Preserving Health Beyond Infection Control: Frailty, Weight, and Cognition in OPAT Patients
Abstract
1. Introduction
2. Results
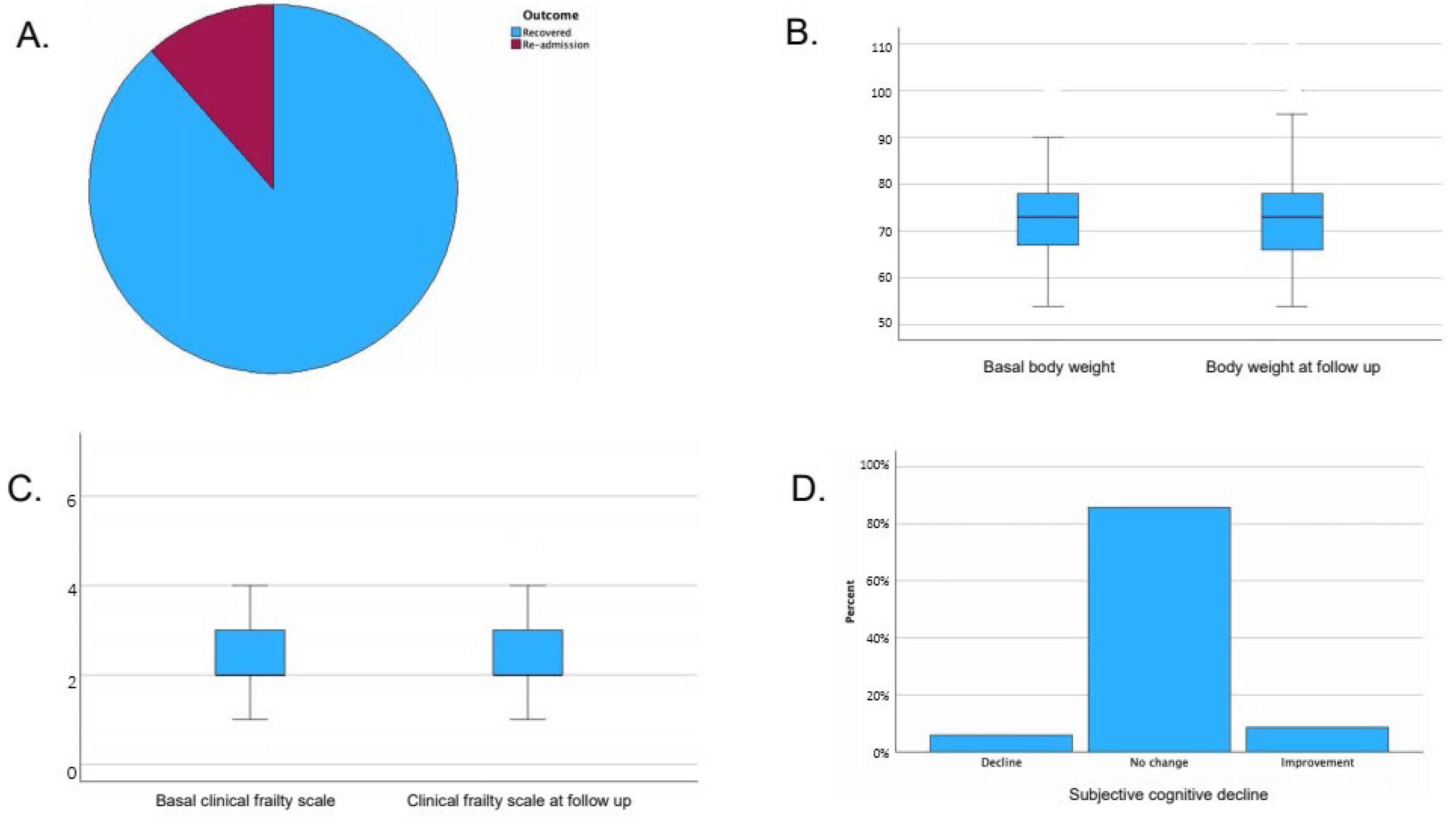
- Median body weight at follow-up was 73 kg (IQR 66–78), with no statistically significant variation compared to baseline (p-value 0.43). Body weight at follow-up was missing for 8 patients.
- Median frailty index at follow-up was 2 (IQR 2–3), with no significant variation compared to baseline (p-value 0.16). Frailty index was missing at follow-up for 20 patients.
- Subjective cognitive status: A total of 85.6% reported no change, 5.9% reported decline, and 8.5% reported improvement. Subjective cognitive status was missing at follow-up for 2 patients.
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCI | Charlson Comorbidity Index |
| CFI | Clinical Frailty Scale |
| DTR | Difficult-to-Treat |
| ESBL | Extended Spectrum Beta-Lactamase |
| KPC | Klebsiella pneumoniae Carbapenemase |
| MDR | Multi-Drug Resistant |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| OPAT | Outpatient Parenteral Antimicrobial Therapy |
| UTI | Urinary Tract Infection |
| CODP | Chronic Obstructive Pulmonary Disease |
| ABSSSI | Acute Bacterial Skin and Skin-Structure Infections |
References
- Mohammed, S.A.; Roberts, J.A.; Cotta, M.O.; Rogers, B.; Pollard, J.; Assefa, G.M.; Erku, D.; Sime, F.B. Safety and efficacy of outpatient parenteral antimicrobial therapy: A systematic review and meta-analysis of randomized clinical trials. Int. J. Antimicrob. Agents 2024, 64, 107263. [Google Scholar] [CrossRef] [PubMed]
- Durojaiye, O.C.; Bell, H.; Andrews, D.; Ntziora, F.; Cartwright, K. Clinical efficacy, cost analysis and patient acceptability of outpatient parenteral antibiotic therapy (OPAT): A decade of Sheffield (UK) OPAT service. Int. J. Antimicrob. Agents 2018, 51, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.A.; Semple, L.; Seaton, R.A. Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital-based practice: A retrospective cohort study describing experience and evolution over 10 years. Int. J. Antimicrob. Agents 2012, 39, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, M.; Gilchrist, M.; Seaton, R.A. Outpatient parenteral antimicrobial therapy (OPAT) versus inpatient care in the UK: A health economic assessment for six key diagnoses. BMJ Open 2021, 11, e049733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Twiddy, M.; Murray, C.J.C.; Mason, S.J.; Meads, D.; Wright, J.M.; Mitchell, E.D.; Minton, J. A qualitative study of patients’ feedback about Outpatient Parenteral Antimicrobial Therapy (OPAT) services in Northern England: Implications for service improvement. BMJ Open 2018, 8, e019099. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, S.; Dawudi, Y.; Cassard, B.; Longuet, P.; Lesprit, P.; Gauzit, R. Home intravenous antibiotherapy and the proper use of elastomeric pumps: Systematic review of the literature and proposals for improved use. Infect. Dis. Now 2021, 51, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bales, C.W.; Ritchie, C.S. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu. Rev. Nutr. 2002, 22, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Botero, L.; Young, A.M.; Banks, M.D.; Bauer, J. Incidence and criteria used in the diagnosis of hospital-acquired malnutrition in adults: A systematic review and pooled incidence analysis. Eur. J. Clin. Nutr. 2023, 77, 23–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zannidi, D.; Patel, P.S.; Leventea, E.; Paciepnik, J.; Dobson, F.; Heyes, C.; Goudie, R.J.B.; Griep, L.M.O.; Preller, J.; Spillman, L.N. Factors Associated with Significant Weight Loss in Hospitalised Patients with COVID-19: A Retrospective Cohort Study in a Large Teaching Hospital. Nutrients 2022, 14, 4195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, H.J.; Rattray, N.J.W.; Quasim, T.; Moug, S.J. Changes in frailty status and discharge destination post emergency laparotomy. World J. Emerg. Surg. 2025, 20, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.; Ye, Y.; Zhang, Y.; Pan, X.F.; Pan, A. Weight change across adulthood in relation to all cause and cause specific mortality: Prospective cohort study. BMJ 2019, 367, l5584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rockwood, K.; Blodgett, J.M.; Theou, O.; Sun, M.H.; Feridooni, H.A.; Mitnitski, A.; Rose, R.A.; Godin, J.; Gregson, E.; Howlett, S.E. A Frailty Index Based On Deficit Accumulation Quantifies Mortality Risk in Humans and in Mice. Sci. Rep. 2017, 7, 43068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aung Thein, M.Z.; Pereira, J.V.; Nitchingham, A.; Caplan, G.A. A call to action for delirium research: Meta-analysis and regression of delirium associated mortality. BMC Geriatr. 2020, 20, 325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Parejo, Y.; Gonzalez-Rubio, J.; Garcia Guerrero, J.; Gomez-Juarez Sango, A.; Cantero Escribano, J.M.; Najera, A. Risk factors for colonisation by Multidrug-Resistant bacteria in critical care units. Intensive Crit. Care Nurs. 2025, 86, 103760. [Google Scholar] [CrossRef] [PubMed]
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital-Acquired Infections. In StatPearls [Internet]; Updated 12 February 2023; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441857/ (accessed on 17 November 2025).
- Available online: https://www.arnascivico.it/attachments/article/34851/Allegato%20Del%20n.%20587.pdf (accessed on 17 November 2025).
| Characteristic | Value |
|---|---|
| Number of patients | 119 |
| Male sex, n (%) | 67 (56.3%) |
| Female sex, n (%) | 52 (43.7%) |
| Age, median (IQR) | 67 (51–76) years |
| Charlson Comorbidity Index, median (IQR) | 3 (2–6) |
| Most frequent comorbidities | Ischemic heart disease (n = 39, 32.8%); Diabetes Mellitus (n = 35, 29.4%); COPD (n = 26, 21.8%); solid tumor (n = 23, 19.3%), |
| Baseline body weight, median (IQR) | 73 kg (67–79) |
| Frailty Index, median (IQR) | 2 (2–3) |
| Most frequent infectious diagnoses | UTIs (n = 35, 29.4%); Ostemyelitis (n = 29, 25.2%); Pneumonia (n = 20, 16.8%), ABSSSI (n = 10, 8.4%) |
| Most common microbiological isolates | P. aeruginosa (n = 27, 29.3%); K. pneumoniae (n = 23, 25%); S. aureus (n = 15, 16.3%), |
| MDR isolates, n (%) | 61 (66%) including MRSA, ESBL, KPC, OXA-48, or DTR P. aeruginosa |
| Treatment | Value |
|---|---|
| Patients treated with once-daily regimens | 88 (most frequent agents: K. pneumoniae, S. aureus, E. coli) |
| Patients treated with continuous infusion via elastomeric pumps | 31 (most frequent agents: P. aeruginosa, K. Pneumoniae) |
| Adverse drug reactions, n (%) | 3 (2.5%)-1 nausea, 1 C. difficile colitis |
| Catheter-related complications, n (%) | 12 (10%) |
| Outcome | Value |
|---|---|
| Recovered, n (%) | 98 (82.5%) |
| Hospital readmission, n (%) | 19 (16%) |
| Deaths, n (%) | 0 (0%) |
| Body weight at follow-up, median (IQR) | 73 kg (66–78) |
| Change in body weight vs. baseline | Non-significant variation (p-value 0.43) |
| Frailty Index at follow-up, median (IQR) | 2.5 (2–3) |
| Change in frailty vs. baseline | Non-significant variation (p-value 0.16) |
| Cognitive status—no change, n (%) | 101 (85.6%) |
| Cognitive status—decline, n (%) | 7 (5.9%) |
| Cognitive status—improvement, n (%) | 10 (8.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciusa, G.; Pipitone, G.; Catania, B.; Coniglione, G.; Imburgia, C.; Marsala, M.G.L.; Scordo, P.; Albanese, A.; Cascio, A.; Guaraldi, G.; et al. Preserving Health Beyond Infection Control: Frailty, Weight, and Cognition in OPAT Patients. Antibiotics 2025, 14, 1173. https://doi.org/10.3390/antibiotics14111173
Ciusa G, Pipitone G, Catania B, Coniglione G, Imburgia C, Marsala MGL, Scordo P, Albanese A, Cascio A, Guaraldi G, et al. Preserving Health Beyond Infection Control: Frailty, Weight, and Cognition in OPAT Patients. Antibiotics. 2025; 14(11):1173. https://doi.org/10.3390/antibiotics14111173
Chicago/Turabian StyleCiusa, Giacomo, Giuseppe Pipitone, Bianca Catania, Giulia Coniglione, Claudia Imburgia, Maria Grazia Laura Marsala, Preziosa Scordo, Antonio Albanese, Antonio Cascio, Giovanni Guaraldi, and et al. 2025. "Preserving Health Beyond Infection Control: Frailty, Weight, and Cognition in OPAT Patients" Antibiotics 14, no. 11: 1173. https://doi.org/10.3390/antibiotics14111173
APA StyleCiusa, G., Pipitone, G., Catania, B., Coniglione, G., Imburgia, C., Marsala, M. G. L., Scordo, P., Albanese, A., Cascio, A., Guaraldi, G., & Iaria, C. (2025). Preserving Health Beyond Infection Control: Frailty, Weight, and Cognition in OPAT Patients. Antibiotics, 14(11), 1173. https://doi.org/10.3390/antibiotics14111173








