Full Validation of Pooled Antibiotic Susceptibility Testing Using CLSI Methods and Performance Criteria in UTI Pathogens
Abstract
1. Introduction
2. Results
2.1. Validation of Monomicrobial Specimens
2.1.1. Monomicrobial Enterobacterales
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Amoxicillin/ Clavulanate | 51 | 98.0 | 0 | 0 | 1 (2.0) | 100.0 |
| Ampicillin | 37 | 100.0 | 0 | 0 | 0 | N/A |
| Ampicillin/ Sulbactam | 51 | 92.2 | 0 | 0 | 4 (7.8) | 75.0 |
| Cefaclor | 51 | 100.0 | 0 | 0 | 0 | N/A |
| Cefazolin | 69 | 100.0 | 0 | 0 | 0 | N/A |
| Cefepime | 51 | 94.1 | 0 | 0 | 3 (5.9) | 0 |
| Ceftazidime | 51 | 96.1 | 0 | 0 | 2 (3.9) | 0 |
| Ceftriaxone | 51 | 98.0 | 0 | 0 | 1 (2.0) | 100.0 |
| Ciprofloxacin | 51 | 98.0 | 0 | 0 | 1 (2.0) | 100.0 |
| Doxycycline | 51 | 98.0 | 0 | 0 | 1 (2.0) | 100.0 |
| Fosfomycin | 41 | 95.1 | 0 | 0 | 2 (4.9) | 100.0 |
| Gentamicin | 50 | 98.0 | 0 | 0 | 1 (2.0) | 100.0 |
| Levofloxacin | 51 | 98.0 | 0 | 0 | 1 (2.0) | 0 |
| Meropenem | 52 | 100.0 | 0 | 0 | 0 | N/A |
| Nitrofurantoin | 51 | 94.1 | 0 | 0 | 3 (5.9) | 100.0 |
| Piperacillin/ Tazobactam | 53 | 96.2 | 0 | 0 | 2 (3.8) | 50.0 |
| Sulfamethoxazole/ Trimethoprim | 51 | 100.0 | 0 | 0 | 0 | N/A |
| Trimethoprim | 63 | 100.0 | 0 | 0 | 0 | N/A |
2.1.2. Monomicrobial Enterococci
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Ampicillin | 52 | 100.0 | 0 | 0 | 0 | N/A |
| Ciprofloxacin | 51 | 98.0 | 0 | 0 | 1 (2.0) | 100.0 |
| Doxycycline | 53 | 94.3 | 0 | 0 | 3 (5.7) | 100.0 |
| Fosfomycin | 51 | 98.0 | 0 | 0 | 1 (2.0) | 100.0 |
| Levofloxacin | 51 | 100.0 | 0 | 0 | 0 | N/A |
| Linezolid | 59 | 98.3 | 0 | 0 | 1 (1.7) | 100.0 |
| Nitrofurantoin | 56 | 98.2 | 0 | 0 | 1 (1.8) | 100.0 |
| Vancomycin | 56 | 100.0 | 0 | 0 | 0 | N/A |
2.1.3. Monomicrobial Staphylococci
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Ciprofloxacin | 40 | 97.5 | 0 | 0 | 1 (2.5) | 0 |
| Doxycycline | 47 | 97.9 | 0 | 0 | 1 (2.1) | 100.0 |
| Gentamicin | 46 | 97.8 | 0 | 0 | 1 (2.2) | 0 |
| Levofloxacin | 40 | 97.5 | 0 | 0 | 1 (2.5) | 0 |
| Linezolid | 46 | 100.0 | 0 | 0 | 0 | N/A |
| Nitrofurantoin | 47 | 100.0 | 0 | 0 | 0 | N/A |
| Sulfamethoxazole/ Trimethoprim | 41 | 97.6 | 0 | 1 (2.9) | 0 | N/A |
| Trimethoprim | 40 | 100.0 | 0 | 0 | 0 | N/A |
| Vancomycin | 39 | 100.0 | --- | 0 | 0 | N/A |
2.1.4. Monomicrobial Pseudomonas aeruginosa
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Cefepime | 42 | 100.0 | 0 | 0 | 0 | N/A |
| Ceftazidime | 46 | 95.7 | 0 | 1 (2.9) | 1 (2.2) | 100.0 |
| Ciprofloxacin | 42 | 95.2 | 0 | 0 | 2 (4.8) | 50.0 |
| Gentamicin | 41 | 100.0 | 0 | 0 | 0 | N/A |
| Levofloxacin | 42 | 100.0 | 0 | 0 | 0 | N/A |
| Meropenem | 42 | 97.6 | 0 | 0 | 1 (2.4) | 0 |
| Piperacillin/ Tazobactam | 43 | 100.0 | 0 | 0 | 0 | N/A |
2.1.5. Monomicrobial Acinetobacter Species
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Ampicillin/ Sulbactam | 39 | 97.4 | 0 | 0 | 1 (2.6) | 100.0 |
| Cefepime | 39 | 89.7 | 0 | 0 | 4 (10.3) | 75.0 |
| Ceftazidime | 39 | 100.0 | 0 | 0 | 0 | N/A |
| Ceftriaxone | 39 | 94.9 | 0 | 0 | 2 (5.1) | 50.0 |
| Ciprofloxacin | 39 | 100.0 | 0 | 0 | 0 | N/A |
| Gentamicin | 39 | 100.0 | 0 | 0 | 0 | N/A |
| Levofloxacin | 39 | 100.0 | 0 | 0 | 0 | N/A |
| Meropenem | 39 | 100.0 | 0 | 0 | 0 | N/A |
| Piperacillin/ Tazobactam | 39 | 97.4 | 0 | 0 | 1 (2.6) | 0 |
| Sulfamethoxazole/ Trimethoprim | 39 | 100.0 | 0 | 0 | 0 | N/A |
2.2. Validation of Polymicrobial Specimens
2.2.1. Polymicrobial Enterobacterales
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Amoxicillin/ Clavulanate | 40 | 90.0 | 0 | 0 | 4 (10.0) | 50.0 |
| Ampicillin | 39 | 97.4 | 1 (2.9) | 0 | 0 | N/A |
| Ampicillin/ Sulbactam | 70 | 90.0 | 0 | 1 (2.9) | 6 (8.6) | 83.3 |
| Cefaclor | 54 | 98.1 | 0 | 0 | 1 (1.9) | 0 |
| Cefazolin | 54 | 98.1 | 1 (2.0) | 0 | 0 | N/A |
| Cefepime | 40 | 97.5 | 0 | 0 | 1 (2.5) | 0 |
| Ceftazidime | 49 | 100.0 | 0 | 0 | 0 | N/A |
| Ceftriaxone | 54 | 100.0 | 0 | 0 | 0 | N/A |
| Ciprofloxacin | 40 | 90.0 | 0 | 0 | 4 (10.0) | 75.0 |
| Doxycycline | 40 | 100.0 | 0 | 0 | 0 | N/A |
| Fosfomycin | 38 | 94.7 | 0 | 0 | 2 (5.3) | 100.0 |
| Gentamicin | 40 | 100.0 | 0 | 0 | 0 | N/A |
| Levofloxacin | 40 | 92.5 | 0 | 0 | 3 (7.5) | 66.7 |
| Meropenem | 40 | 97.5 | 0 | 0 | 1 (2.5) | 100.0 |
| Nitrofurantoin | 51 | 90.2 | 0 | 0 | 5 (9.8) | 80.0 |
| Piperacillin/ Tazobactam | 45 | 93.3 | 0 | 1 (2.9) | 2 (4.4) | 50.0 |
| Sulfamethoxazole/ Trimethoprim | 40 | 100.0 | 0 | 0 | 0 | N/A |
| Trimethoprim | 51 | 100.0 | 0 | 0 | 0 | N/A |
2.2.2. Polymicrobial Enterococci
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Ampicillin | 46 | 97.8 | 1 (2.9) | 0 | 0 | N/A |
| Ciprofloxacin | 40 | 90.0 | 0 | 0 | 4 (10.0) | 75.0 |
| Doxycycline | 37 | 100.0 | 0 | 0 | 0 | N/A |
| Fosfomycin | 37 | 100.0 | 0 | 0 | 0 | N/A |
| Levofloxacin | 50 | 92.0 | 0 | 0 | 4 (8.0) | 75.0 |
| Linezolid | 43 | 100.0 | 0 | 0 | 0 | N/A |
| Nitrofurantoin | 41 | 90.2 | 0 | 0 | 4 (9.8) | 100.0 |
| Vancomycin | 42 | 100.0 | 0 | 0 | 0 | N/A |
2.2.3. Polymicrobial Staphylococci
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Ciprofloxacin | 29 | 100.0 | 0 | 0 | 0 | N/A |
| Doxycycline | 31 | 93.5 | 0 | 0 | 2 (6.5) | 100.0 |
| Gentamicin | 30 | 96.7 | 0 | 0 | 1 (3.3) | 100.0 |
| Levofloxacin | 31 | 100.0 | 0 | 0 | 0 | N/A |
| Linezolid | 30 | 100.0 | 0 | 0 | 0 | N/A |
| Nitrofurantoin | 29 | 100.0 | 0 | 0 | 0 | N/A |
| Sulfamethoxazole/ Trimethoprim | 29 | 100.0 | 0 | 0 | 0 | N/A |
| Trimethoprim | 29 | 100.0 | 0 | 0 | 0 | N/A |
| Vancomycin | 31 | 100.0 | 0 | 0 | 0 | N/A |
2.2.4. Polymicrobial Pseudomonas aeruginosa
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Cefepime | 30 | 100.0 | 0 | 0 | 0 | N/A |
| Ceftazidime | 31 | 100.0 | 0 | 0 | 0 | N/A |
| Ciprofloxacin | 30 | 100.0 | 0 | 0 | 0 | N/A |
| Gentamicin | 31 | 96.8 | 0 | 0 | 1 (3.2) | 100.0 |
| Levofloxacin | 31 | 100.0 | 0 | 0 | 0 | N/A |
| Meropenem | 32 | 93.8 | 0 | 0 | 2 (6.2) | 50.0 |
| Piperacillin/ Tazobactam | 30 | 100.0 | 0 | 0 | 0 | N/A |
2.2.5. Polymicrobial Acinetobacter Species
| Antibiotic | Total n | CA % | VME n (%) | ME n (%) | mE n (%) | mE w/EA (% of mE) |
|---|---|---|---|---|---|---|
| Ampicillin/ Sulbactam | 31 | 100.0 | 0 | 0 | 0 | N/A |
| Cefepime | 30 | 100.0 | 0 | 0 | 0 | N/A |
| Ceftazidime | 32 | 96.9 | 0 | 0 | 1 (3.1) | 100.0 |
| Ceftriaxone | 31 | 96.8 | 0 | 0 | 1 (3.2) | 100.0 |
| Ciprofloxacin | 30 | 100.0 | 0 | 0 | 0 | N/A |
| Gentamicin | 31 | 96.8 | 0 | 0 | 1 (3.2) | 100.0 |
| Levofloxacin | 31 | 100.0 | 0 | 0 | 0 | N/A |
| Meropenem | 32 | 100.0 | 0 | 0 | 0 | N/A |
| Piperacillin/ Tazobactam | 30 | 100.0 | 0 | 0 | 0 | N/A |
| Sulfamethoxazole/ Trimethoprim | 30 | 100.0 | 0 | 0 | 0 | N/A |
2.3. Precision (Reproducibility)
3. Discussion
4. Materials and Methods
4.1. Study Design and Specimen Selection
4.2. Bacterial Identification with Multiplex-Polymerase Chain Reaction
4.3. Bacterial Identification with Standard Urine Culture
4.4. Pooled Antibiotic Susceptibility Testing
4.5. Disk Diffusion Antibiotic Susceptibility Testing
4.6. Broth Microdilution Antibiotic Susceptibility Testing
4.7. Considerations for Comparing Results for Polymicrobial Specimens
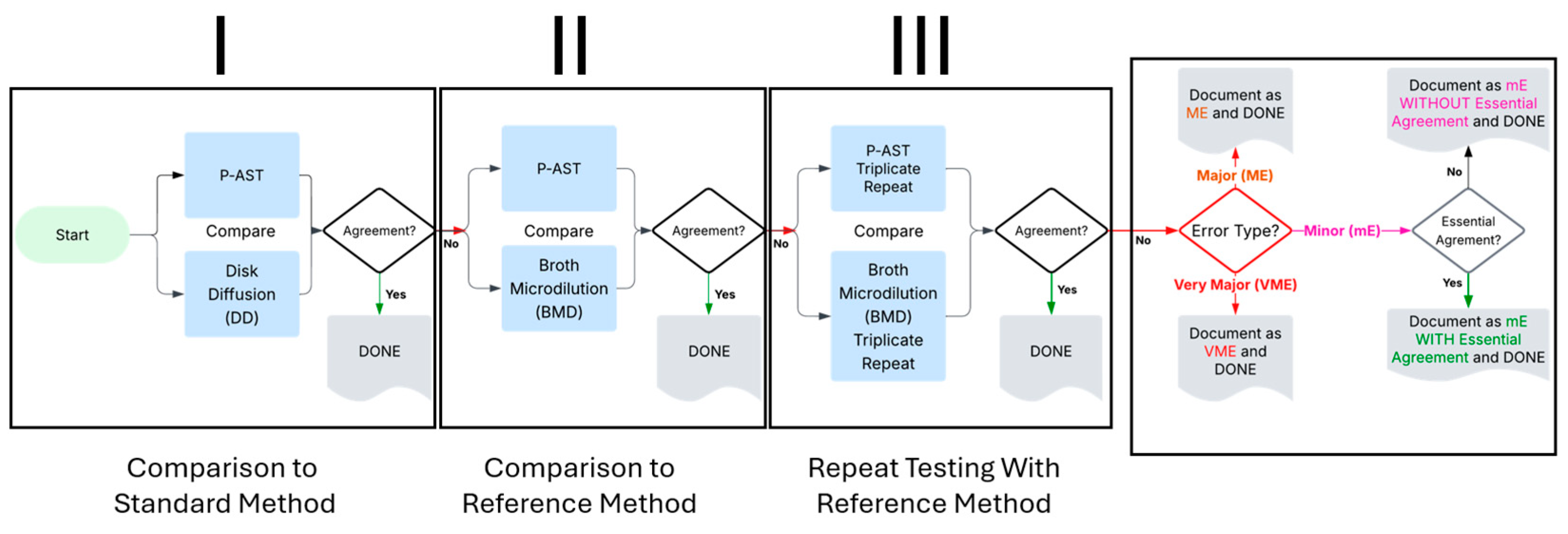
4.8. Workflow
4.9. Precision (Reproducibility)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABR | Antibiotic Resistance |
| AST | Antibiotic Susceptibility Test(ing) |
| BIT | Breakpoint Implementation Toolkit |
| BMD | Broth Microdilution |
| CA | Categorical Agreement |
| CDC | United States Centers for Disease Control |
| CFU | Colony Forming Unit |
| CLSI | Clinical and Laboratory Standards Institute |
| DALY | Disability Adjusted Life Years |
| DD | Disk Diffusion |
| EA | Essential Agreement |
| FDA | United States Food and Drug Administration |
| MD | Major Discrepancy |
| mD | Minor Discrepancy |
| ME | Major Error |
| mE | Minor Error |
| MIC | Minimum Inhibitory Concentration |
| M-PCR | Multiplex-Polymerase Chain Reaction |
| N/A | Not Applicable |
| P-AST | Pooled Antibiotic Susceptibility Test(ing) |
| STIC | Susceptibility Test Interpretive Criteria |
| SUC | Standard Urine Culture |
| UTI | Urinary Tract Infection |
| VMD | Very Major Discrepancy |
| VME | Very Major Error |
Appendix A
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Shorr, A.F. Descriptive Epidemiology and Outcomes of Hospitalizations with Complicated Urinary Tract Infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab591. [Google Scholar] [CrossRef]
- Haley, E.; Luke, N.; Korman, H.; Baunoch, D.; Wang, D.; Zhao, X.; Mathur, M. Improving Patient Outcomes While Reducing Empirical Treatment with Multiplex-Polymerase-Chain-Reaction/Pooled-Antibiotic-Susceptibility-Testing Assay for Complicated and Recurrent Urinary Tract Infections. Diagnostics 2023, 13, 3060. [Google Scholar] [CrossRef] [PubMed]
- Fromer, D.L.; Luck, M.E.; Cheng, W.Y.; Mahendran, M.; Costa, W.L.; da Pinaire, M.; Duh, M.S.; Preib, M.T.; Ellis, J.J. Risk Factors for Empiric Treatment Failure in US Female Outpatients with Uncomplicated Urinary Tract Infection: An Observational Study. J. Gen. Intern. Med. 2025, 40, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.W.; Puttagunta, S.; Aronin, S.I.; Brossette, S.; Murray, J.; Gupta, V. Impact of Empirical Antibiotic Therapy on Outcomes of Outpatient Urinary Tract Infection Due to Nonsusceptible Enterobacterales. Microbiol. Spectr. 2022, 10, e0235921. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Fasolino, T.; Ivankovic, D.; Davis, N.J.; Brownlee, N. Genetic Factors That Contribute to Antibiotic Resistance through Intrinsic and Acquired Bacterial Genes in Urinary Tract Infections. Microorganisms 2023, 11, 1407. [Google Scholar] [CrossRef]
- Bard, J.; Lee, F. Why Can’t We Just Use PCR? The Role of Genotypic versus Phenotypic Testing for Antimicrobial Resistance Testing. Clin. Microbiol. Newsl. 2018, 40, 87–95. [Google Scholar] [CrossRef]
- Gasiorek, M.; Hsieh, M.H.; Forster, C.S. Utility of DNA Next-Generation Sequencing and Expanded Quantitative Urine Culture in Diagnosis and Management of Chronic or Persistent Lower Urinary Tract Symptoms. J. Clin. Microbiol. 2019, 58, e00204-19. [Google Scholar] [CrossRef]
- Baunoch, D.; Luke, N.; Wang, D.; Vollstedt, A.; Zhao, X.; Ko, D.S.C.; Huang, S.; Cacdac, P.; Sirls, L.T. Concordance Between Antibiotic Resistance Genes and Susceptibility in Symptomatic Urinary Tract Infections. Infect. Drug Resist. 2021, 14, 3275–3286. [Google Scholar] [CrossRef]
- Szlachta-McGinn, A.; Douglass, K.M.; Chung, U.Y.R.; Jackson, N.J.; Nickel, J.C.; Ackerman, A.L. Molecular Diagnostic Methods Versus Conventional Urine Culture for Diagnosis and Treatment of Urinary Tract Infection: A Systematic Review and Meta-Analysis. Eur. Urol. Open Sci. 2022, 44, 113–124. [Google Scholar] [CrossRef]
- Multiplex Panel Capabilities for Real-Time PCR Workflows|Thermo Fisher Scientific—US. Available online: https://www.thermofisher.com/us/en/home/clinical/clinical-genomics/pathogen-detection-solutions/custom-capabilities/multiplex-panels.html#antimicrobial (accessed on 7 October 2025).
- Bedenić, B.; Meštrović, T. Mechanisms of Resistance in Gram-Negative Urinary Pathogens: From Country-Specific Molecular Insights to Global Clinical Relevance. Diagnostics 2021, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Cross, B.J.; Partridge, S.R.; Sheppard, A.E. Impacts of Mobile Genetic Elements on Antimicrobial Resistance Genes in Gram-Negative Pathogens: Current Insights and Genomic Approaches. Microbiol. Res. 2026, 302, 128340. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.S.-C.; Lukacz, E.S.; Juster, I.A.; Niecko, T.; Ashok, A.; Vollstedt, A.J.; Baunoch, D.; Mathur, M. Real-World Evidence That a Novel Diagnostic Combining Molecular Testing with Pooled Antibiotic Susceptibility Testing Is Associated with Reduced Infection Severity and Lower Cost Compared with Standard Urine Culture in Patients with Complicated or Persistently Recurrent Urinary Tract Infections. JU Open Plus 2023, 1, e00021. [Google Scholar] [CrossRef]
- Haley, E.; Luke, N.; Korman, H.; Rao, G.S.; Baunoch, D.; Chen, X.; Havrilla, J.; Mathur, M. Comparing Prescribing Behaviors and Clinician Experiences Between Multiplex PCR/Pooled Antibiotic Susceptibility Testing and Standard Urine Culture in Complicated UTI Cases. J. Clin. Med. 2024, 13, 7453. [Google Scholar] [CrossRef]
- Siegman-Igra, Y. The Significance of Urine Culture with Mixed Flora. Curr. Opin. Nephrol. Hypertens. 1994, 3, 656–659. [Google Scholar] [CrossRef]
- Festa, R.A.; Cockerill, F.R.; Pesano, R.L.; Haley, E.; Luke, N.; Mathur, M.; Chen, X.; Havrilla, J.; Percaccio, M.; Magallon, J.; et al. Pooled Antibiotic Susceptibility Testing for Polymicrobial UTI Performs Within CLSI Validation Standards. Antibiotics 2025, 14, 143. [Google Scholar] [CrossRef]
- Haley, E.; Cockerill, F.R.; Pesano, R.L.; Festa, R.A.; Luke, N.; Mathur, M.; Chen, X.; Havrilla, J.; Baunoch, D. Pooled Antibiotic Susceptibility Testing Performs Within CLSI Standards for Validation When Measured Against Broth Microdilution and Disk Diffusion Antibiotic Susceptibility Testing of Cultured Isolates. Antibiotics 2024, 13, 1214. [Google Scholar] [CrossRef]
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing, 35th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025.
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 12th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024.
- CLSI Standard M02; Performance Standards for Antimicrobial Disk Susceptibility Tests, 14th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024.
- CLSI Guideline M52; Verification of Commercial Microbial Identification and Antimicrobial Susceptibility Testing Systems, 1st ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015.
- CLSI Breakpoint Implementation Toolkit (BIT)|Resources|CLSI. Available online: https://clsi.org/resources/breakpoint-implementation-toolkit/ (accessed on 6 August 2025).
- Sievert, D.M.; Rudrik, J.T.; Patel, J.B.; McDonald, L.C.; Wilkins, M.J.; Hageman, J.C. Vancomycin-Resistant Staphylococcus Aureus in the United States, 2002–2006. Clin. Infect. Dis. 2008, 46, 668–674. [Google Scholar] [CrossRef]
- Brennan, B.; McNamara, S.; McCullor, K.; Soehnlen, M.; Campbell, D.; Gargis, A.S.; Halpin, A.L.; Karlsson, M.; Walters, M.S.; Ham, D.C. Investigation and Laboratory Characterization of the Fifteenth U.S. Case of Vancomycin-Resistant Staphylococcus aureus—Michigan, 2021. Infect. Control Hosp. Epidemiol. 2025, 46, 954–955. [Google Scholar] [CrossRef]
- Kass, E. Asymptomatic Infections of the Urinary Tract. Trans. Assoc. Am. Physicians 1956, 69, 56–64. [Google Scholar]
- Kass, E.H. Bacteriuria and Pyelonephritis of Pregnancy. Arch. Intern. Med. 1960, 105, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Chai, T.C.; Horsley, H.; Khasriya, R.; Moreland, R.B.; Wolfe, A. Tarnished Gold—The “Standard” Urine Culture: Reassessing the Characteristics of a Criterion Standard for Detecting Urinary Microbes. Front. Urol. 2023, 3, 1206046. [Google Scholar] [CrossRef] [PubMed]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques to Detect Resident Bacterial Flora in the Adult Female Bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.; Favour, C.; Mao, F.; Harrison, J. Evaluation of the “Positive” Urine Culture: An Approach to the Differentiation of Significant Bacteria from Contaminants. Am. J. Med. 1956, 20, 88–93. [Google Scholar] [CrossRef]
- Complicated Urinary Tract Infections—StatPearls—NCBI Bookshelf. Available online: https://www.ncbi.nlm.nih.gov/books/NBK436013/ (accessed on 13 January 2023).
- Peck, J.; Shepherd, J.P. Recurrent Urinary Tract Infections Diagnosis, Treatment, and Prevention. Obstet. Gynecol. Clin. N. Am. 2021, 48, 501–513. [Google Scholar] [CrossRef]
- Vazquez-Montes, M.D.L.A.; Fanshawe, T.R.; Stoesser, N.; Walker, A.S.; Butler, C.; Hayward, G. Epidemiology and Microbiology of Recurrent UTI in Women in the Community in Oxfordshire, UK. JAC-Antimicrob. Resist. 2024, 6, dlad156. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Pilatz, A.; Weidner, W. Urosepsis—From the View of the Urologist. Int. J. Antimicrob. Agents 2011, 38, 51–57. [Google Scholar] [CrossRef]
- Waller, T.A.; Pantin, S.A.L.; Yenior, A.L.; Pujalte, G.G.A. Urinary Tract Infection Antibiotic Resistance in the United States. Prim. Care 2018, 45, 455–466. [Google Scholar] [CrossRef]
- Matthews, S.J.; Lancaster, J.W. Urinary Tract Infections in the Elderly Population. Am. J. Geriatr. Pharmacother. 2011, 9, 286–309. [Google Scholar] [CrossRef]
- Liu, F.; Ling, Z.; Xiao, Y.; Yang, Q.; Zheng, L.; Jiang, P.; Li, L.; Wang, W. Characterization of the Urinary Microbiota of Elderly Women and the Effects of Type 2 Diabetes and Urinary Tract Infections on the Microbiota. Oncotarget 2017, 8, 100678–100690. [Google Scholar] [CrossRef]
- Fagan, M.; Lindbæk, M.; Grude, N.; Reiso, H.; Romøren, M.; Skaare, D.; Berild, D. Antibiotic Resistance Patterns of Bacteria Causing Urinary Tract Infections in the Elderly Living in Nursing Homes versus the Elderly Living at Home: An Observational Study. BMC Geriatr. 2015, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Paul, M.; Tiseo, G.; Yahav, D.; Prendki, V.; Friberg, L.E.; Guerri, R.; Gavazzi, G.; Mussini, C.; Tinelli, M.; et al. Considerations for the Optimal Management of Antibiotic Therapy in Elderly Patients. J. Glob. Antimicrob. Resist. 2020, 22, 325–333. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L. Urinary Tract Infections in the Elderly: A Review of Disease Characteristics and Current Treatment Options. Drugs Context 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.K.; Verma, S. Quality of Life in Women with Urinary Tract Infections: Is Benign Disease a Misnomer? J. Am. Board Fam. Pract. 2000, 13, 392–397. [Google Scholar] [CrossRef]
- Thompson, J.; Marijam, A.; Mitrani-Gold, F.S.; Wright, J.; Joshi, A.V. Activity Impairment, Health-Related Quality of Life, Productivity, and Self-Reported Resource Use and Associated Costs of Uncomplicated Urinary Tract Infection among Women in the United States. PLoS ONE 2023, 18, e0277728. [Google Scholar] [CrossRef]
- Ernst, E.J.; Ernst, M.E.; Hoehns, J.D.; Bergus, G.R. Women’s Quality of Life Is Decreased by Acute Cystitis and Antibiotic Adverse Effects Associated with Treatment. Health Qual. Life Outcomes 2005, 3, 45. [Google Scholar] [CrossRef]
- Grigoryan, L.; Mulgirigama, A.; Powell, M.; Schmiemann, G. The Emotional Impact of Urinary Tract Infections in Women: A Qualitative Analysis. BMC Women’s Health 2022, 22, 182. [Google Scholar] [CrossRef]
- Renard, J.; Ballarini, S.; Mascarenhas, T.; Zahran, M.; Quimper, E.; Choucair, J.; Iselin, C.E. Recurrent Lower Urinary Tract Infections Have a Detrimental Effect on Patient Quality of Life: A Prospective, Observational Study. Infect. Dis. Ther. 2015, 4, 125–135. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Wullt, B.; Ballarini, S.; Zingg, D.; Naber, K.G. Social and Economic Burden of Recurrent Urinary Tract Infections and Quality of Life: A Patient Web-Based Study (GESPRIT). Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 107–117. [Google Scholar] [CrossRef]
- Naber, K.G.; Tirán-Saucedo, J.; Wagenlehner, F.M.E.; RECAP Group. Psychosocial Burden of Recurrent Uncomplicated Urinary Tract Infections. GMS Infect. Dis. 2022, 10, Doc01. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.C.S.; Thum, L.W.; Sadun, T.; Markowitz, M.; Maliski, S.L.; Ackerman, A.L.; Anger, J.T.; Kim, J.-H. Fear and Frustration Among Women with Recurrent Urinary Tract Infections: Findings from Patient Focus Groups. J. Urol. 2021, 206, 688–695. [Google Scholar] [CrossRef]
- Maxwell, K.; Roberts, L.; Kramer, M.; Price, J.; Newlands, A.; Finlay, K.A. Psychosocial Burden and Healthcare Disillusionment in Recurrent UTI: A Large-Scale International Survey of Patient Perspectives. Front. Urol. 2023, 3, 1264299. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Roberts, L.; Kramer, M.; Finlay, K. Using the Working Model of Adjustment to Chronic Illness to Explain the Burden of Recurrent Urinary Tract Infection: A Survey-Based Study. Int. J. Pharm. Pract. 2021, 29, i5–i6. [Google Scholar] [CrossRef]
- O’Brien, M.; Marijam, A.; Mitrani-Gold, F.S.; Terry, L.; Taylor-Stokes, G.; Joshi, A.V. Unmet Needs in Uncomplicated Urinary Tract Infection in the United States and Germany: A Physician Survey. BMC Infect. Dis. 2023, 23, 281. [Google Scholar] [CrossRef] [PubMed]
- Price, T.K.; Hilt, E.E.; Dune, T.J.; Mueller, E.R.; Wolfe, A.J.; Brubaker, L. Urine Trouble: Should We Think Differently about UTI? Int. Urogynecol. J. 2018, 29, 205–210. [Google Scholar] [CrossRef]
- Moreland, R.B.; Choi, B.I.; Geaman, W.; Gonzalez, C.; Hochstedler-Kramer, B.R.; John, J.; Kaindl, J.; Kesav, N.; Lamichhane, J.; Lucio, L.; et al. Beyond the Usual Suspects: Emerging Uropathogens in the Microbiome Age. Front. Urol. 2023, 3, 1212590. [Google Scholar] [CrossRef]
- Price, T.K.; Dune, T.; Hilt, E.E.; Thomas-White, K.J.; Kliethermes, S.; Brincat, C.; Brubaker, L.; Wolfe, A.J.; Mueller, E.R.; Schreckenberger, P.C. The Clinical Urine Culture: Enhanced Techniques Improve Detection of Clinically Relevant Microorganisms. J. Clin. Microbiol. 2016, 54, 1216–1222. [Google Scholar] [CrossRef]
- Hooton, T.M.; Roberts, P.L.; Cox, M.E.; Stapleton, A.E. Voided Midstream Urine Culture and Acute Cystitis in Premenopausal Women. N. Engl. J. Med. 2013, 369, 1883–1891. [Google Scholar] [CrossRef]
- Brecher, S.M. Commentary: Complicated Urinary Tract Infections: What’s a Lab to Do? J. Clin. Microbiol. 2016, 54, 1189–1190. [Google Scholar] [CrossRef]
- Kline, K.A.; Lewis, A.L. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Hansen, M.A.; Valentine-King, M.; Zoorob, R.; Schlueter, M.; Matas, J.L.; Willis, S.E.; Danek, L.C.K.; Muldrew, K.L.; Zare, M.; Hudson, F.; et al. Prevalence and Predictors of Urine Culture Contamination in Primary Care: A Cross-Sectional Study. Int. J. Nurs. Stud. 2022, 134, 104325. [Google Scholar] [CrossRef]
- Whelan, P.S.; Nelson, A.; Kim, C.J.; Tabib, C.; Preminger, G.M.; Turner, N.A.; Lipkin, M.; Advani, S.D. Investigating Risk Factors for Urine Culture Contamination in Outpatient Clinics: A New Avenue for Diagnostic Stewardship. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e29. [Google Scholar] [CrossRef] [PubMed]
- Moreland, R.B.; Brubaker, L.; Wolfe, A.J. Polymicrobial Urine Cultures: Reconciling Contamination with the Urobiome While Recognizing the Pathogens. Front. Cell Infect. Microbiol. 2025, 15, 1562687. [Google Scholar] [CrossRef]
- Kunin, C.; White, L.; Hua, T. A Reassessment of the Importance of “Low-Count” Bacteriuria in Young Women with Acute Urinary Symptoms. Ann. Intern. Med. 1993, 119, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Parnell, L.K.D.; Luke, N.; Mathur, M.; Festa, R.A.; Haley, E.; Wang, J.; Jiang, Y.; Anderson, L.; Baunoch, D. Elevated UTI Biomarkers in Symptomatic Patients with Urine Microbial Densities of 10,000 CFU/mL Indicate a Lower Threshold for Diagnosing UTIs. Diagnostics 2023, 13, 2688. [Google Scholar] [CrossRef] [PubMed]
- Hilt, E.E.; Parnell, L.K.; Wang, D.; Stapleton, A.E.; Lukacz, E.S. Microbial Threshold Guidelines for UTI Diagnosis: A Scoping Systematic Review. Pathol. Lab. Med. Int. 2023, 15, 43–63. [Google Scholar] [CrossRef]
- Al-Shebiny, A.; Shawky, R.; Emara, M. Heteroresistance: A Gray Side of Antimicrobial Susceptibility Testing. J. Adv. Pharm. Res. 2023, 7, 101–110. [Google Scholar] [CrossRef]
- Vollstedt, A.; Baunoch, D.; Wolfe, A.; Luke, N.; Wojno, K.J.; Cline, K.; Belkoff, L.; Milbank, A.; Sherman, N.; Haverkorn, R.; et al. Bacterial Interactions as Detected by Pooled Antibiotic Susceptibility Testing (P-AST) in Polymicrobial Urine Specimens. J. Surg. Urol. 2020, 1, 101. [Google Scholar]
- De Vos, M.G.; Zagorski, M.; McNally, A.; Bollenbach, T. Interaction Networks, Ecological Stability, and Collective Antibiotic Tolerance in Polymicrobial Infections. Proc. Natl. Acad. Sci. USA 2017, 114, 10666–10671. [Google Scholar] [CrossRef]
- Gaston, J.R.; Johnson, A.O.; Bair, K.L.; White, A.N.; Armbruster, C.E. Polymicrobial Interactions in the Urinary Tract: Is the Enemy of My Enemy My Friend? Infect. Immun. 2021, 89, e00652-20. [Google Scholar] [CrossRef]
- Galván, E.M.; Mateyca, C.; Ielpi, L. Role of Interspecies Interactions in Dual-Species Biofilms Developed In Vitro by Uropathogens Isolated from Polymicrobial Urinary Catheter-Associated Bacteriuria. Biofouling 2016, 32, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Zandbergen, L.E.; Halverson, T.; Brons, J.K.; Wolfe, A.J.; de Vos, M.G.J. The Good and the Bad: Ecological Interaction Measurements Between the Urinary Microbiota and Uropathogens. Front. Microbiol. 2021, 12, 659450. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Granados, M.C.; Zenick, B.; Englander, H.E.; Mok, W.W.K. The Social Network: Impact of Host and Microbial Interactions on Bacterial Antibiotic Tolerance and Persistence. Cell. Signal. 2020, 75, 109750. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mo, X.; Zhang, H.; Wan, F.; Luo, Q.; Xiao, Y. Epidemiology, mechanisms, and clinical impact of bacterial heteroresistance. NPJ Antimicrob. Resist. 2025, 3, 7. [Google Scholar] [CrossRef]
- Korman, H.J.; Baunoch, D.; Luke, N.; Wang, D.; Zhao, X.; Levin, M.; Wenzler, D.L.; Mathur, M. A Diagnostic Test Combining Molecular Testing with Phenotypic Pooled Antibiotic Susceptibility Improved the Clinical Outcomes of Patients with Non-E. coli or Polymicrobial Complicated Urinary Tract Infections. Res. Rep. Urol. 2023, 15, 141–147. [Google Scholar] [CrossRef]
- Festa, R.A.; Luke, N.; Mathur, M.; Parnell, L.; Wang, D.; Zhao, X.; Magallon, J.; Remedios-Chan, M.; Nguyen, J.; Cho, T.; et al. A Test Combining Multiplex-PCR with Pooled Antibiotic Susceptibility Testing Has High Correlation with Expanded Urine Culture for Detection of Live Bacteria in Urine Samples of Suspected UTI Patients. Diagn. Microbiol. Infect. Dis. 2023, 107, 116015. [Google Scholar] [CrossRef]
- Haley, E.; Luke, N.; Mathur, M.; Festa, R.A.; Wang, J.; Jiang, Y.; Anderson, L.; Baunoch, D. Comparison Shows That Multiplex Polymerase Chain Reaction Identifies Infection-Associated Urinary Biomarker–Positive Urinary Tract Infections That Are Missed by Standard Urine Culture. Eur. Urol. Open Sci. 2023, 58, 73–81. [Google Scholar] [CrossRef]
- Wojno, K.J.; Baunoch, D.; Luke, N.; Opel, M.; Korman, H.; Kelly, C.; Jafri, S.M.A.; Keating, P.; Hazelton, D.; Hindu, S.; et al. Multiplex PCR Based Urinary Tract Infection (UTI) Analysis Compared to Traditional Urine Culture in Identifying Significant Pathogens in Symptomatic Patients. Urology 2020, 136, 119–126. [Google Scholar] [CrossRef]
- Korman, H.J.; Mathur, M.; Luke, N.; Wang, D.; Zhao, X.; Levin, M.; Wenzler, D.L.; Baunoch, D. Multiplex Polymerase Chain Reaction/Pooled Antibiotic Susceptibility Testing Was Not Associated with Increased Antibiotic Resistance in Management of Complicated Urinary Tract Infections. Infect. Drug Resist. 2023, 16, 2841–2848. [Google Scholar] [CrossRef]
- Vollstedt, A.; Baunoch, D.; Wojno, K.; Luke, N.; Cline, K.; Belkoff, L.; Sirls, L. Multisite Prospective Comparison of Multiplex Polymerase Chain Reaction Testing with Urine Culture for Diagnosis of Urinary Tract Infections in Symptomatic Patients. J. Sur. Urol. 2020, JSU-102. [Google Scholar] [CrossRef]
- Daly, A.; Baunoch, D.; Rehling, K.; Luke, N.; Campbell, M.; Cacdac, P.; Penaranda, M.; Opel, M.; Huang, S.; Zhao, X. Utilization of M-PCR and P-AST for Diagnosis and Management of Urinary Tract Infections in Home-Based Primary Care. JOJ Uro Nephron 2020, 7, 555707. [Google Scholar]
- Barnes, H.C.; Wolff, B.; Abdul-Rahim, O.; Harrington, A.; Hilt, E.E.; Price, T.K.; Halverson, T.; Hochstedler, B.R.; Pham, T.; Acevedo-Alvarez, M.; et al. A Randomized Clinical Trial of Standard Versus Expanded Cultures to Diagnose Urinary Tract Infections in Women. J. Urol. 2021, 206, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Bhalodi, A.A.; Oppermann, N.; Campeau, S.A.; Humphries, R.M. Variability of Beta-Lactam Broth Microdilution for Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2021, 65, e0064021. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K.; Hardy, D.; Zimmer, B.; et al. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef]
- Elias, R.; Melo-Cristino, J.; Lito, L.; Pinto, M.; Gonçalves, L.; Campino, S.; Clark, T.G.; Duarte, A.; Perdigão, J. Klebsiella Pneumoniae and Colistin Susceptibility Testing: Performance Evaluation for Broth Microdilution, Agar Dilution and Minimum Inhibitory Concentration Test Strips and Impact of the “Skipped Well” Phenomenon. Diagnostics 2021, 11, 2352. [Google Scholar] [CrossRef]
- Reszetnik, G.; Hammond, K.; Mahshid, S.; AbdElFatah, T.; Nguyen, D.; Corsini, R.; Caya, C.; Papenburg, J.; Cheng, M.P.; Yansouni, C.P. Next-Generation Rapid Phenotypic Antimicrobial Susceptibility Testing. Nat. Commun. 2024, 15, 9719. [Google Scholar] [CrossRef]
- Haley, E.; Luke, N.; Mathur, M.; Festa, R.A.; Wang, J.; Jiang, Y.; Anderson, L.A.; Baunoch, D. The Prevalence and Association of Different Uropathogens Detected by M-PCR with Infection-Associated Urine Biomarkers in Urinary Tract Infections. Res. Rep. Urol. 2024, 16, 19–29. [Google Scholar] [CrossRef]
- Leber, A.L. Clinical Microbiology Procedures Handbook; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Gentamicin—Injection Products|FDA. Available online: https://www.fda.gov/drugs/development-resources/gentamicin-injection-products (accessed on 19 September 2025).
| Microorganism Analysis Group | Remnant Clinical Specimens | Challenge Stocks n (%) | Total Specimens n | |
|---|---|---|---|---|
| Fresh n (%) | Frozen (Biobanked) n (%) | |||
| Monomicrobial | ||||
| Enterobacterales | 60 (75) | 17 (21) | 3 (4) | 80 |
| Enterococci | 35 (58) | 25 (42) | 0 | 60 |
| Staphylococci | 24 (50) | 14 (29) | 10 (21) | 48 |
| P. aeruginosa | 22 (47) | 18 (38) | 7 (15) | 47 |
| Acinetobacter spp. | 3 (8) | 4 (10) | 32 (82) | 39 |
| Polymicrobial | ||||
| Enterobacterales | 44 (63) | 6 (9) | 20 (29) | 70 |
| Enterococci | 38 (76) | 6 (12) | 6 (12) | 50 |
| Staphylococci | 7 (23) | 2 (6) | 22 (71) | 31 |
| P. aeruginosa | 8 (25) | 0 | 24 (75) | 32 |
| Acinetobacter spp. | 2 (6) | 0 | 30 (94) | 32 |
| P-AST Performance Metric | Calculation Formula |
|---|---|
| Categorical Agreement (%) | NCA/NT × 100 |
| Minor Errors (%) | NmE/NT × 100 |
| Minor Errors with Essential Agreement (%) | NmEEA/NME × 100 |
| Very Major Errors (%) | NVME/NRefR × 100 |
| Major Errors (%) | NME/NRefS × 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Festa, R.A.; Cockerill, F.R.; Pesano, R.L.; Haley, E.; Luke, N.; Mathur, M.; Chen, X.; Havrilla, J.; Percaccio, M.; Rosas, A.; et al. Full Validation of Pooled Antibiotic Susceptibility Testing Using CLSI Methods and Performance Criteria in UTI Pathogens. Antibiotics 2025, 14, 1168. https://doi.org/10.3390/antibiotics14111168
Festa RA, Cockerill FR, Pesano RL, Haley E, Luke N, Mathur M, Chen X, Havrilla J, Percaccio M, Rosas A, et al. Full Validation of Pooled Antibiotic Susceptibility Testing Using CLSI Methods and Performance Criteria in UTI Pathogens. Antibiotics. 2025; 14(11):1168. https://doi.org/10.3390/antibiotics14111168
Chicago/Turabian StyleFesta, Richard A., Frank R. Cockerill, Rick L. Pesano, Emery Haley, Natalie Luke, Mohit Mathur, Xiaofei Chen, Jim Havrilla, Michael Percaccio, Alain Rosas, and et al. 2025. "Full Validation of Pooled Antibiotic Susceptibility Testing Using CLSI Methods and Performance Criteria in UTI Pathogens" Antibiotics 14, no. 11: 1168. https://doi.org/10.3390/antibiotics14111168
APA StyleFesta, R. A., Cockerill, F. R., Pesano, R. L., Haley, E., Luke, N., Mathur, M., Chen, X., Havrilla, J., Percaccio, M., Rosas, A., Magallon, J., Erickson, S., Ghashghaie, M., Sinatra, J., Gonzalez, V., & Baunoch, D. (2025). Full Validation of Pooled Antibiotic Susceptibility Testing Using CLSI Methods and Performance Criteria in UTI Pathogens. Antibiotics, 14(11), 1168. https://doi.org/10.3390/antibiotics14111168








