Knowledge, Attitudes, and Practices of Antibiotic Use and AMR in Low-Income Urban Delhi, India: A Community-Based Cross-Sectional Study
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
2.2. Knowledge and Misconceptions Regarding Antibiotics and AMR
2.3. Antibiotic Use and Self-Medication Practices
2.4. Sociodemographic Correlates of Knowledge, Attitudes, and Practices Related to Antibiotics
3. Discussion
Strengths and Limitations
4. Methods
4.1. Study Outcomes
4.2. Sample Size and Sampling
4.3. Measurement of Outcomes
- (i).
- Knowledge Assessment: A total of 13 questions were used to compute the knowledge score. Each response was dichotomously coded, with correct answers assigned a value of 1 and other responses (including wrong True/False selections and “Don’t know”/”Can’t remember” answers) coded as 0. This approach yielded a total knowledge score ranging from 0 to 13 points, with higher scores indicating greater antibiotic-related knowledge (Cronbach’s alpha, α = 0.80).
- (ii).
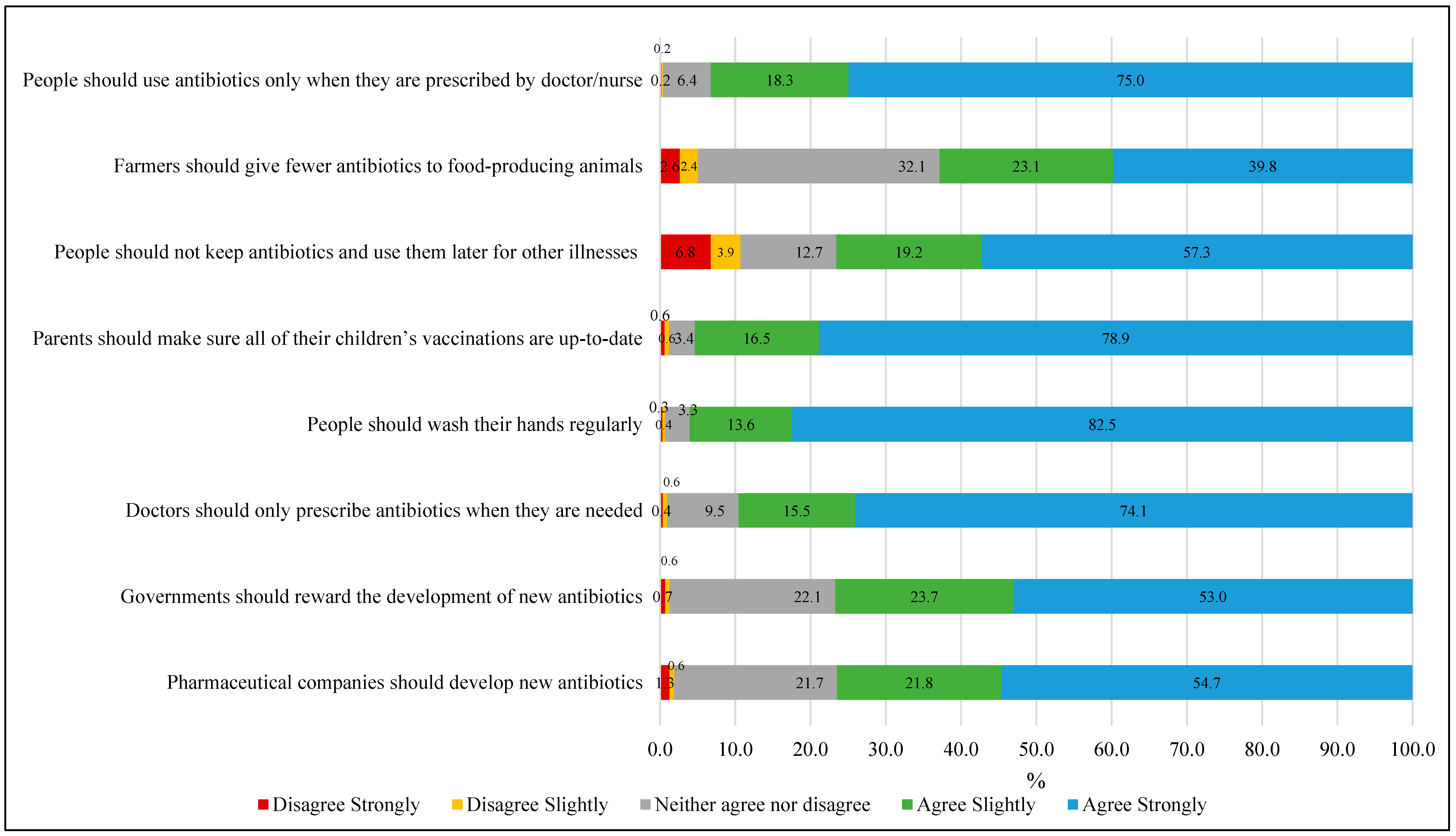
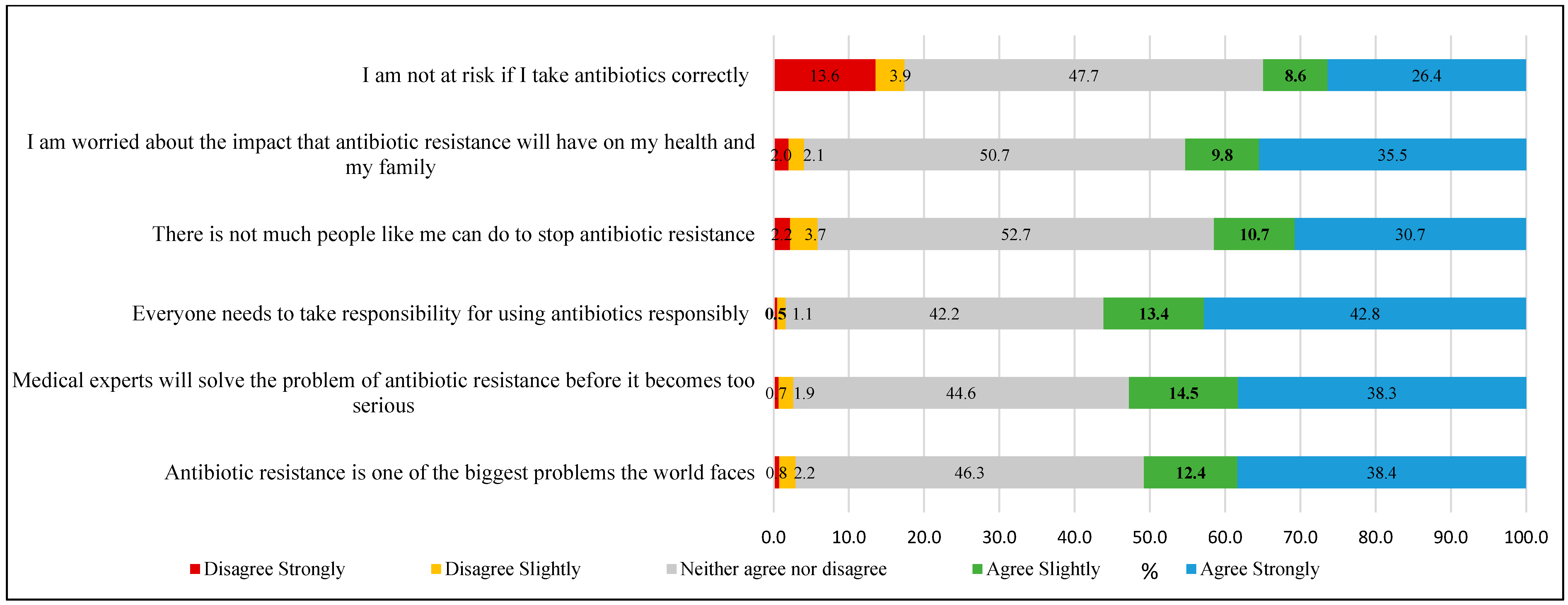
- Attitude assessment: Participants’ attitude was assessed using 14 statements (presented in Figure 1 and Figure 2) measured on a 5-point Likert scale ranging from 1 (“Strongly disagree”) to 5 (“Strongly agree”). The total attitude score was calculated by summing responses across all statements, yielding a possible range of 14 to 70 points. Higher scores indicated more positive attitudes toward appropriate antibiotic use and antimicrobial resistance management (Cronbach’s alpha, α = 0.85).
- (iii).
- Practice assessment: A total of 5 practice-related questions were used to generate a practice score, which included behaviors such as obtaining prescriptions from a physician when last used antibiotics, receiving advice from a physician on antibiotic use, purchasing medicines from a medical store, self-medication, and checking medicine expiry. Each reported practice was scored dichotomously, with appropriate behaviors coded as 1 and inappropriate practices or cannot remember responses coded as 0, yielding a total score ranging from 0 to 5, where higher scores indicated more medically appropriate behaviors (Cronbach’s alpha, α = 0.72).
4.4. Ethical Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 26 November 2024).
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial resistance: A silent pandemic. Nat. Commun. 2024, 15, 6198. [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. In Review on Antimicrobial Resistance; AMR Review: St Lucia, QLD, Australia, 2016. [Google Scholar]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global Increase and Geographic Convergence in Antibiotic Consumption Between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Elmahi, R.H.; Alrasheed, N.A.; Al Sayegh, A.H.; Almobark, A.A.; Banu, N.; Ali, M.D. Knowledge, Attitude, and Practice of using Antibiotics among the Community in Eastern Province, Saudi Arabia. J. Pharm. Bioallied Sci. 2023, 15, 132–138. [Google Scholar] [CrossRef]
- Gualano, M.R.; Gili, R.; Scaioli, G.; Bert, F.; Siliquini, R. General population’s knowledge and attitudes about antibiotics: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2015, 24, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Licata, F.; Zucco, R.; Papadopoli, R.; Pavia, M. Knowledge and practices regarding antibiotics use: Findings from a cross-sectional survey among Italian adults. Evol. Med. Public Health 2020, 2020, 129–138. [Google Scholar] [CrossRef]
- World Health Organization. Antibiotic Resistance: Multi-Country Public Awareness Survey; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Gunasekaran, K. Antimicrobial Resistance in India—“A Silent Pandemic within the Pandemic”. Curr. Med. Issues 2022, 20, 123–124. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- Basu, S.; Santra, S.; Bhatnagar, N.; Laul, A. Outpatient antibiotic prescribing behavior and their psychosocial predictors among early-career clinicians in Delhi, India. Int. J. Acad. Med. 2022, 8, 11–15. [Google Scholar] [CrossRef]
- Gandra, S.; Joshi, J.; Trett, A.; Lamkang, A.; Laxminarayan, R. Scoping Report on Antimicrobial Resistance in India. Washington, DC: Center for Disease Dynamics, Economics & Policy. 2017. Available online: https://www.researchgate.net/publication/321421339_Scoping_Report_on_Antimicrobial_Resistance_in_India (accessed on 1 October 2025).
- Pattnaik, M.; Nayak, A.K.; Karna, S.; Sahoo, S.K.; Palo, S.K.; Kanungo, S.; Kshatri, J.S.; Parai, D.; Walia, K.; Singh, T.; et al. Perception and determinants leading to antimicrobial (mis)use: A knowledge, attitude, and practices study in the rural communities of Odisha, India. Front. Public Health 2023, 10, 1074154. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.V.; Marothi, Y.; Sharma, M. Knowledge, Attitude, and Practice Regarding Antibiotic Use and Resistance for Upper Respiratory Tract Infections among the Population Attending a Mass Gathering in Central India: A Cross-Sectional Study. Antibiotics 2022, 11, 1473. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Shenoy, M.S.; Baliga, S.; Unnikrishnan, B.; Baliga, B.S. Knowledge, attitude, and practices related to antibiotic use and resistance among the general public of coastal south Karnataka, India—A cross-sectional survey. Clin. Epidemiol. Glob. Health 2021, 11, 100717. [Google Scholar] [CrossRef]
- Government of India. National Policy for AMR Containment in India. 2011. Available online: https://ncdc.mohfw.gov.in/wp-content/uploads/2024/07/National-Policy-for-AMR-Containment-in-India.pdf (accessed on 1 October 2025).
- Ferrinho, P.; Viveiros, M.; Fronteira, I. Antimicrobial resistance, society and environment: A global syndemic. One Health 2023, 16, 100512. [Google Scholar] [CrossRef] [PubMed]
- Ndagire, R.; Obuku, E.A.; Segawa, I.; Atim, F.; Lwanira, C.N.; Wangi, R.N.; Ocan, M. Knowledge, attitude, and practices regarding antibiotic use and antimicrobial resistance among urban slum dwellers in Uganda. Antimicrob. Resist. Infect. Control 2025, 14, 12. [Google Scholar] [CrossRef]
- Ministry of Statistics and Programme Implementation, Government of India. Literacy Rate (in per Cent) of Persons of Different Age-Groups. Available online: https://www.mospi.gov.in/literacy-rate-cent-persons-different-age-groups-5 (accessed on 1 October 2025).
- Muflih, S.M.; Al-Azzam, S.; Karasneh, R.A.; Conway, B.R.; Aldeyab, M.A. Public Health Literacy, Knowledge, and Awareness Regarding Antibiotic Use and Antimicrobial Resistance during the COVID-19 Pandemic: A Cross-Sectional Study. Antibiotics 2021, 10, 1107. [Google Scholar] [CrossRef]
- Heid, C.; Knobloch, M.J.; Schulz, L.T.; Safdar, N. Use of the Health Belief Model to Study Patient Perceptions of Antimicrobial Stewardship in the Acute Care Setting. Infect. Control Hosp. Epidemiol. 2016, 37, 576–582. [Google Scholar] [CrossRef]
- Basu, S.; Garg, S. Antibiotic prescribing behavior among physicians: Ethical challenges in resource-poor settings. J. Med. Ethics Hist. Med. 2018, 11, 5. [Google Scholar]
- Shehadeh, M.; Suaifan, G.; Darwish, R.M.; Wazaify, M.; Zaru, L.; Alja’fari, S. Knowledge, attitudes and behavior regarding antibiotics use and misuse among adults in the community of Jordan. A pilot study. Saudi Pharm. J. 2012, 20, 125–133. [Google Scholar] [CrossRef]
- Gashaw, T.; Yadeta, T.A.; Weldegebreal, F.; Demissie, L.; Jambo, A.; Assefa, N. The global prevalence of antibiotic self-medication among the adult population: Systematic review and meta-analysis. Syst. Rev. 2025, 14, 49. [Google Scholar] [CrossRef]
- Krishna, M.; Makwana, N.; Kakde, G.S.; Puri, S.; Kharat, A.S. Knowledge and Attitude toward Antibiotic Use and Identification of Financially Feasible Options to Curb the Spread of Antibiotics in Environment. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 6403250. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Sabuncu, E.; David, J.; Bernède-Bauduin, C.; Pépin, S.; Leroy, M.; Boëlle, P.-Y.; Watier, L.; Guillemot, D. Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med. 2009, 6, e1000084. [Google Scholar] [CrossRef]
- Bauraind, I.; Lopez-Lozano, J.M.; Beyaert, A.; Marchal, J.-L.; Seys, B.; Yane, F.; Hendrickx, E.; Goossens, H.; Tulkens, P.M.; Verbist, L. Association between antibiotic sales and public campaigns for their appropriate use. JAMA 2004, 292, 2468–2470. [Google Scholar] [CrossRef]
- Lambert, M.F.; Masters, G.A.; Brent, S.L. Can mass media campaigns change antimicrobial prescribing? A regional evaluation study. J. Antimicrob. Chemother. 2007, 59, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Palo, S.K.; Bhimarasetty, D.M.; Kandipudi, K.L.P.; Purty, A.J.; Kumar, T.; Basu, S.; Alice, A.; Velavan, A.; Madhavan, S.; et al. Community Dynamics and Engagement Strategies in Establishing Demographic Development and Environmental Surveillance Systems: A Multi-Site Report from India. Healthcare 2023, 11, 411. [Google Scholar] [CrossRef]
- Singh, M.M.; Basu, S.; Lalwani, H.; Rao, S.; Maheshwari, V.; Garg, S.; Sharma, N. Hypertension care cascade in an urban resettlement colony and slum in Delhi, India: A cross-sectional survey. BMC Public Health 2023, 23, 2116. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.N.; Williams, K.A. Sample Size Considerations: Basics for Preparing Clinical or Basic Research. Ann. Nucl. Cardiol. 2020, 6, 81–85. [Google Scholar] [CrossRef] [PubMed]
| N = 1601 | |
|---|---|
| n (%) | |
| Age, mean (SD) | 34.7 (13.7) |
| Gender | |
| Male | 697 (43.5) |
| Female | 904 (56.5) |
| Education | |
| Below high school | 323 (20.2) |
| High school graduate | 189 (11.8) |
| 12th grade with technical/vocational training/associate degree | 549 (34.3) |
| Bachelor’s degree | 398 (24.9) |
| Master’s/Professional degree/Doctorate | 142 (8.9) |
| Total household income (monthly) | |
| Below ₹10,000 | 188 (11.7) |
| ₹10,001–₹25,000 | 793 (49.5) |
| ₹25,001–₹50,000 | 367 (22.9) |
| Above ₹50,001 | 253 (15.8) |
| Household size, mean (SD) | 5.4 (2.1) |
| N = 1601 | |
|---|---|
| n (%) | |
| Knowledge of antibiotics | |
| Heard/know of antibiotics | 1088 (68.0) |
| When should antibiotics be stopped once started? | |
| When you’ve taken all of the antibiotics as directed | 556 (34.7) |
| When you feel better | 190 (11.8) |
| Don’t know | 855 (53.4) |
| Okay to use antibiotics prescribed to a friend/family member if used to treat the same illness | |
| True | 380 (23.7) |
| False | 1044 (65.2) |
| Don’t know | 177 (11.1) |
| Okay to buy the same antibiotics, or request from a doctor, for a similar illness if it has helped get better in the past | |
| True | 388 (24.2) |
| False | 989 (61.8) |
| Don’t know | 224 (14.0) |
| Knowledge about antibiotic resistance | |
| Heard of the following terms * | |
| Antibiotic resistance | 147 (9.2) |
| Superbugs | 29 (1.8) |
| Antimicrobial resistance | 42 (2.6) |
| AMR | 67 (4.2) |
| Drug resistance | 143 (8.9) |
| Antibiotic-resistant bacteria | 119 (7.4) |
| None | 1291 (80.6) |
| Antibiotic resistance occurs when your body becomes resistant to antibiotics, and they no longer work as well | |
| True | 517 (32.3) |
| False | 143 (8.9) |
| Don’t know | 941 (58.8) |
| Many infections are becoming increasingly resistant to treatment with antibiotics | |
| True | 502 (31.4) |
| False | 104 (6.5) |
| Don’t know | 995 (62.1) |
| If bacteria are resistant to antibiotics, it can be very difficult or impossible to treat the infections they cause | |
| True | 540 (33.7) |
| False | 133 (8.3) |
| Don’t know | 928 (58.0) |
| Antibiotic resistance is an issue that could affect me or my family | |
| True | 594 (37.1) |
| False | 128 (8.0) |
| Don’t know | 879 (54.9) |
| Antibiotic resistance is an issue in other countries, but not here | |
| True | 260 (16.2) |
| False | 363 (22.7) |
| Don’t know | 978 (61.1) |
| Antibiotic resistance is only a problem for people who take antibiotics | |
| True | 353 (22.1) |
| False | 331 (20.7) |
| Don’t know | 916 (57.2) |
| Bacteria that are resistant to antibiotics can be spread from person to person | |
| True | 526 (32.9) |
| False | 195 (12.2) |
| Don’t know | 880 (54.9) |
| Antibiotic-resistant infections could make medical procedures like surgery, organ transplants and cancer treatment much more dangerous | |
| True | 417 (26.1) |
| False | 135 (8.4) |
| Don’t know | 1049 (65.5) |
| N = 1601 | |
|---|---|
| n (%) | |
| Use of antibiotics | |
| The last time antibiotics were taken (N = 1601) | |
| In the last month | 266 (16.6) |
| In the last 6 months | 227 (14.2) |
| In the last year | 90 (5.6) |
| More than a year ago | 143 (8.9) |
| Never | 236 (14.7) |
| Can’t remember | 639 (39.9) |
| On that occasion, they had received antibiotics or a prescription from a doctor/nurse (N = 1365 a) | 651 (47.7) |
| On that occasion, they had received advice from a doctor/pharmacist on how to take them (N = 1365 a) | 663 (48.6) |
| On that occasion, antibiotics were obtained from (N = 1365 a) | |
| Medical store | 549 (40.2) |
| Other | 38 (2.8) |
| Can’t remember | 781 (57.2) |
| % that self-medicate with antibiotics (N = 1601) | 394 (24.6) |
| Common symptoms for which participants self-medicate (N = 394) * | |
| Headache | 153 (38.8) |
| Fever | 313 (79.4) |
| Cough | 157 (39.8) |
| Pain | 182 (46.2) |
| Diarrhea | 16 (4.1) |
| Acidity | 68 (17.3) |
| Others | 7 (1.8) |
| Sources of information about self-medication (N = 368) | |
| Internet | 90 (24.5) |
| Friends/Family | 145 (39.4) |
| Healthcare professionals | 276 (75.0) |
| Old prescription | 39 (10.6) |
| Advertisement | 24 (6.5) |
| Neighbor | 10 (2.72) |
| Check the expiry of the medication (N = 1601) | 975 (60.9) |
| Variable | Crude OR (95% CI) | Adjusted OR a (95% CI) | p-Value | Adjusted OR b (95% CI) | p-Value |
|---|---|---|---|---|---|
| I. Knowledge | N = 1598 | N = 1598 | |||
| Age | 0.97 (0.96, 0.98) | 0.99 (0.98, 1.00) | 0.003 | 0.99 (0.98, 1.00) | 0.003 |
| Gender | |||||
| Male | Ref | Ref | Ref | ||
| Female | 0.65 (0.54, 0.79) | 0.82 (0.67, 1.00) | 0.05 | 0.82 (0.67, 1.00) | 0.05 |
| Education | |||||
| Below high school | 0.09 (0.07, 0.13) | 0.16 (0.11, 0.22) | <0.001 | 0.16 (0.11, 0.22) | <0.001 |
| High school | 0.40 (0.29, 0.56) | 0.38 (0.26, 0.57) | <0.001 | 0.60 (0.40, 0.90) | 0.012 |
| 12th grade | 0.31 (0.24, 0.39) | 0.35 (0.27, 0.46) | <0.001 | 0.35 (0.27, 0.46) | <0.001 |
| College degree and above | Ref | Ref | Ref | ||
| Income | |||||
| <₹10,000 | 0.14 (0.10, 0.20) | 0.21 (0.12, 0.37) | <0.001 | 0.21 (0.12, 0.37) | 0.001 |
| ₹10,001–₹25,000 | 0.38 (0.29, 0.50) | 0.33 (0.20, 0.54) | <0.001 | 1.20 (0.84, 1.70) | 0.317 |
| ₹25,001–₹50,000 | 0.70 (0.52, 0.95) | 0.49 (0.29, 0.83) | 0.008 | 1.41 (0.98, 2.04) | 0.066 |
| >₹50,000 | Ref | Ref | Ref | ||
| II. Attitude | N = 1598 | N = 1598 | |||
| Age | 0.98 (0.97, 0.99) | 0.98 (0.97, 0.99) | <0.001 | 0.98 (0.97, 0.99) | <0.001 |
| Gender | |||||
| Male | Ref | ||||
| Female | 0.96 (0.79, 1.16) | ||||
| Education | |||||
| Below high school | 0.56 (0.42, 0.74) | 2.36 (1.63, 3.41) | <0.001 | 2.36 (1.63, 3.41) | <0.001 |
| High school | 0.88 (0.63, 1.22) | 1.51 (1.04, 2.19) | 0.03 | 1.51 (1.04, 2.19) | 0.03 |
| 12th grade | 0.33 (0.26, 0.42) | 0.53 (0.40, 0.70) | <0.001 | 0.53 (0.40, 0.70) | <0.001 |
| College degree and above | Ref | (1.00) Ref | (1.00) Ref | ||
| Income (per month) | |||||
| <₹10,000 | 0.09 (0.06, 0.13) | 0.10 (0.06, 0.17) | <0.001 | 0.10 (0.06, 0.17) | <0.001 |
| ₹10,001–₹25,000 | 0.58 (0.43, 0.77) | 0.71 (0.51, 1.00) | 0.047 | 0.71 (0.51, 1.00) | 0.047 |
| ₹25,001–₹50,000 | 0.68 (0.49, 0.94) | 0.77 (0.54, 1.10) | 0.147 | 0.77 (0.54, 1.10) | 0.147 |
| >₹50,000 | Ref | (1.00) Ref | (1.00) Ref | ||
| Knowledge of antibiotics and antimicrobial resistance | 1.21 (1.17, 1.26) | 1.28 (1.21, 1.35) | <0.001 | 1.05 (1.00, 1.11) | 0.075 |
| III Practice | N = 1363 | N = 1363 | |||
| Age | 1.00 (0.99, 1.00) | ||||
| Gender | |||||
| Male | Ref | ||||
| Female | 0.93 (0.76, 1.13) | ||||
| Education | |||||
| Below high school | 0.39 (0.30, 0.52) | 0.98 (0.67, 1.42) | 0.902 | 0.57 (0.35, 0.93) | 0.024 |
| High school | 0.41 (0.30, 0.58) | 0.89 (0.59, 1.34) | 0.579 | 0.28 (0.14, 0.58) | 0.001 |
| 12th grade | 0.78 (0.60, 1.00) | 1.02 (0.76, 1.36) | 0.91 | 1.02 (0.76, 1.36) | 0.91 |
| College degree and above | Ref | Ref | |||
| Income | |||||
| <₹10,000 | 0.69 (0.47, 1.02) | 0.58 (0.35, 0.97) | 0.037 | 0.94 (0.56, 1.58) | 0.814 |
| ₹10,001–₹25,000 | 0.28 (0.21, 0.39) | 0.37 (0.26, 0.53) | <0.001 | 0.37 (0.26, 0.53) | <0.001 |
| ₹25,001–₹50,000 | 0.47 (0.33, 0.67) | 0.52 (0.35, 0.75) | 0.001 | 0.52 (0.35, 0.75) | 0.001 |
| >₹50,000 | Ref | Ref | |||
| Knowledge score | 1.14 (1.11, 1.18) | 1.30 (1.23, 1.37) | <0.001 | 1.08 (1.02, 1.15) | 0.01 |
| Attitude score | 0.95 (0.94, 0.96) | 0.93 (0.92, 0.95) | <0.001 | 0.93 (0.92, 0.95) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.; Basu, S.; Rajaura, S.; Zode, M.; Singh, M.M.; Sharma, N. Knowledge, Attitudes, and Practices of Antibiotic Use and AMR in Low-Income Urban Delhi, India: A Community-Based Cross-Sectional Study. Antibiotics 2025, 14, 1184. https://doi.org/10.3390/antibiotics14121184
Rao S, Basu S, Rajaura S, Zode M, Singh MM, Sharma N. Knowledge, Attitudes, and Practices of Antibiotic Use and AMR in Low-Income Urban Delhi, India: A Community-Based Cross-Sectional Study. Antibiotics. 2025; 14(12):1184. https://doi.org/10.3390/antibiotics14121184
Chicago/Turabian StyleRao, Shivani, Saurav Basu, Srishty Rajaura, Mrunali Zode, Mongjam Meghachandra Singh, and Nandini Sharma. 2025. "Knowledge, Attitudes, and Practices of Antibiotic Use and AMR in Low-Income Urban Delhi, India: A Community-Based Cross-Sectional Study" Antibiotics 14, no. 12: 1184. https://doi.org/10.3390/antibiotics14121184
APA StyleRao, S., Basu, S., Rajaura, S., Zode, M., Singh, M. M., & Sharma, N. (2025). Knowledge, Attitudes, and Practices of Antibiotic Use and AMR in Low-Income Urban Delhi, India: A Community-Based Cross-Sectional Study. Antibiotics, 14(12), 1184. https://doi.org/10.3390/antibiotics14121184





