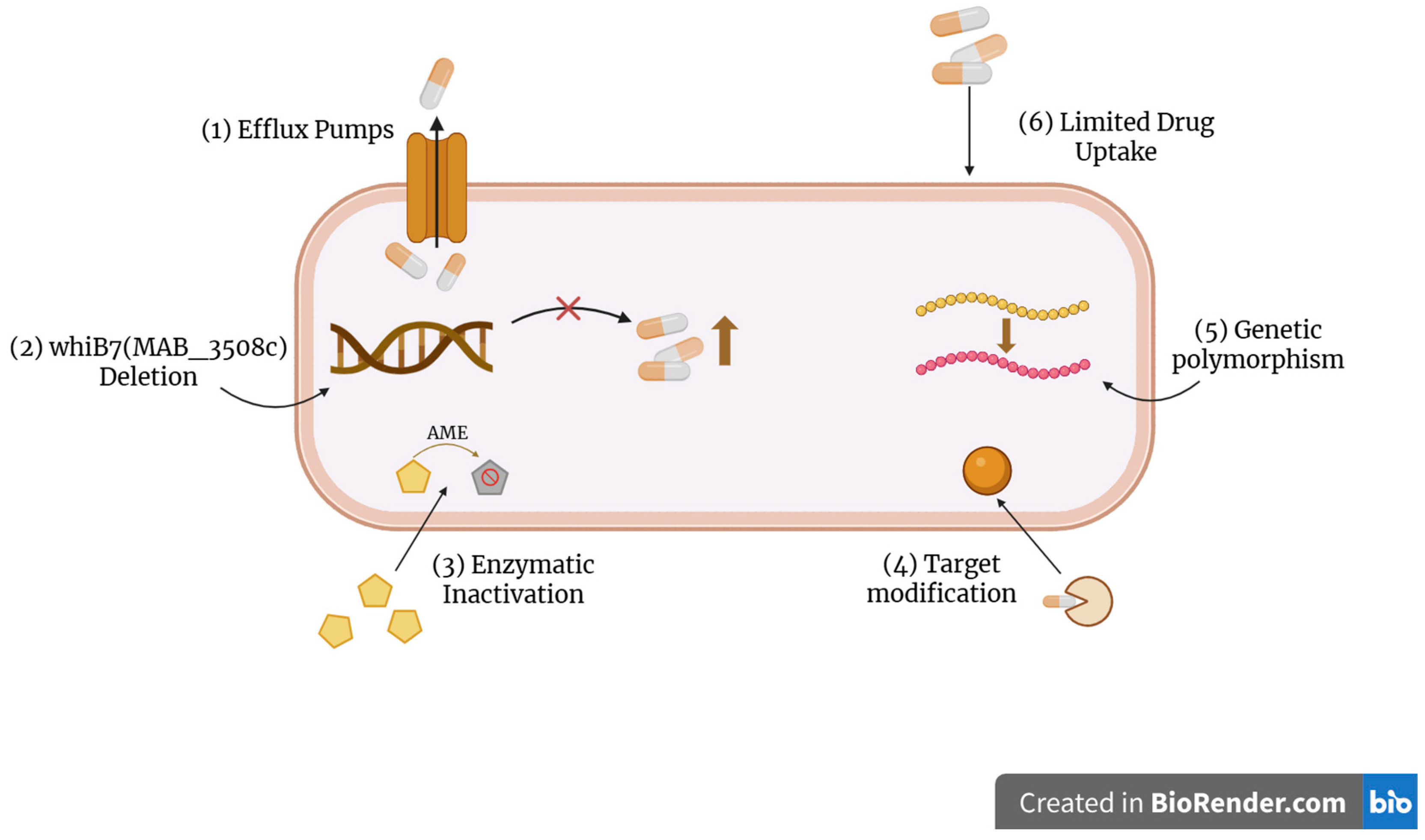
3. Resistance Mechanisms in NTM and Current Treatment Options
4. Advances in Drug Discovery and Development for NTM
4.1. New Small-Molecule Compounds
4.2. Scaffolds of Existing Drugs
4.3. Other Antibiotics
5. Other Investigational Approaches
5.1. Peptide-Based Therapies
5.2. Adjunctive Therapies
5.3. Alternative and Supportive Therapies
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| AMOX | Amoxicillin |
| AMP(s) | Antimicrobial peptide(s) |
| ATCC | American Type Culture Collection |
| BCS | Biopharmaceutics classification system |
| CF | Cystic fibrosis |
| CFU | Colony-forming units |
| CHDP | Cationic host defence peptide |
| COPD | Chronic obstructive pulmonary disease |
| CXM | Cefuroxime |
| DBO(s) | Diazabicyclooctane(s) |
| DNA | Deoxyribonucleic acid |
| DUR | Durlobactam |
| FDA | U.S. Food and Drug Administration |
| FICI | Fractional inhibitory concentration index |
| GSK | GlaxoSmithKline |
| HIV | Human immunodeficiency virus |
| IMI | Imipenem |
| MAC | Mycobacterium avium complex |
| Mab | Mycobacterium abscessus |
| MIC | Minimum inhibitory concentration |
| MIC50 | MIC required to inhibit 50% of organisms |
| MIC90 | MIC required to inhibit 90% of organisms |
| Mmpl3 | Mycobacterial membrane protein large 3 |
| NCE | New chemical entity |
| NLRP3 | NLR family pyrin domain containing 3 |
| NTM | Non-tuberculous mycobacteria |
| NTM-PD | Non-tuberculous mycobacterial pulmonary disease |
| P4C | Piperidine-4-carboxamide |
| QIDPD | Qualified Infectious Diseases Product Designation |
| WGS | Whole-genome sequencing |
References
- Thornton, C.S.; Mellett, M.; Jarand, J.; Barss, L.; Field, S.K.; Fisher, D.A. The respiratory microbiome and nontuberculous mycobacteria: An emerging concern in human health. Eur. Respir. Rev. 2021, 30, 200299. [Google Scholar] [CrossRef]
- Non-Tuberculous Mycobacterial Infection (NTM). Available online: https://www.asthmaandlung.org.uk/conditions/non-tuberculous-mycobacterial-ntm-infections (accessed on 20 December 2024).
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef]
- Richter, A.; Shapira, T.; Av-Gay, Y. THP-1 and Dictyostelium Infection Models for Screening and Characterization of Anti-Mycobacterium abscessus Hit Compounds. Antimicrob. Agents Chemother. 2019, 64, e01601-19. [Google Scholar] [CrossRef]
- Johansen, M.D.; Herrmann, J.-L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef]
- Johnston, J.C.; Chiang, L.; Elwood, K. Mycobacterium kansasii. Microbiol. Spectr. 2017, 5, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Kim, K.M.; Chin, B.S.; Choi, S.H.; Lee, H.S.; Kim, M.S.; Jeong, S.J.; Choi, H.K.; Kim, C.O.; Choi, J.Y.; et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: A case report and comprehensive review of the literature. J. Korean Med. Sci. 2010, 25, 304–308. [Google Scholar] [CrossRef]
- Sparks, I.L.; Derbyshire, K.M.; Jacobs, W.R., Jr.; Morita, Y.S. Mycobacterium smegmatis: The Vanguard of Mycobacterial Research. J. Bacteriol. 2023, 205, e0033722. [Google Scholar] [CrossRef] [PubMed]
- Obregón-Henao, A.; Arnett Kimberly, A.; Henao-Tamayo, M.; Massoudi, L.; Creissen, E.; Andries, K.; Lenaerts Anne, J.; Ordway Diane, J. Susceptibility of Mycobacterium abscessus to Antimycobacterial Drugs in Preclinical Models. Antimicrob. Agents Chemother. 2015, 59, 6904–6912. [Google Scholar] [CrossRef]
- Johansen, M.D.; Spaink, H.P.; Oehlers, S.H.; Kremer, L. Modeling nontuberculous mycobacterial infections in zebrafish. Trends Microbiol. 2024, 32, 663–677. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Winthrop, K.L. You Gotta Make Me See, What Does It Mean to Have an MIC? Chest 2021, 159, 462–464. [Google Scholar] [CrossRef]
- Trust, W. The Growing Crisis for Antibiotic R&D. Available online: https://wellcome.org/sites/default/files/the-growing-crisis-for-antibiotic-r-and-d.pdf (accessed on 26 August 2025).
- Hay, M.; Thomas, D.W.; Craighead, J.L.; Economides, C.; Rosenthal, J. Clinical development success rates for investigational drugs. Nat. Biotechnol. 2014, 32, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Brode, S.K.; Daley, C.L.; Marras, T.K. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: A systematic review. Int. J. Tuberc. Lung Dis. 2014, 18, 1370–1377. [Google Scholar] [CrossRef]
- Dahl, V.N.; Mølhave, M.; Fløe, A.; van Ingen, J.; Schön, T.; Lillebaek, T.; Andersen, A.B.; Wejse, C. Global trends of pulmonary infections with nontuberculous mycobacteria: A systematic review. Int. J. Infect. Dis. 2022, 125, 120–131. [Google Scholar] [CrossRef]
- Tursun, E.G.; Bozok, T.; Aslan, G. Antimicrobial resistance mechanisms in non-tuberculous mycobacteria. Folia Microbiol. 2025, 70, 729–738. [Google Scholar] [CrossRef]
- Nasiri, M.J.; Haeili, M.; Ghazi, M.; Goudarzi, H.; Pormohammad, A.; Imani Fooladi, A.A.; Feizabadi, M.M. New Insights in to the Intrinsic and Acquired Drug Resistance Mechanisms in Mycobacteria. Front. Microbiol. 2017, 8, 681. [Google Scholar] [CrossRef]
- Saxena, S.; Spaink, H.P.; Forn-Cuní, G. Drug Resistance in Nontuberculous Mycobacteria: Mechanisms and Models. Biology 2021, 10, 96. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Heo, B.E.; Jeon, S.; Ash, A.; Lee, H.; Moon, C.; Jang, J. Exploring antibiotic resistance mechanisms in Mycobacterium abscessus for enhanced therapeutic approaches. Front. Microbiol. 2024, 15, 1331508. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.M.; Gomes, M.S.; Silva, T. Looking beyond Typical Treatments for Atypical Mycobacteria. Antibiotics 2020, 9, 18. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium avium and Mycobacterium abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef]
- Lee, D.G.; Kim, H.J.; Lee, Y.; Kim, J.H.; Hwang, Y.; Ha, J.; Ryoo, S. 10-DEBC Hydrochloride as a Promising New Agent against Infection of Mycobacterium abscessus. Int. J. Mol. Sci. 2022, 23, 591. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, B.; Akhter, G.; Hamid, H.; Kesharwani, P.; Alam, M.S. Benzoxaboroles: New emerging and versatile scaffold with a plethora of pharmacological activities. J. Mol. Struct. 2022, 1252, 132057. [Google Scholar] [CrossRef]
- Ganapathy, U.S.; Del Rio, R.G.; Cacho-Izquierdo, M.; Ortega, F.; Lelièvre, J.; Barros-Aguirre, D.; Lindman, M.; Dartois, V.; Gengenbacher, M.; Dick, T. A Leucyl-tRNA Synthetase Inhibitor with Broad-Spectrum Anti-Mycobacterial Activity. Antimicrob. Agents Chemother. 2023, 95, e02420-20. [Google Scholar] [CrossRef]
- Dong, W.; Li, S.; Wen, S.; Jing, W.; Shi, J.; Ma, Y.; Huo, F.; Gao, F.; Pang, Y.; Lu, J. In Vitro Susceptibility Testing of GSK656 against Mycobacterium Species. Antimicrob. Agents Chemother. 2020, 64, e01577-19. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study to Evaluate GSK656 in Tuberculosis. Available online: https://clinicaltrials.gov/study/NCT03557281?intr=GSK656&rank=1 (accessed on 30 April 2024).
- Madani, A.; Negatu, D.A.; El Marrouni, A.; Miller, R.R.; Boyce, C.W.; Murgolo, N.; Bungard, C.J.; Zimmerman, M.D.; Dartois, V.; Gengenbacher, M.; et al. Activity of Tricyclic Pyrrolopyrimidine Gyrase B Inhibitor against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2022, 66, e0066922. [Google Scholar] [CrossRef]
- Degiacomi, G.; Chiarelli, L.R.; Riabova, O.; Loré, N.I.; Muñoz-Muñoz, L.; Recchia, D.; Stelitano, G.; Postiglione, U.; Saliu, F.; Griego, A.; et al. The novel drug candidate VOMG kills Mycobacterium abscessus and other pathogens by inhibiting cell division. Int. J. Antimicrob. Agents 2024, 64, 107278. [Google Scholar] [CrossRef] [PubMed]
- GSK. Gepotidacin Approved by US FDA for Treatment of Uncomplicated Urinary Tract Infections. Available online: https://www.gsk.com/en-gb/media/press-releases/blujepa-gepotidacin-approved-by-us-fda-for-treatment-of-uncomplicated-urinary-tract-infections/ (accessed on 28 August 2025).
- Ahmad, M.N.; Garg, T.; Singh, S.; Shukla, R.; Malik, P.; Krishnamurthy, R.V.; Kaur, P.; Chopra, S.; Dasgupta, A. In Vitro and In Vivo Activity of Gepotidacin against Drug-Resistant Mycobacterial Infections. Antimicrob. Agents Chemother. 2022, 66, e0056422. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.C.; Madani, A.; Santucci, P.; Martin, B.P.; Paudel, R.R.; Delattre, S.; Herrmann, J.-L.; Spilling, C.D.; Kremer, L.; Canaan, S.; et al. Cyclophostin and Cyclipostins analogues, new promising molecules to treat mycobacterial-related diseases. Int. J. Antimicrob. Agents 2018, 51, 651–654. [Google Scholar] [CrossRef]
- Sarrazin, M.; Poncin, I.; Fourquet, P.; Audebert, S.; Camoin, L.; Denis, Y.; Santucci, P.; Spilling, C.D.; Kremer, L.; Le Moigne, V.; et al. Cyclophostin and Cyclipostins analogues counteract macrolide-induced resistance mediated by erm(41) in Mycobacterium abscessus. J. Biomed. Sci. 2024, 31, 103. [Google Scholar] [CrossRef]
- Raynaud, C.; Daher, W.; Johansen, M.D.; Roquet-Banères, F.; Blaise, M.; Onajole, O.K.; Kozikowski, A.P.; Herrmann, J.L.; Dziadek, J.; Gobis, K.; et al. Active Benzimidazole Derivatives Targeting the MmpL3 Transporter in Mycobacterium abscessus. ACS Infect. Dis. 2020, 6, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; Flume, P.; Hamed, K.A. Nontuberculous mycobacterial pulmonary disease and the potential role of SPR720. Expert. Rev. Anti Infect. Ther. 2023, 21, 1177–1187. [Google Scholar] [CrossRef]
- Locher, C.P.; Jones, S.M.; Hanzelka, B.L.; Perola, E.; Shoen, C.M.; Cynamon, M.H.; Ngwane, A.H.; Wiid, I.J.; van Helden, P.D.; Betoudji, F.; et al. A novel inhibitor of gyrase B is a potent drug candidate for treatment of tuberculosis and nontuberculosis mycobacterial infections. Antimicrob. Agents Chemother. 2015, 59, 1455–1465. [Google Scholar] [CrossRef]
- Pidot, S.J.; Porter, J.L.; Lister, T.; Stinear, T.P. In vitro activity of SPR719 against Mycobacterium ulcerans, Mycobacterium marinum and Mycobacterium chimaera. PLoS Negl. Trop. Dis. 2021, 15, e0009636. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study Details | NCT03796910 | A Study of the Safety, Tolerability, and Pharmacokinetics of SPR720 in Healthy Volunteers | ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03796910?term=spr720&viewType=Table&checkSpell=&rank=4 (accessed on 16 November 2025).
- Therapeutics, S. SPR720—Spero Therapeutics. Available online: https://s3.amazonaws.com/b2icontent.irpass.cc/2748/rl139704.pdf (accessed on 16 November 2025).
- Cotroneo, N.; Stokes, S.S.; Pucci, M.J.; Rubio, A.; Hamed, K.A.; Critchley, I.A. Efficacy of SPR720 in murine models of non-tuberculous mycobacterial pulmonary infection. J. Antimicrob. Chemother. 2024, 79, 875–882. [Google Scholar] [CrossRef]
- Negatu, D.A.; Beuchel, A.; Madani, A.; Alvarez, N.; Chen, C.; Aragaw, W.W.; Zimmerman, M.D.; Laleu, B.; Gengenbacher, M.; Dartois, V.; et al. Piperidine-4-Carboxamides Target DNA Gyrase in Mycobacterium abscessus. Antimicrob. Agents Chemother. 2021, 65, e0067621. [Google Scholar] [CrossRef]
- Pennings, L.J.; Ruth, M.M.; Wertheim, H.F.L.; van Ingen, J. The Benzimidazole SPR719 Shows Promising Concentration-Dependent Activity and Synergy against Nontuberculous Mycobacteria. Antimicrob. Agents Chemother. 2021, 65, e02469-20. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Viljoen, A.; Dubar, F.; Blaise, M.; Bernut, A.; Pawlik, A.; Bouchier, C.; Brosch, R.; Guérardel, Y.; Lelièvre, J.; et al. A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol. Microbiol. 2016, 101, 515–529. [Google Scholar] [CrossRef] [PubMed]
- de Ruyck, J.; Dupont, C.; Lamy, E.; Le Moigne, V.; Biot, C.; Guérardel, Y.; Herrmann, J.L.; Blaise, M.; Grassin-Delyle, S.; Kremer, L.; et al. Structure-Based Design and Synthesis of Piperidinol-Containing Molecules as New Mycobacterium abscessus Inhibitors. ChemistryOpen 2020, 9, 351–365. [Google Scholar] [CrossRef]
- Abdalla, M.Y.; Switzer, B.L.; Goss, C.H.; Aitken, M.L.; Singh, P.K.; Britigan, B.E. Gallium Compounds Exhibit Potential as New Therapeutic Agents against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2015, 59, 4826–4834. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Study of Gallium Nitrate in CF Patients with NTM. Available online: https://clinicaltrials.gov/study/NCT04294043 (accessed on 8 May 2024).
- Bermudez, L.E.; Inderlied, C.B.; Kolonoski, P.; Chee, C.B.; Aralar, P.; Petrofsky, M.; Parman, T.; Green, C.E.; Lewin, A.H.; Ellis, W.Y.; et al. Identification of (+)-erythro-mefloquine as an active enantiomer with greater efficacy than mefloquine against Mycobacterium avium infection in mice. Antimicrob. Agents Chemother. 2012, 56, 4202–4206. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, L.E.; Kolonoski, P.; Seitz, L.E.; Petrofsky, M.; Reynolds, R.; Wu, M.; Young, L.S. SRI-286, a thiosemicarbazole, in combination with mefloquine and moxifloxacin for treatment of murine Mycobacterium avium complex disease. Antimicrob. Agents Chemother. 2004, 48, 3556–3558. [Google Scholar] [CrossRef]
- Mann, L.; Ganapathy, U.S.; Abdelaziz, R.; Lang, M.; Zimmerman, M.D.; Dartois, V.; Dick, T.; Richter, A. In Vitro Profiling of the Synthetic RNA Polymerase Inhibitor MMV688845 against Mycobacterium abscessus. Microbiol. Spectr. 2022, 10, e0276022. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Ganapathy, U.S.; Mann, L.; Abdelaziz, R.; Seidel, R.W.; Goddard, R.; Sequenzia, I.; Hoenke, S.; Schulze, P.; Aragaw, W.W.; et al. Synthesis and Characterization of Phenylalanine Amides Active against Mycobacterium abscessus and Other Mycobacteria. J. Med. Chem. 2023, 66, 5079–5098. [Google Scholar] [CrossRef] [PubMed]
- Holt, E. Phase 2 trial of a novel tuberculosis drug launched. The Lancet Microbe. 2024, 5, e316. [Google Scholar] [CrossRef]
- Sarathy, J.P.; Ganapathy, U.S.; Zimmerman, M.D.; Dartois, V.; Gengenbacher, M.; Dick, T. TBAJ-876, a 3,5-Dialkoxypyridine Analogue of Bedaquiline, Is Active against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2020, 64, e02404–e02419. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Evaluation of the Safety, Tolerability, PK of TBAJ-587 in Healthy Adults. Available online: https://clinicaltrials.gov/study/NCT04890535 (accessed on 16 November 2025).
- Fan, J.; Tan, Z.; He, S.; Li, A.; Jia, Y.; Li, J.; Zhang, Z.; Li, B.; Chu, H. TBAJ-587, a novel diarylquinoline, is active against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2024, 68, e0094524. [Google Scholar] [CrossRef]
- Xu, J.; Converse, P.J.; Upton, A.M.; Mdluli, K.; Fotouhi, N.; Nuermberger, E.L. Comparative Efficacy of the Novel Diarylquinoline TBAJ-587 and Bedaquiline against a Resistant Rv0678 Mutant in a Mouse Model of Tuberculosis. Antimicrob. Agents Chemother. 2021, 65, e02418-20. [Google Scholar] [CrossRef]
- Zhu, R.; Shang, Y.; Chen, S.; Xiao, H.; Ren, R.; Wang, F.; Xue, Y.; Li, L.; Li, Y.; Chu, N.; et al. In Vitro Activity of the Sudapyridine (WX-081) against Non-Tuberculous Mycobacteria Isolated in Beijing, China. Microbiol. Spectr. 2022, 10, e0137222. [Google Scholar] [CrossRef]
- Nie, W.; Gao, S.; Su, L.; Liu, L.; Geng, R.; You, Y.; Chu, N. Antibacterial activity of the novel compound Sudapyridine (WX-081) against Mycobacterium abscessus. Front. Cell Infect. Microbiol. 2023, 13, 1217975. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, L.; Fu, L.; Zhang, W.; Dong, S.; Chen, X.; Lu, Y. In vitro and in vivo antibacterial activity of sudapyridine (WX-081) combined with other drugs against Mycobacterium abscessus. Tuberculosis 2025, 154, 102655. [Google Scholar] [CrossRef]
- Krátký, M.; Bősze, S.; Baranyai, Z.; Szabó, I.; Stolaříková, J.; Paraskevopoulos, G.; Vinšová, J. Synthesis and in vitro biological evaluation of 2-(phenylcarbamoyl)phenyl 4-substituted benzoates. Bioorganic Med. Chem. 2015, 23, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xu, L.; Tan, F.; Zhang, Y.; Fan, J.; Wang, X.; Zhang, Z.; Li, B.; Chu, H. A Novel Oxazolidinone, Contezolid (MRX-I), Expresses Anti-Mycobacterium abscessus Activity In Vitro. Antimicrob. Agents Chemother. 2021, 65, e0088921. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Innovating Shorter, All- Oral, Precised, Individualized Treatment Regimen for Rifampicin Resistant Tuberculosis: Contezolid, Delamanid and Bedaquiline Cohort (INSPIRE-CODA). Available online: https://www.clinicaltrials.gov/study/NCT06081361 (accessed on 10 September 2025).
- Trials, E.C. Study on the Safety and Effectiveness of Contezolid Acefosamil, Contezolid, and Linezolid for Adults with Moderate or Severe Diabetic Foot Infections. Available online: https://clinicaltrials.eu/trial/study-on-the-safety-and-effectiveness-of-contezolid-acefosamil-contezolid-and-linezolid-for-adults-with-moderate-or-severe-diabetic-foot-infections/ (accessed on 10 September 2025).
- ClinicalTrials.gov. Phase II, Multicenter, Randomized, Double-Blind Study Comparing Contezolid Acefosamil with Linezolid in Adults with Acute Bacterial Skin and Skin Structure Infections (ABSSSI). Available online: https://clinicaltrials.gov/study/NCT03747497 (accessed on 30 August 2025).
- Kim, T.S.; Choe, J.H.; Kim, Y.J.; Yang, C.S.; Kwon, H.J.; Jeong, J.; Kim, G.; Park, D.E.; Jo, E.K.; Cho, Y.L.; et al. Activity of LCB01-0371, a Novel Oxazolidinone, against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2017, 61, e02752-16. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lim, H.S.; Cho, Y.L.; Nam, H.S.; Bae, K.S. Multiple-dose Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Oral LCB01-0371 in Healthy Male Volunteers. Clin. Ther. 2018, 40, 2050–2064. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Phase II Clinical Study of LCB01-0371 to Evaluate the EBA, Safety and PK. Available online: https://clinicaltrials.gov/study/NCT02836483 (accessed on 30 August 2025).
- Madani, A.; Mallick, I.; Guy, A.; Crauste, C.; Durand, T.; Fourquet, P.; Audebert, S.; Camoin, L.; Canaan, S.; Cavalier, J.F. Dissecting the antibacterial activity of oxadiazolone-core derivatives against Mycobacterium abscessus. PLoS ONE 2020, 15, e0238178. [Google Scholar] [CrossRef]
- Batchelder, H.R.; Story-Roller, E.; Lloyd, E.P.; Kaushik, A.; Bigelow, K.M.; Maggioncalda, E.C.; Nuermberger, E.L.; Lamichhane, G.; Townsend, C.A. Development of a penem antibiotic against Mycobacteroides abscessus. Commun. Biol. 2020, 3, 741. [Google Scholar] [CrossRef] [PubMed]
- Dousa, K.M.; Nguyen, D.C.; Kurz, S.G.; Taracila, M.A.; Bethel, C.R.; Schinabeck, W.; Kreiswirth, B.N.; Brown, S.T.; Boom, W.H.; Hotchkiss, R.S.; et al. Inhibiting Mycobacterium abscessus Cell Wall Synthesis: Using a Novel Diazabicyclooctane β-Lactamase Inhibitor To Augment β-Lactam Action. mbio 2022, 13, e0352921. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M. Sulbactam-Durlobactam: The “Double Beta-Lactamase Inhibitor” Drug. Available online: https://clsi.org/about/news/ast-news-update-january-2024-sulbactam-durlobactam-the-double-beta-lactamase-inhibitor-drug/ (accessed on 10 September 2025).
- ClinicalTrials.gov. Study to Evaluate the Efficacy and Safety of Intravenous Sulbactam-ETX2514 in the Treatment of Patients With Infections Caused by Acinetobacter Baumannii-calcoaceticus Complex (ATTACK). Available online: https://clinicaltrials.gov/study/NCT03894046 (accessed on 30 August 2025).
- Disratthakit, A.; Doi, N. In vitro activities of DC-159a, a novel fluoroquinolone, against Mycobacterium species. Antimicrob. Agents Chemother. 2010, 54, 2684–2686. [Google Scholar] [CrossRef]
- Garcia, P.K.; Annamalai, T.; Wang, W.; Bell, R.S.; Le, D.; Martin Pancorbo, P.; Sikandar, S.; Seddek, A.; Yu, X.; Sun, D.; et al. Mechanism and resistance for antimycobacterial activity of a fluoroquinophenoxazine compound. PLoS ONE 2019, 14, e0207733. [Google Scholar] [CrossRef]
- Pflégr, V.; Horváth, L.; Stolaříková, J.; Pál, A.; Korduláková, J.; Bősze, S.; Vinšová, J.; Krátký, M. Design and synthesis of 2-(2-isonicotinoylhydrazineylidene)propanamides as InhA inhibitors with high antitubercular activity. Eur. J. Med. Chem. 2021, 223, 113668. [Google Scholar] [CrossRef]
- Fahel, J.S.; Vieira, R.P.; Marinho, F.V.; Santos, V.C.; de Assis, J.V.; Corsetti, P.P.; Ferreira, R.S.; de Almeida, M.V.; Oliveira, S.C. JVA, an isoniazid analogue, is a bioactive compound against a clinical isolate of the Mycobacterium avium complex. Tuberculosis 2019, 115, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wan, M.; Zhang, L.; Dai, Y.; Hai, Y.; Yue, C.; Xu, J.; Ding, Y.; Wang, M.; Xie, J.; et al. ALA_PDT Promotes Ferroptosis-Like Death of Mycobacterium abscessus and Antibiotic Sterilization via Oxidative Stress. Antioxidants 2022, 11, 546. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef]
- da Silva, J.L.; Gupta, S.; Olivier, K.N.; Zelazny, A.M. Antimicrobial peptides against drug resistant Mycobacterium abscessus. Res. Microbiol. 2020, 171, 211–214. [Google Scholar] [CrossRef]
- Sudadech, P.; Roytrakul, S.; Kaewprasert, O.; Sirichoat, A.; Chetchotisakd, P.; Kanthawong, S.; Faksri, K. Assessment of in vitro activities of novel modified antimicrobial peptides against clarithromycin resistant Mycobacterium abscessus. PLoS ONE 2021, 16, e0260003. [Google Scholar] [CrossRef]
- Rao, K.U.; Henderson, D.I.; Krishnan, N.; Puthia, M.; Glegola-Madejska, I.; Brive, L.; Bjarnemark, F.; Millqvist Fureby, A.; Hjort, K.; Andersson, D.I.; et al. A broad spectrum anti-bacterial peptide with an adjunct potential for tuberculosis chemotherapy. Sci. Rep. 2021, 11, 4201. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.; Maresca, M.; Poncin, I.; Point, V.; Olleik, H.; Boidin-Wichlacz, C.; Tasiemski, A.; Mabrouk, K.; Cavalier, J.F.; Canaan, S. Promising antibacterial efficacy of arenicin peptides against the emerging opportunistic pathogen Mycobacterium abscessus. J. Biomed. Sci. 2024, 31, 18. [Google Scholar] [CrossRef] [PubMed]
- Roh, T.; Seo, W.; Won, M.; Yang, W.S.; Sapkota, A.; Park, E.-J.; Yun, S.-H.; Jeon, S.M.; Kim, K.T.; Lee, B.; et al. The inflammasome-activating poxvirus peptide IAMP29 promotes antimicrobial and anticancer responses. Exp. Mol. Med. 2024, 56, 2475–2490. [Google Scholar] [CrossRef]
- Iannuzo, N.; Haller, Y.A.; McBride, M.; Mehari, S.; Lainson, J.C.; Diehnelt, C.W.; Haydel, S.E. High-Throughput Screening Identifies Synthetic Peptides with Antibacterial Activity against Mycobacterium abscessus and Serum Stability. ACS Omega 2022, 7, 23967–23977. [Google Scholar] [CrossRef]
- Felicetti, T.; Machado, D.; Cannalire, R.; Astolfi, A.; Massari, S.; Tabarrini, O.; Manfroni, G.; Barreca, M.L.; Cecchetti, V.; Viveiros, M.; et al. Modifications on C6 and C7 Positions of 3-Phenylquinolone Efflux Pump Inhibitors Led to Potent and Safe Antimycobacterial Treatment Adjuvants. ACS Infect. Dis. 2019, 5, 982–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.; Guo, Q.; He, S.; Fan, J.; Xu, L.; Zhang, Z.; Wu, W.; Chu, H. Antibacterial peptide RP557 increases the antibiotic sensitivity of Mycobacterium abscessus by inhibiting biofilm formation. Sci. Total Environ. 2022, 807, 151855. [Google Scholar] [CrossRef]
- Vianna, J.S.; Ramis, I.B.; Bierhals, D.; von Groll, A.; Ramos, D.F.; Zanatta, N.; Lourenço, M.C.; Viveiros, M.; Almeida da Silva, P.E. Tetrahydropyridine derivative as efflux inhibitor in Mycobacterium abscessus. J. Glob. Antimicrob. Resist. 2019, 17, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Millar, B.C.; Nelson, D.; Moore, R.E.; Rao, J.R.; Moore, J.E. Antimicrobial properties of basidiomycota macrofungi to Mycobacterium abscessus isolated from patients with cystic fibrosis. Int. J. Mycobacteriology 2019, 8, 93–97. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, J.W.; Yu, A.R.; Yoon, H.S.; Kang, M.; Lee, B.S.; Park, H.W.; Lee, S.K.; Whang, J.; Kim, J.S. Isoegomaketone exhibits potential as a new Mycobacterium abscessus inhibitor. Front. Microbiol. 2024, 15, 1344914. [Google Scholar] [CrossRef]
- Watanabe, M.; Hasegawa, N.; Ishizaka, A.; Asakura, K.; Izumi, Y.; Eguchi, K.; Kawamura, M.; Horinouchi, H.; Kobayashi, K. Early pulmonary resection for Mycobacterium avium complex lung disease treated with macrolides and quinolones. Ann. Thorac. Surg. 2006, 81, 2026–2030. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Smith, B.E.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Whitney Brown, A.; Cohen, K.A.; Davidson, R.M.; van Duin, D.; et al. Phage Therapy of Mycobacterium Infections: Compassionate Use of Phages in 20 Patients With Drug-Resistant Mycobacterial Disease. Clin. Infect. Dis. 2023, 76, 103–112. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.Y.; Lee, J.J.; Jeon, S.M.; Silwal, P.; Kim, I.S.; Kim, H.J.; Park, C.R.; Chung, C.; Han, J.E.; et al. Arginine-mediated gut microbiome remodeling promotes host pulmonary immune defense against nontuberculous mycobacterial infection. Gut Microbes 2022, 14, 2073132. [Google Scholar] [CrossRef]
- Murthy, M.K.; Gupta, V.K.; Maurya, A.P. Diagnosis of nontuberculous mycobacterial infections using genomics and artificial intelligence-machine learning approaches: Scope, progress and challenges. Front. Microbiol. 2025, 16, 1665685. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Xu, W.; Ren, S.; Xu, Q.; Zhang, S.; Zhang, R.; Jiang, M.; Zhang, Y.; Xu, D.; Li, R. Machine Learning Accelerates De Novo Design of Antimicrobial Peptides. Interdiscip. Sci. Comput. Life Sci. 2024, 16, 392–403. [Google Scholar] [CrossRef]
- Schmalstig, A.A.; Zorn, K.M.; Murcia, S.; Robinson, A.; Savina, S.; Komarova, E.; Makarov, V.; Braunstein, M.; Ekins, S. Mycobacterium abscessus drug discovery using machine learning. Tuberculosis 2022, 132, 102168. [Google Scholar] [CrossRef] [PubMed]
- U.S. F.D.A. Qualified Infectious Disease Product Designation Questions and Answers. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/qualified-infectious-disease-product-designation-questions-and-answers (accessed on 31 August 2025).


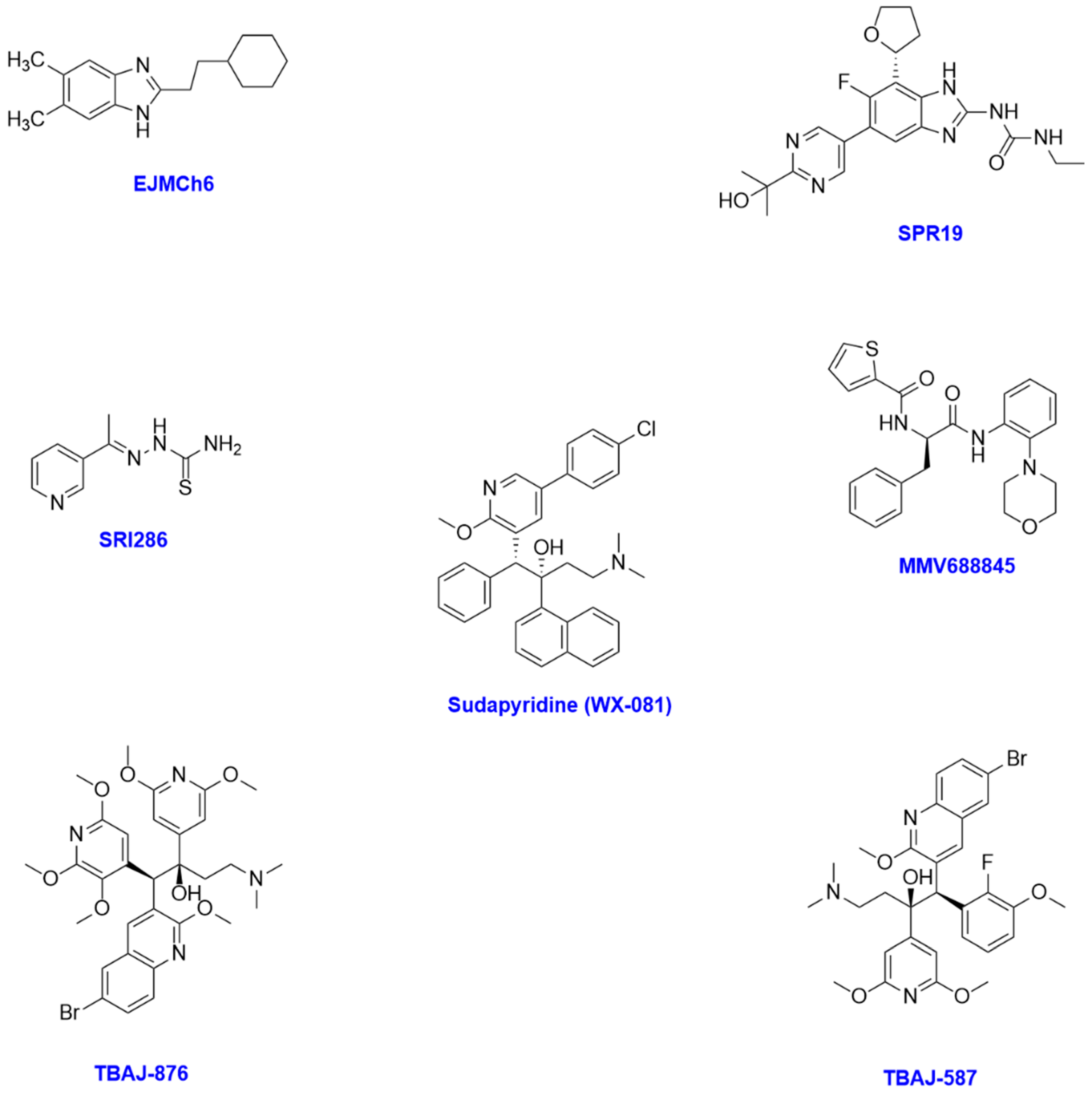
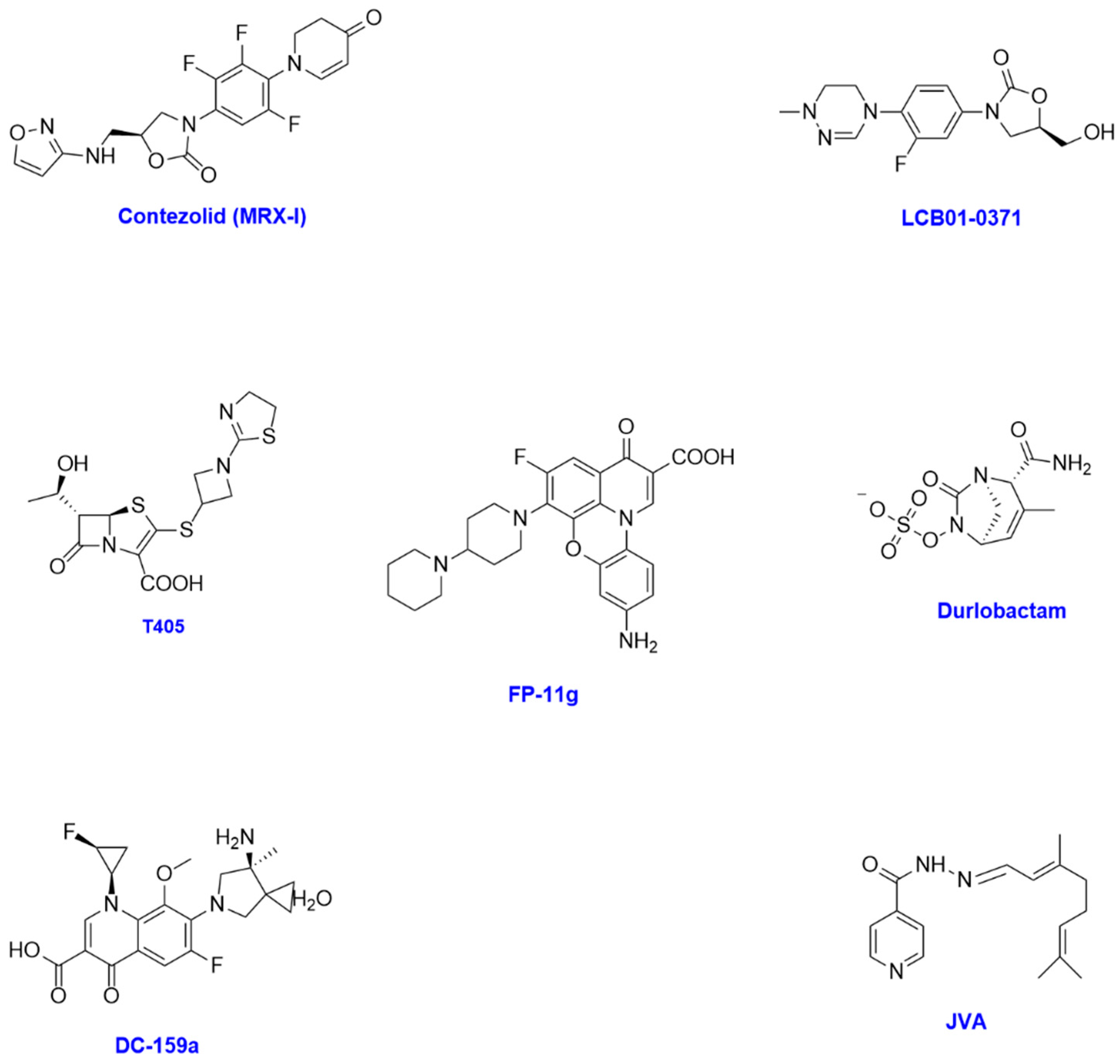
| Compound | Bacterial Strain(s) | MIC | Bacterial Target and Mechanism of Action | Development Status |
|---|---|---|---|---|
| EC/11770 | M. abscessus Bamboo (subsp. abscessus) M. abscessus subsp. Abscessus (no. of strains = 7) M. abscessus subsp. Massiliense (no. of strains = 2) M. abscessus subsp. Bolletii (no. of strains = 3) M. avium 11 (subsp. hominissuis)) M. intracellulare (no. of strains = 1) M. chimaera (no. of strains = 1) | 0.7–1.2 μM 0.33–0.93 μM 0.71–0.95 μM 0.48–1.3 μM 4.0 μM 0.37 μM 1.7 μM | Leucyl-tRNA synthetase inhibitor, interfering with bacterial protein biosynthesis | Preclinical stage |
| GSK656 | M. intercellulare (no. of strains = 30) M. avium (no. of strains = 16) M. abscessus (no. of strains = 36) | >0.8 mg/L >0.8 mg/L 0.016–0.25 mg/L | Leucyl-tRNA synthetase inhibitor, interfering with bacterial protein biosynthesis | Phase II clinical trials |
| TPP8 | M. abscessus subsp. Abscessus (no. of strains = 7) M. abscessus subsp. Bolletii (no. of strains = 3) M. abscessus subsp. Massiliense (no. of strains = 2) | 0.02–0.2 μM 0.04–0.2 μM 0.1–0.2 μM | DNA gyrase, causing bacterial DNA damage | Preclinical stage |
| VOMG | M. abscessus subsp. Abscessus (no. of strains = 8) M. abscessus subsp. Bolletii (no. of strains = 1) M. abscessus subsp. Massiliense (no. of strains = 1) M. avium (no. of strains = 5) M. bovis (no. of strains = 5) M. smegmatis (no. of strains = 5) | 0.25–0.5 µg/mL 0.5 µg/mL 0.5 µg/mL 0.5 µg/mL 0.0625 µg/mL 1 µg/mL | FtsZ enzyme, interfering with bacterial cell division | Preclinical stage |
| 10-DEBC hydrochloride | M. abscessus (no. of strains = 9) | 2.38–4.77 µg/mL | Akt inhibitor; unknown mechanism of action | Lead optimisation/preclinical stage |
| Gepotidacin | M. fortuitum ATCC 6841 M. chelonae ATCC 35752 M. abscessus ATCC 19977 M. avium ATCC 19698 M. gordonae ATCC 14470 M. nonchromogenicum ATCC 19530 M. kansasii ATCC 12478 M. intracellulare ATCC 13950 | 2 mg/L 2 mg/L 2 mg/L 16 mg/L 16 mg/L 32 mg/L 32 mg/L 16 mg/L | Triazaacenapthylene topoisomerase inhibitor, inhibiting bacterial type II topoisomerase and interfering with bacterial DNA replication | Preclinical stage |
| CyC17 | M. smegmatis mc2155 M. abscessus CIP 104536T M. marinum M. bovis BCG M. abscessus (no. of strains = 10) M. massiliense (no. of strains = 4) M. bolletii (no. of strains = 2) M. chelonae (no. of strains = 10) | 0.81 µg/mL 0.18 µg/mL 0.74 µg/mL 0.58 µg/mL 10 µg/mL 10 µg/mL <2 µg/mL 40 µg/mL | Serine/cysteine hydrolase inhibitors, impairing bacterial lipid metabolism and cell wall assembly | Hit-to-lead identification |
| Compound | Bacterial Strain(s) | MIC | Bacterial Target and Mechanism of Action | Development Status |
|---|---|---|---|---|
| EJMCh-6 | M. abscessus (no. of strains = 12) M. massiliense (no. of strains = 12) M. bolletii (no. of strains = 9) | 0.031–0.5 µg/mL 0.062–0.5 µg/mL 0.062–1 µg/mL | MmpL3 transporter, blocking cell wall mycolylation | Hit-to-lead identification/lead optimisation stage |
| SPR719 | M. avium complex M. avium M. intracellulare M. chimaera MAC-X M. abscessus M. abscessus subspecies abscessus M. abscessus subspecies massiliense M. abscessus/massiliense hybrid M. kansasii M. chelonae M. fortuitum M. immunogenum M. mucogenicum M. marinum M. simiae M. xenopi M. ulcerans | 0.002–4 µg/mL 0.23–2 µg/mL 0.12–2 µg/mL <0.03–2 µg/mL 0.12–1 µg/mL 0.03–>32 µg/mL 0.25–8 µg/mL 0.12–4 µg/mL 0.06–2 µg/mL 0.002–0.25 µg/mL 2–4 µg/mL 0.06–1 µg/mL 4–8 µg/mL 0.015–0.25 µg/mL 0.12–1 µg/mL 0.5–8 µg/mL 0.06–0.5 µg/mL 0.125–0.25 µg/mL | ATPase activity of DNA gyrase B, inhibiting bacterial DNA replication | Phase I clinical trials |
| SPR20 | N/A | N/A | ATPase activity of DNA gyrase B, inhibiting bacterial DNA replication | Phase I clinical trials |
| 844 | M. abscessus ATCC 19977 M. abscessus clinical isolates (no. of strains = 7) M. bolletii CCUG 50184T M. bolletii clinical isolates (no. of strains = 2) M. massiliense CCUG 48898T M. massiliense clinical isolates (no. of strains = 1) | 8 μM 6.3–12 μM 14 μM 6.3 μM 14 μM 12.5 μM | DNA gyrase inhibitor, causing bacterial DNA damage | Lead optimisation/preclinical stage |
| PIPD1 | M. abscessus (no. of strains = 12) M. massiliense (no. of strains = 12) M. bolletii (no. of strains = 8) | 0.125 0.125 0.125 | MmpL3 transporter, blocking cell wall mycolylation | Lead optimisation/preclinical stage |
| Ga(NO3)3 & Ga-protoporphyrin | N/A | N/A | Interference with bacterial iron metabolism, causing metabolic dysfunction | Phase I clinical trials |
| SRI286 | MAC | 2 μg/mL | Mycolic acid synthesis inhibitor, disrupting function and integrity of bacterial cell wall | Preclinical stage |
| MMV688845 | M. abscessus subsp. abscessus ATCC 19977 M. abscessus clinical isolates (no. of strains = 7) M. abscessus subsp. bolletii CCUG 50184T M. bolletii clinical isolates (no. of strains = 2) M. abscessus subsp. massiliense CCUG 48898T M. massiliense clinical isolates (no. of strains = 1) | 7.5 μM 5.4–8.4 μM 10 μM 4.5–6.9 μM 10 μM 8.4 μM | RNA polymerase inhibitor, exhibiting bactericidal activity | Lead optimisation/preclinical stage |
| TBAJ-876 | M. abscessus subsp. abscessus ATCC 19977 M. abscessus clinical isolates (no. of strains = 6) M. abscessus subsp. bolletii CCUG 50184-T M. bolletii clinical isolates (no. of strains = 2) M. abscessus subsp. massiliense CCUG 48898-T M. massiliense clinical isolates (no. of strains = 1) | 0.48 μM 0.14–0.46 μM 0.53 μM 0.30–0.45 μM 0.42 μM 0.30 μM | F-ATP synthase inhibitor, preventing ATP synthesis and thus bactericidal activity | Phase II clinical trials |
| TBAJ-587 | M. abscessus ATCC 19977 M. abscessus clinical isolates (no. of strains = 148) M. massiliense CIP 108297 M. massiliense clinical isolates (no. of strains = 46) M. smegmatis ATCC 607 M. fortuitum ATCC 35855 M. peregrinum ATCC 700686 M. avium ATCC 25291 M. intracellulare ATCC 13950 M. kansasii ATCC 12478 M. gordonae ATCC 14470 M. szulgai ATCC 35799 M. scrofulaceum ATCC 19981 | 0.031 mg/L 0.0625 mg/L 0.031 mg/L 0.0625 mg/L 0.004 mg/L 0.008 mg/L ≤0.002 mg/L ≤0.002 mg/L ≤0.002 mg/L ≤0.002 mg/L ≤0.002 mg/L ≤0.002 mg/L 0.008 mg/L | Inhibitor of F-ATP synthase c-chain, exhibiting bactericidal activity | Phase I clinical trials |
| Sudapyridine (WX-081) | Rapidly growing Mycobacterial species (no of strains = 26) Slowly growing Mycobacterial species (no of strains = 24) | 0.0078–0.5 μg/mL 0.0039–>2 μg/mL | ATP synthase inhibitor, preventing ATP production required for cellular activities | Phase III clinical trials |
| Salicylanilide | M. avium 330/8 M. kansaii 235/80 M. kansasii 6509/96 | 8 μM 1 μM 2 μM | Multiple mechanisms of action, inhibiting mycobacterial enzymes, regulatory systems, and impairing bacterial energy production | Hit-to-lead identification stage |
| Compound | Bacterial Strain(s) | MIC | Bacterial Target and Mechanism of Action | Development Status |
|---|---|---|---|---|
| Contezolid | M. abscessus subsp. abscessus (ATCC 19977) M. abscessus subsp. massiliense (CIP108297) Mycobacterium fortuitum (ATCC 6841) Mycobacterium smegmatis (ATCC 19420) Mycobacterium peregrinum (ATCC 700686) M. avium (ATCC 25291) M. intracellulare (ATCC 13950) Mycobacterium kansasii (ATCC 12478) Mycobacterium gordonae (ATCC 14470) Mycobacterium scrofulaceum (ATCC 19981) Mycobacterium marinum (ATCC 927) Mycobacterium xenopi (ATCC 19250) M. abscessus subsp. abscessus (no. of strains = 148) M. abscessus subsp. massiliense (no. of strains = 46) | 16 mg/L 16 mg/L 8 mg/L 1 mg/L 1 mg/L 32 mg/L 64 mg/L 1 mg/L 2 mg/L 1 mg/L 4 mg/L 1 mg/L 0.5–64 mg/L 0.25–64 mg/L | Bacterial protein synthesis inhibitor, interfering with bacterial growth and replication | Phase III clinical trials |
| LCB01-0371 | M. abscessus ATCC 19977 M. abscessus clinical isolates (no. of strains = 8) | 1.2 μg/mL 0.7–22.3 μg/mL | Bacterial protein synthesis inhibitor, interfering with bacterial growth and replication | Phase II clinical trials |
| Oxadiazolone derivatives | M. abscessus S-variant M. abscessus R-variant | 3.9–>200 μM 7.4–>200 μM | Inhibits multiple bacterial enzymes, interfering with lipid metabolism and cell wall biosynthesis | Hit-to-lead identification stage |
| T405 | M. abscessus ATCC 19977 M. abscessus clinical isolates (no. of strains = 20) | 2 μg/mL 1–8 μg/mL | Inhibits penicillin-binding proteins and l,d-transpeptidases, inhibiting cell wall synthesis | Lead optimisation/preclinical stage |
| Durlobactam | M. abscessus ATCC 19977 | 2–8 μg/mL | Beta-lactamase inhibitor, improves beta-lactam activity and inhibits cell wall synthesis | Phase III clinical trials |
| DC-159a | M. kansasii (no. of strains = 22) M. avium (no. of strains = 33) M. intracellulare (no. of strains = 17) M. fortuitum (no. of strains = 10) M. chelonae (no. of strains = 10) M. abscessus (no. of strains = 12) | 0.03–025 μg/mL 0.25–8 μg/mL 0.25–8 μg/mL 0.03–0.25 μg/mL 4–16 μg/mL 4–32 μg/mL | DNA gyrase, causing bacterial DNA damage and resulting in bactericidal effect | Lead optimisation/preclinical stage |
| FP-11g | M. smegmatis mc2155 M. abscessus | 0.31 μM 50 μM | Bacterial topoisomerase and DNA gyrase, exhibiting bactericidal effect | Lead optimisation stage |
| Isoniazid derivatives | M. avium 330/88 M. kansasii 6509/96 M. kansasii 235/80 | 250–1000 μM 2–1000 μM 8–>250 μM | InhA enzyme inhibitor, inhibiting mycolic acid production and thus bacterial cell wall biosynthesis | Hit-to-lead identification stage |
| JVA | M. avium 2447 | 320 μM | Isoniazid derivative, which gets hydrolysed to isoniazid, inhibiting bacterial cell wall biosynthesis | Lead optimisation/preclinical stage |
| Peptide Name | Bacterial Strain(s) | MIC | Development Status |
|---|---|---|---|
| AMP1-AMP-6 AMP1 AMP2 | M. abscessus ATCC 19977 M. abscessus subsp. massiliense MAB_062600_1635 M. abscessus subsp. massiliense MAB_030804_1651 M. abscessus subsp. massiliense MAB_010708_1655 M. abscessus clinical isolates (no. of strains = 25) M. abscessus clinical isolates (no. of strains = 25) M. abscessus clinical isolates (no. of strains = 25) M. abscessus clinical isolates (no. of strains = 25) | 3.1–>50 μg/mL 1.6–>50 μg/mL 1.6–>50 μg/mL 1.6–>50 μg/mL 1.5–6.2 μg/mL >50 μg/mL 1.5–6.2 μg/mL >50 μg/mL | Hit-to-lead identification stage |
| S61, S62, S63 KLK1 S61 S62 S63 KLK1 | M. abscessus ATCC 19977 M. abscessus ATCC 19977 M. abscessus clinical isolates (no. of strains = 16) M. abscessus clinical isolates (no. of strains = 16) M. abscessus clinical isolates (no. of strains = 16) M. abscessus clinical isolates (no. of strains = 16) | 200 μg/mL 400 μg/mL 6.25–>400 μg/mL 12.5–>400 μg/mL 6.25–>400 μg/mL 25–>400 μg/mL | Hit-to-lead identification stage |
| NZX | M. abscessus (no. of strains = 3) M. abscessus subsp abscessus (no. of strains = 3) M. abscessus subsp boletti (no. of strains = 3) M. gordonae (no. of strains = 3) M. xenopi (no. of strains = 3) M. kansasii (no. of strains = 3) M. lentiflavum (no. of strains = 3) M. avium (no. of strains = 3) M. shimodeii (no. of strains = 3) M. szulgai (no. of strains = 3) M. chimaera (no. of strains = 3) M. scrofulaceum (no. of strains = 3) M. intracellulare (no. of strains = 3) M. marinum (no. of strains = 3) M. chelonae (no. of strains = 3) | 12.5–25 mg/L 6.3–25 mg/L 3.2–25 mg/L 0.4–12.5 mg/L 0.4–0.8 mg/L 1.6–6.3 mg/L 3.2–25 mg/L 1.6–3.2 mg/L 0.4–3.2 mg/L 12.5–25 mg/L 0.4–1.6 mg/L 0.8–1.2 mg/L 0.4–3.2 mg/L 6.3–25 mg/L 0.4–3.2 mg/L | Preclinical stage |
| Arenicin peptides * Ar-1 Ar-1-Abu Ar-2 Ar-2-Abu Ar-3 Ar-3-Abu Ar-3(3–20) Ar-3(7–16) | M. abscessus CIP 104536T M. abscessus CIP 104536T M. abscessus CIP 104536T M. abscessus CIP 104536T M. abscessus CIP 104536T M. abscessus CIP 104536T M. abscessus CIP 104536T M. abscessus CIP 104536T | 11.4/17.5–11.6/20.2 μM >100 μM 19.8/29.3–53.1/>100 μM 89.3/ > 100–>100 μM 5.3/12.2–44.7/> 100 μM >100 μM 48.3/> 100–77.8/ > 100 μM 17.2/21.3–24.6/ > 100 μM | Lead optimisation stage |
| Antimicrobial Agent | Bacterial Strain(s) | MIC | Bacterial Target and Mechanism of Action | Development Status |
|---|---|---|---|---|
| 16a | M. smegmatis mc2155 M. avium | 32 μg/mL 128 μg/mL | Bacterial efflux pump inhibitor, boosting the activity of co-administered antibiotics | Hit-to-lead identification/lead optimisation |
| RP557 | N/A | N/A | Inhibitor of bacterial biofilm formation | Lead optimisation/preclinical stage |
| NUNLO2 | M. abscessus subsp. abscessus ATCC 19977 M. abscessus subsp. abscessus (AT 07) M. abscessus subsp. bolletii (AT 46) M. abscessus subsp. bolletii (AT 52) | 200 μg/mL 100 μg/mL 100 μg/mL 50 μg/mL | Bacterial efflux pump inhibitor, boosting the activity of co-administered antibiotics | Lead optimisation |
| Basidiomycota macrofungi | N/A | N/A | Unknown bacterial target and mechanism of action | Hit discovery/exploratory phase |
| Isoegomaketone | M. abscessus ATCC 19977 M. abscessus clinical isolates (no. of strains = 8) | 128 μg/mL 32–128 μg/mL | Exact mechanism of action is unknown, but bactericidal and anti-biofilm activity, and disruption of cell membrane is observed | Lead optimisation/preclinical stage |
| Intervention | Description | Challenges |
|---|---|---|
| Pulmonary resection surgery | Subjects resistant to multiple antibiotics received either a lobectomy, partial lung resection, or a segmentectomy, and were free of MAC sputum four months post-surgery. | Potential for resistance emerging to surgery and risk in operating on very sick people. Labour-intensive and requires more resources. |
| Phage therapy | A total of 11 out of 20 subjects in the study demonstrated favourable clinical responses with limited side effects. | Lack of phages available for NTM treatment and risk for emergence of resistance. Potential translational and regulatory approval challenges. |
| Gut microbe remodelling | Oral administration of arginine in mice boosted pulmonary immune defence against NTM and faecal microbiota transplants showed increased protective host defence. | Complexity and differences in microbiota limits reproducibility of this intervention. |
| ALA_PDT | Promotes ferroptosis-like death of M. abscessus and antibiotic sterilisation through oxidative stress. | Further animal and clinical experiments are required to define exact molecular basis and clinical utility. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cross, J.; Gargate, N.; Rahman, K.M. Current Advances in Developing New Antimicrobial Agents Against Non-Tuberculous Mycobacterium. Antibiotics 2025, 14, 1189. https://doi.org/10.3390/antibiotics14121189
Cross J, Gargate N, Rahman KM. Current Advances in Developing New Antimicrobial Agents Against Non-Tuberculous Mycobacterium. Antibiotics. 2025; 14(12):1189. https://doi.org/10.3390/antibiotics14121189
Chicago/Turabian StyleCross, Jane, Nupur Gargate, and Khondaker Miraz Rahman. 2025. "Current Advances in Developing New Antimicrobial Agents Against Non-Tuberculous Mycobacterium" Antibiotics 14, no. 12: 1189. https://doi.org/10.3390/antibiotics14121189
APA StyleCross, J., Gargate, N., & Rahman, K. M. (2025). Current Advances in Developing New Antimicrobial Agents Against Non-Tuberculous Mycobacterium. Antibiotics, 14(12), 1189. https://doi.org/10.3390/antibiotics14121189







