2.1. Structural Characterization of Modified Analogues of NaD1
Previously, it has been shown that PIP4,5 binding and the tobacco defensin NaD1 oligomerization play an important role in fungal cell killing and are critical for cytotoxic activity of the peptide [
10]. The NaD1 oligomer is formed as a horseshoe-like assembly of seven NaD1 dimers cooperatively binding 14 PIP4,5 molecules. The NaD1 dimer contains a “cationic grip” capable of interacting with anionic headgroups of PIP4,5. The “cationic grip” and interaction with PIP4,5 are carried out by the amino acid residues K4 and H33, as well as by K36, I37, L38, and R40 in the L5 loop (S
35KILRR
40) of NaD1 [
11]. It has been shown, that NaD1 is also able to interact with phosphatidic acid (PA), forming nearly flat, carpet-like larger oligomeric protein–lipid complexes. These complexes are considered to be weaker compared to the NaD1–PIP4,5 complexes, since PA lacks the inositol head group and S35, K36 I37, L38, R39, and R40 of the peptide take part in PA binding [
15].
Previously, mutant analogues of NaD1 containing substitutions of the basic amino acid residues from L5 loop, involved in the oligomerization of the peptide and its interaction with target lipids, with alanine or acidic amino acid residues were obtained. Such substitution typically resulted in a decrease in the lipid binding capacity of NaD1 and its cytotoxic properties but also led to a decrease in the antifungal activity of tobacco defensin. For example, the modified analogues NaD1 K36E and NaD1 R39A lost the ability to form oligomers with PA and exhibited reduced antifungal activity against
C. albicans [
15]. The Arg40 substitution with glutamic acid resulted in a decrease, but not a loss, of the peptide ability to bind PIP4,5, but was critical for its oligomerization and the ability to inhibit growth of
F. oxysporum [
11]. A chimeric peptide containing the replacement of the L5 loop part (R
35GFR
38 instead of S
35KIL
38 by analogy with the tobacco defensin NaD2 (
Figure 1) did not bind PIP4,5, and was less active against
Fusarium oxysporum and tumour cells, as compared to NaD1 [
14]. We aimed to develop modified analogues of NaD1 with high antifungal activity and reduced cytotoxicity relative to the tobacco defensin.
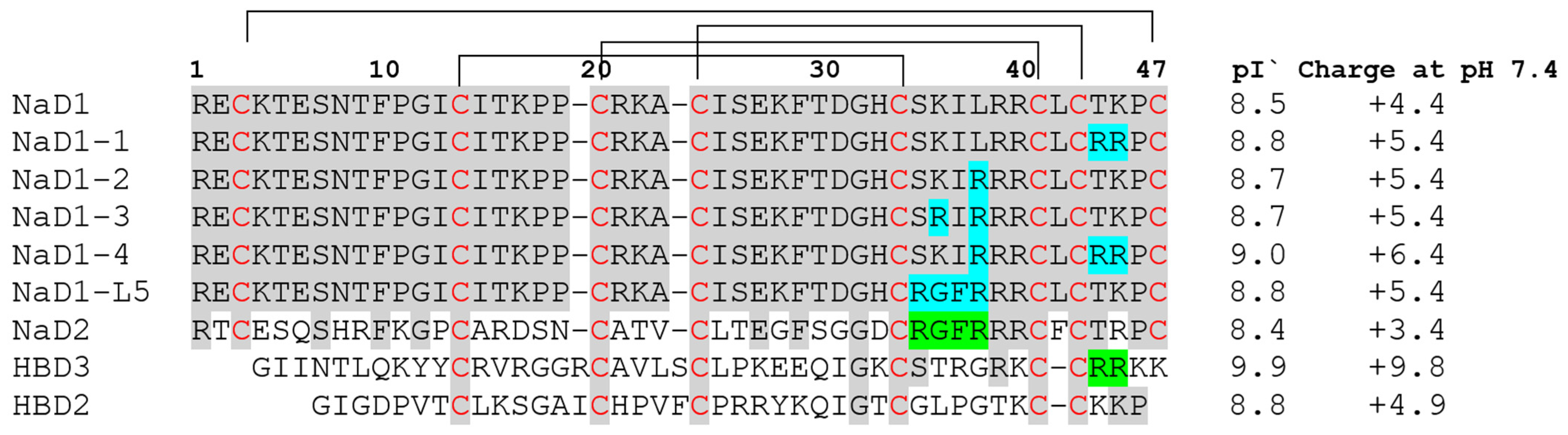
Here, we investigated modified analogues of the tobacco defensin NaD1 containing substitutions with arginine of amino acid residues in the L5 loop responsible for the “cationic grip” and binding of PIP4,5 as well as in the nearby C-terminal region of the peptide. It is well known that substitutions with arginine enhance the cationic properties of AMPs that lead to an increased antimicrobial activity by improving their ability to penetrate the cytoplasmic membrane and increasing their tolerance to high salt concentrations [
16,
17]. We hypothesized that the substitution of various amino acids with arginine may affect the conformation of the “cationic grip” and the affinity of NaD1 for PIP4,5. Nonetheless, these effects might not be crucial for antifungal activity, as they could be offset by additional positive charges and possible changes in the peptide mechanism of action. As is known, some human β-defensins have a spatial structure similar to that of plant defensins and also bind to PIP4,5 [
6,
18,
19]. It has been shown that the C-terminal Arg42 and Arg43, which are absent in the structure of the human β-defensin HBD2, are responsible for a more pronounced antimicrobial activity and a lower sensitivity to the presence of salts of the human β-defensin HBD3 (
Figure 1) [
20]. Therefore, we obtained the analogue NaD1-1 T44R/K45R, suggesting the possibility that this peptide will be more active than NaD1. The analogues NaD1-2 L38R and NaD1-3 K36R/L38R contained the substitutions in the “cationic grip”. The combined analogue NaD1-4 L38R/T44R/K45R with substitutions both in the C-terminal region of NaD1 and in the L5 loop was also studied (
Figure 1). The chimeric peptide R
35GFR
38, designated as NaD1-L5, was also obtained and used in all experiments as a comparison (
Figure 1).
In order to determine the effects of amino acid substitutions on the peptide structure, computer modelling by using the mutagenesis wizard tool in PyMOL was performed. The spatial structure of the tobacco defensin NaD1 (PDB 1MR4), previously solved by NMR in solution, was used as a template in the current study (
Figure 2A). Computer visualization of modified analogues of NaD1 demonstrated that all amino acid substitutions had no significant effect on the overall peptide structure (
Figure 2A–F). However, the T44R/K45R substitutions in NaD1-1 also caused minor structural changes in the β3-sheet and affected such amino acid residues as Leu42 and Cys43 (
Figure 2G). Moreover, even a single L38R substitution in NaD1-2 caused minor conformational changes that also influenced other amino acid residues in the L5 loop including Lys36, Ile37, and Arg39 (
Figure 2H). It was also noted that all of the introduced positively charged arginine residues were located on the surface of the peptide structure (
Figure 2B–F). Arg44 and Arg45 in the C-terminal regions of NaD1-1 and NaD1-4 were oriented in different directions on the surface of the modified analogues; a similar phenomenon was observed for Arg36 and Arg38 in NaD1-3, which were introduced into the L5 loop (
Figure 2B,E). It is worth noting that PyMOL does not modify the underlying scaffold and does not consider steric clashes and therefore may not fully capture all changes in the peptide structure.
The modified analogues NaD1-(1-4) as well as NaD1-L5 were obtained by heterologous expression in
E. coli cells (
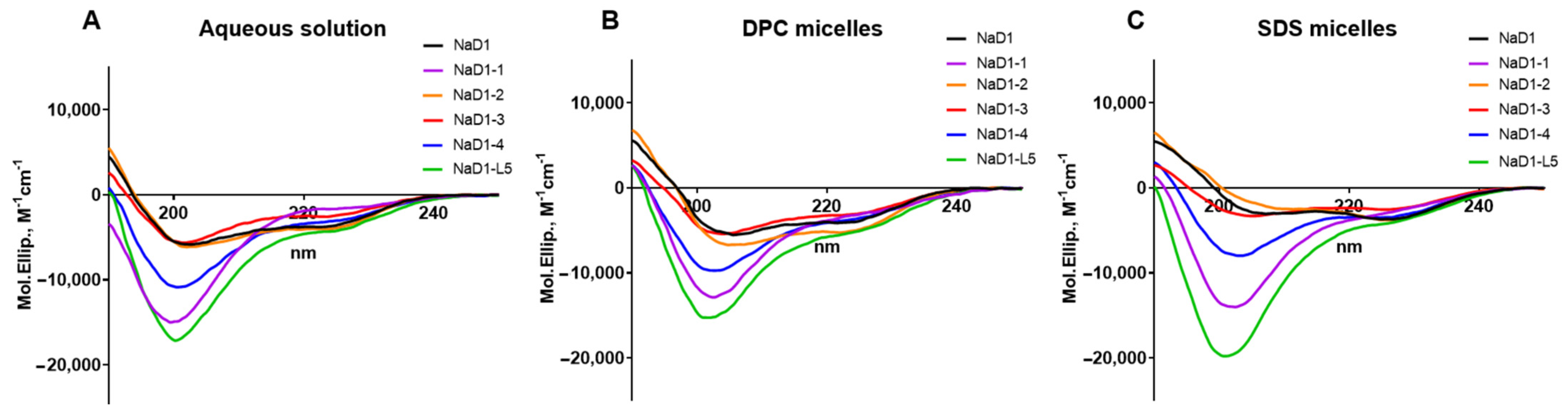
Supplementary Materials, Figure S1–S4, Table S1). The secondary structure of NaD1 and its analogues in an aqueous solution and in the presence of DPC or SDS micelles was examined by circular dichroism (CD) spectroscopy (
Figure 3,
Table 1). The far-UV CD spectra of all peptides in aqueous solution are characterized by a high content of β-sheet structure and a low content of α-helices. No significant differences were observed in the secondary structures of the obtained peptides under these conditions, although NaD1-L5 exhibited a higher percentage of α-helices (
Figure 3A,
Table 1). However, the secondary structure of NaD1 and its modified analogues changed differently in the presence of detergent micelles. In the presence of zwitterionic DPC micelles, the secondary structures of NaD1 and NaD1-3 remained virtually unchanged, whereas an increase in the proportion of α-helices was found for NaD1-1, NaD1-2, NaD1-4, and NaD1-L5 (
Figure 3B). Surprisingly, in the presence of anionic SDS micelles, the proportion of α-helices increased for all peptides, except NaD1-3 for which a small increase in β-sheet content was recorded (
Figure 3C). We proposed that the interaction of the NaD1-3 analogue with detergent micelles and cell membranes may occur in a different manner compared to those of the tobacco defensin and other modified analogues possibly due to the presence of Arg36 in its structure (
Figure 2D).
It is known that plant defensins, unlike many other AMPs, are characterized by high stability, due to their compact structure stabilized by four disulfide bonds [
6]. It was shown that NaD1 retains high antifungal activity after boiling or DTT treatment [
13]. In addition, tobacco defensin demonstrates significant resistance to proteolytic enzymes of the human gastrointestinal tract [
21]. Here, we investigated the stability of NaD1 analogues by using trypsin and chymotrypsin, digestive enzymes with differing cleavage specificities. For that purpose, peptides were incubated for 24 h at 50 mM ammonium bicarbonate buffer, pH 8.0, with or without protease. All modified analogues showed high stability to trypsin and chymotrypsin digestion for 24 h, similar to that of NaD1 (
Supplementary Materials, Figure S5).
2.2. Modified Analogues Effectively Inhibit the Growth of C. albicans Cells, but Act Less Fungicidally than NaD1
Using the two-fold serial dilution method, antifungal activities of the obtained analogues were tested against different
C. albicans strains: susceptible collection strain ATCC 18804; resistant to azoles and anidulafungin collection strain ATCC 10231 and clinical isolates 9.1 and 8.2 [
7]. All four analogues NaD1-(1-4) effectively inhibited fungal growth, and minimal inhibitory concentrations (MICs) of 6.25 μM were observed for strains ATCC 18804, ATCC 10231, and 9.1, as in the case of NaD1 (
Table 2;
Supplementary Materials, Figure S6A–F). The activities of NaD1-3 and NaD1-4 were lower against the resistant strain 8.2, with MIC values twice as high (12.5 μM). At the same time, actions of all analogues except NaD1-1, having no substitutions in the L5 loop, were less fungicidal, and higher minimal fungicidal concentrations (MFCs) were observed. This effect was less pronounced for the peptides NaD1-2 and NaD1-4 for which some MFC values were comparable to those of NaD1 (
Table 2). NaD1-L5 was overall the least active of all the peptides tested (
Table 2).
Previously, we have shown that NaD1 acted synergistically in combination with the conventional antimycotic caspofungin, which inhibits fungal cell wall biosynthesis, as well as with the membrane-acting human cathelicidin LL-37 [
7]. Here we investigated the combined action of NaD1-2, NaD1-3, and NaD1-4 with these antifungals against
C. albicans ATCC 18804. All three selected analogues acted synergistically with caspofungin, and the FICI value for NaD1-2 was lower than that of the tobacco defensin. At the same time, combinations of all three modified analogues with LL-37 acted less effectively, and only additive effects were observed in the case of NaD1-2 and NaD1-4.
Thus, we demonstrated that four modified analogues NaD1-(1-4), in general, effectively inhibited the growth of both susceptible and resistant strains of
C. albicans. However, all analogues except NaD1-1, having no substitutions in the L5 loop, involved in the “cationic grip”, are less fungicidal than NaD1. A difference in the effectiveness of some modified analogues against various strains of
C. albicans unlike NaD1 could be associated with variations in the lipid composition of the cell membranes of these susceptible and resistant fungi [
15]. Together with the different potencies of modified analogue combinations with caspofungin and LL-37 compared to NaD1, this fact indicates that the peptides may act differently.
2.3. Modified Analogues Differ from NaD1 in Their Sensitivity to Various Salts
The antifungal activity of many AMPs is markedly reduced in the presence of salts at physiological concentrations; however, the underlying mechanisms of this phenomenon remain incompletely understood. Previously, we demonstrated that the anticandidal activity of NaD1 decreased dramatically in the presence of different salts including 150 mM NaCl, 1.25 mM CaCl
2, and 1.25 mM MgCl
2 [
7]. In this work, we assessed the effects of these salts on the activity of modified analogues of NaD1 against
C. albicans ATCC 18804 in Sabouraud broth. NaCl was used at concentrations of 50, 100, and 150 mM since its content varies in different biological fluids (for example, the concentration of sodium chloride is much lower in vaginal fluid and saliva than in blood and interstitial fluid [
22,
23]). We showed that in the presence of 50 mM NaCl the activity of NaD1 and all its modified analogues, except NaD1-3, significantly decreased. The MIC values of NaD1 and NaD1-3 were 50 μM and 12.5 μM, respectively (
Table 3;
Supplementary Materials, Figure S7). A lower sensitivity of NaD1-3 to the presence of salt was also observed at 100 and 150 mM NaCl (MIC 50 μM), where the activity of other analogues and NaD1 itself was not detected (
Table 3). Interestingly, different effects were observed when calcium and magnesium salts were added to the growth medium. All analogues, except for NaD1-1, were less active than NaD1 in the presence of MgCl
2 (
Table 3). At the same time, all analogues in general and especially NaD1-4 (IC
50 and MIC are 12.5 and 50 μM, respectively) showed greater antifungal activity than NaD1 in a medium containing CaCl
2 (
Table 3). It has been shown that Ca
2+ and Mg
2+ ions can have diverse effects on the antimicrobial activity of various AMPs by influencing their interactions with cell membranes, promoting peptide aggregation, or, conversely, stabilizing their conformation [
24,
25].
Thus, we showed that modified analogues differ from NaD1 in their sensitivity to various salts, suggesting a different mode of action. The antifungal activity of NaD1-3 K36R/L38R decreased significantly less in the presence of sodium chloride at various concentrations than those of all other peptides tested. In our opinion, this correlated with the CD spectroscopy data which showed that NaD1-3 undergoes virtually no structural changes in the presence of zwitterionic and negatively charged micelles unlike all other peptides. We suggested that ionic interactions of NaD1-3 with the fungal cell wall or cell membrane are either stronger or play a less important role in its mechanism of action compared to NaD1 and other modified analogues. To gain insights into the mechanisms of action of the modified analogues of NaD1, we further examined their ability to disrupt the integrity of the fungal cell membrane, induce permeabilization of PIP4,5-containing liposomes, form oligomeric complexes, and bind to zymosan.
2.4. All Modified Analogues Are Able to Dimerize and Form Oligomeric Complexes in the Presence of PA and PIP4,5
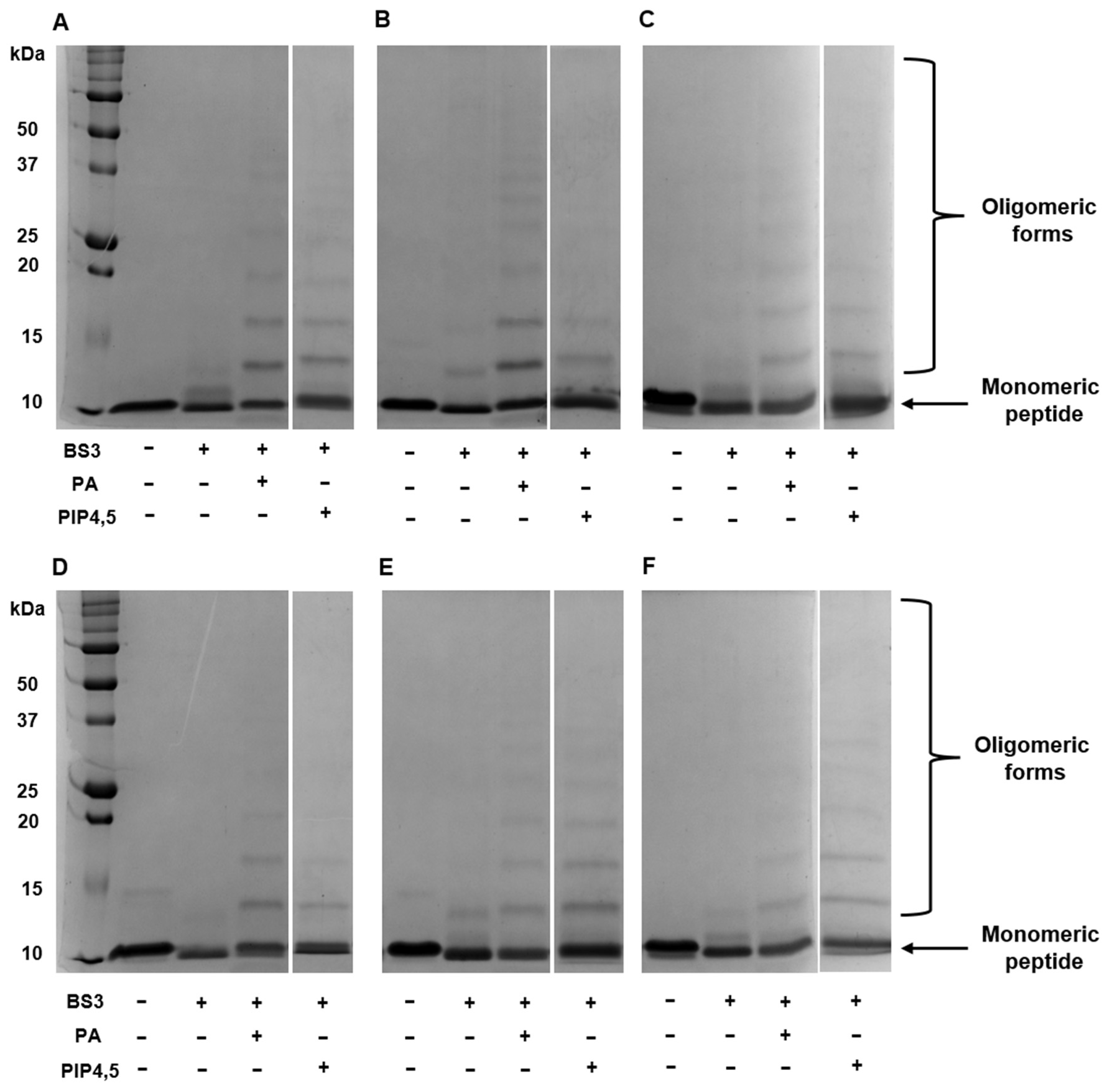
As mentioned above, dimerization and the formation of “cationic grip” play a key role in the binding of PIP4,5 and PA and the subsequent formation of oligomeric protein–lipid complexes. Here, we studied the influence of amino acid substitutions on the ability of modified analogues of NaD1 to form oligomeric complexes with PA and PIP4,5 using the cross-linker bis[sulfosuccinimidyl] suberate (BS3) and reducing SDS-PAGE as described earlier for the tobacco defensin [
26]. We showed that in the presence of PA, NaD1 and all its modified analogues, including NaD1-L5, formed large oligomeric assemblies as evidenced by the distinct laddering patterns in the electropherograms (
Figure 4A–F). In the presence of PIP4,5, NaD1 and all its modified analogues formed oligomers; however, high-order oligomeric aggregates were less pronounced in some cases, particularly with NaD1-1 and NaD1-2, compared to NaD1 (
Figure 4A,B,D). The formation of oligomeric complexes with PIP4,5 also occurred in the case of NaD1-L5, which, as was shown, practically did not bind PIP4,5 in experiments with lipid strips (
Figure 4C) [
14].
Thus, we concluded that arginine substitutions of amino acids from the C-terminal region of NaD1 and its L5 loop responsible for the “cationic grip” did not result in a loss of the ability of peptides to dimerize and form oligomeric complexes in the presence of lipids. However, in the case of PIP4,5, this effect was less pronounced for some analogues compared to PA.
2.5. All Modified Analogues Cause Calcein Leakage from PIP4,5-Containing Liposomes Less Effectively than NaD1
To determine the influence of amino acid substitutions on the ability of modified analogues of NaD1 to cause the permeabilization of artificial membranes, calcein release assays were conducted using liposomes of defined lipid composition. Calcein-loaded PC:PG liposomes in PBS, with or without 5% of PIP4,5 or PA, were used in these experiments. The effectiveness of the peptides was assessed by the release of calcein from liposomes relatively to 0.1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA), which was used as positive control. Melittin from bee venom, which exhibits high non-specific membranotropic activity, was used for comparison.
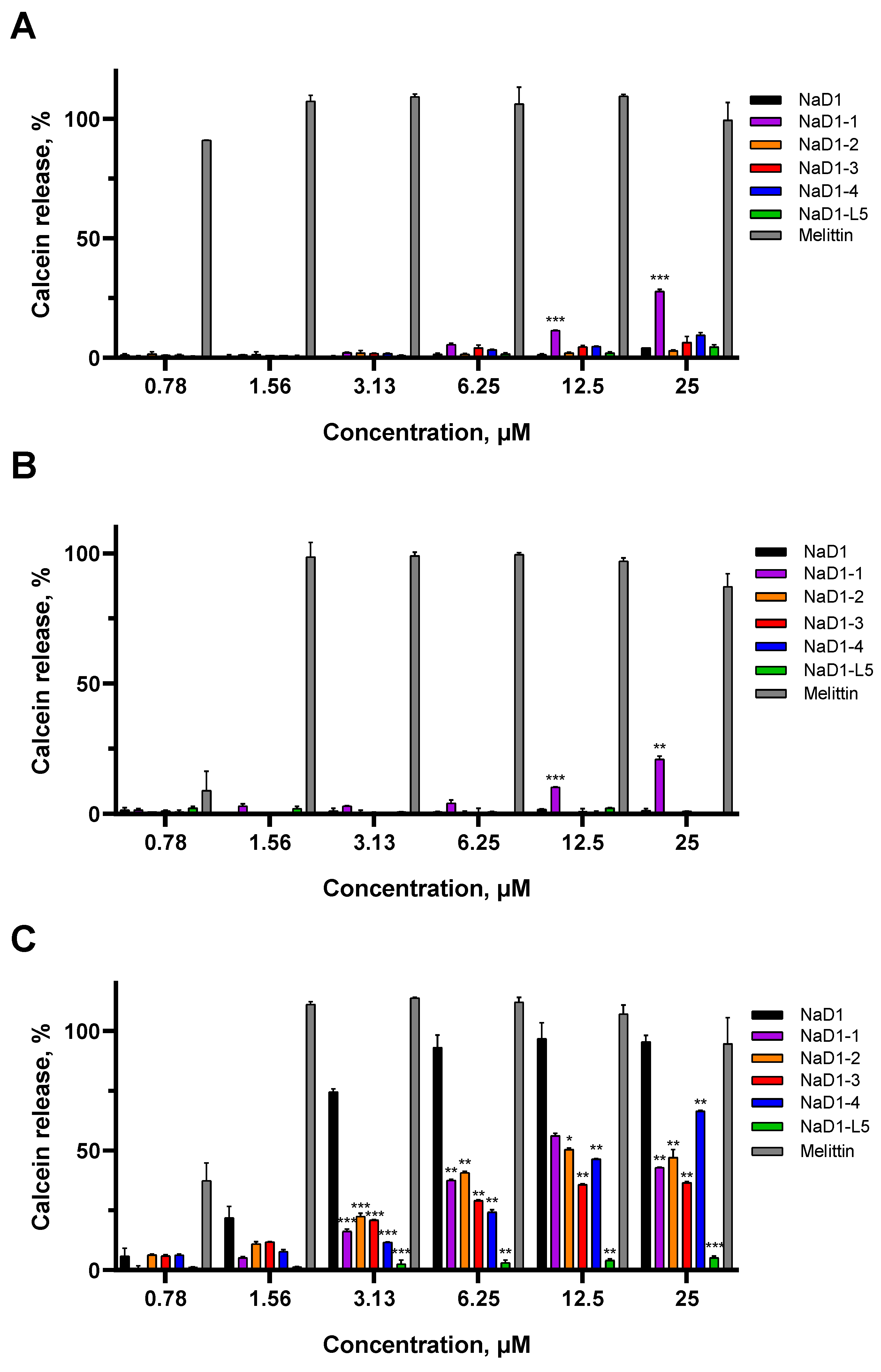
We showed that effects of NaD1 on artificial membranes in PBS which contained NaCl at a concentration of 150 mM were specific, as previously described [
10], since the peptide did not affect the PC:PG liposomes without a target lipid and caused almost 100% calcein release at a concentration of 6.25 μM from PIP4,5-containing liposomes (
Figure 5A,C). The absence of effects was observed with PA-containing liposomes, although NaD1 forms multimeric complexes with this lipid [
15]. Possibly, the lower stability of NaD1–PA complexes compared to those formed with PIP4,5 is the reason for the lack of permeabilizing activity in this case (
Figure 5B). In contrast, melittin at a concentration of 1.56 μM induced 100% calcein release from all liposomes tested, regardless of their lipid composition (
Figure 5A–C).
All modified analogues except NaD1-1 did not affect PC:PG or PC:PG:PA liposomes like NaD1. NaD1-1 slightly interacted with both PC:PG and PC:PG:PA liposomes and caused the release of approximately 28% and 21% of calcein, respectively, at a concentration of 25 μM (
Figure 5A,B). This effect was not observed for NaD1-4 which also contains Arg44 and Arg45 in its C-terminal region. Interestingly, all analogues interacted less efficiently with PIP4,5-containing liposomes than NaD1, with calcein release below 50% at a peptide concentration of 6.25 μM. NaD1-L5, as expected, showed virtually no permeabilizing activity (calcein release below 10% at 25 μM) (
Figure 5C) [
14]. The reduced ability of NaD1-1, which has no substitutions in the “cationic grip”, to disrupt the permeability of PIP4,5-containing liposomes is consistent with its diminished capacity to form oligomers in the presence of this lipid. Possibly, the T44R/K45R substitutions lead to conformational changes in the NaD1 structure, reducing its ability to oligomerize.
Thus, all modified analogues, except NaD1-1, disrupted the permeability of only PIP4,5-containing liposomes in PBS, similar to NaD1. NaD1-1 also exhibited weak non-specific permeabilizing activity, causing calcein leakage from PC:PG and PC:PG:PA liposomes in the presence of salt. All modified analogues, including NaD1-1, which has no substitutions in the “cationic grip”, caused calcein leakage from PIP4,5-containing liposomes less effectively than NaD1. However, this effect was much less pronounced, as expected, than in the case of NaD1-L5.
2.6. All Modified Analogues Disrupt the Integrity of the Fungal Cell Membrane with the Same Efficacy as That of NaD1
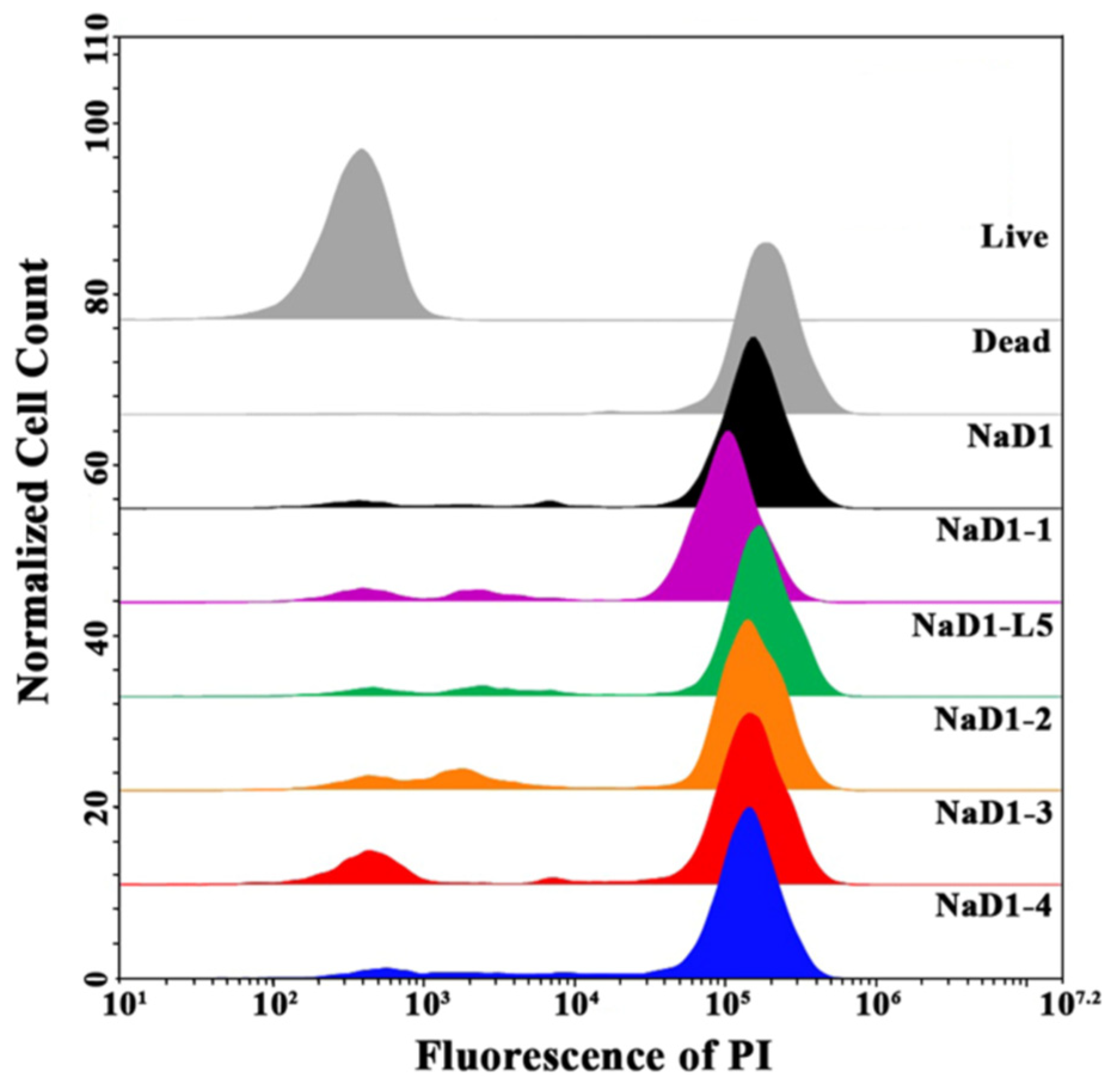
Further, we used flow cytometry to compare an ability of modified analogues of NaD1 to affect fungal membrane permeability and kill fungal cells.
C. albicans ATCC 18804 cells were treated with NaD1 or one of its analogues at MICs (6.25 μM for all peptides) for 4 h, followed by staining with the fluorescent intercalating dye propidium iodide (PI), which penetrates dead or dying cells with damaged membranes. Flow cytometry with PI staining is considered a high-throughput method that allows for the quantification of the effect of antimicrobial peptides (AMPs) on the cell membrane integrity within a large population of cells [
27]. The rate of PI uptake during a short incubation time of antimicrobial agent with cells can serve as an indicator to distinguish rapidly membranolytic agents from antibiotics whose lethal effects on membrane integrity are secondary to other targets.
We demonstrated that NaD1 at MIC effectively damaged the fungal cell membrane and killed fungal cells after incubation for 4 h and PI-stained cells accounted for 90.6% (
Figure 6;
Supplementary Materials, Figure S8). This effect was more pronounced than in the case of incubation for 2 h, when the percentage of PI-stained cells was 52%, as we showed earlier [
7]. Surprisingly, the efficiency of all modified analogues of NaD1 was comparable to that of the tobacco defensin, and after 4 h of incubation, the percentages of PI-stained cells were 82.2, 80.1, 80, 85.3, and 87.3% for NaD1-1, NaD1-2, NaD1-3, NaD1-4, and NaD1-L5, respectively (
Figure 6;
Supplementary Materials, Figure S8).
Our results correlated with previously published data showing that NaD1-L5, with a substitution of the part of the L5 loop, permeabilized
Fusarium oxysporum hyphae as effectively as NaD1 [
14]. Considering that all modified analogues tested here caused calcein leakage from PIP4,5-containing liposome to a lesser extent than NaD1, we suggested that their antifungal activity and the ability to permeabilize
C. albicans cells may be mediated not only through interaction with PIP4,5.
2.7. Modified Analogues of NaD1 Exhibit Varying Affinities for Zymosan Under Saline Conditions
As mentioned above, the mechanism of action of the tobacco defensin NaD1 involves several steps, the first of which is its interaction with the fungal cell wall, followed by the peptide reaching the cell membrane. This peptide binds to fungal cell wall polysaccharides such as β-glucan and, to a lesser extent, chitin. It is proposed that the interaction of NaD1 with negatively charged β-glucan is primarily electrostatic in nature, involving such amino acids as R1, E2, K4, K17, R21, D31, H33, K36, and T44 [
8]. It is assumed that salts impair NaD1 interaction with the cell wall, resulting in a significant decrease in its antifungal activity [
8].
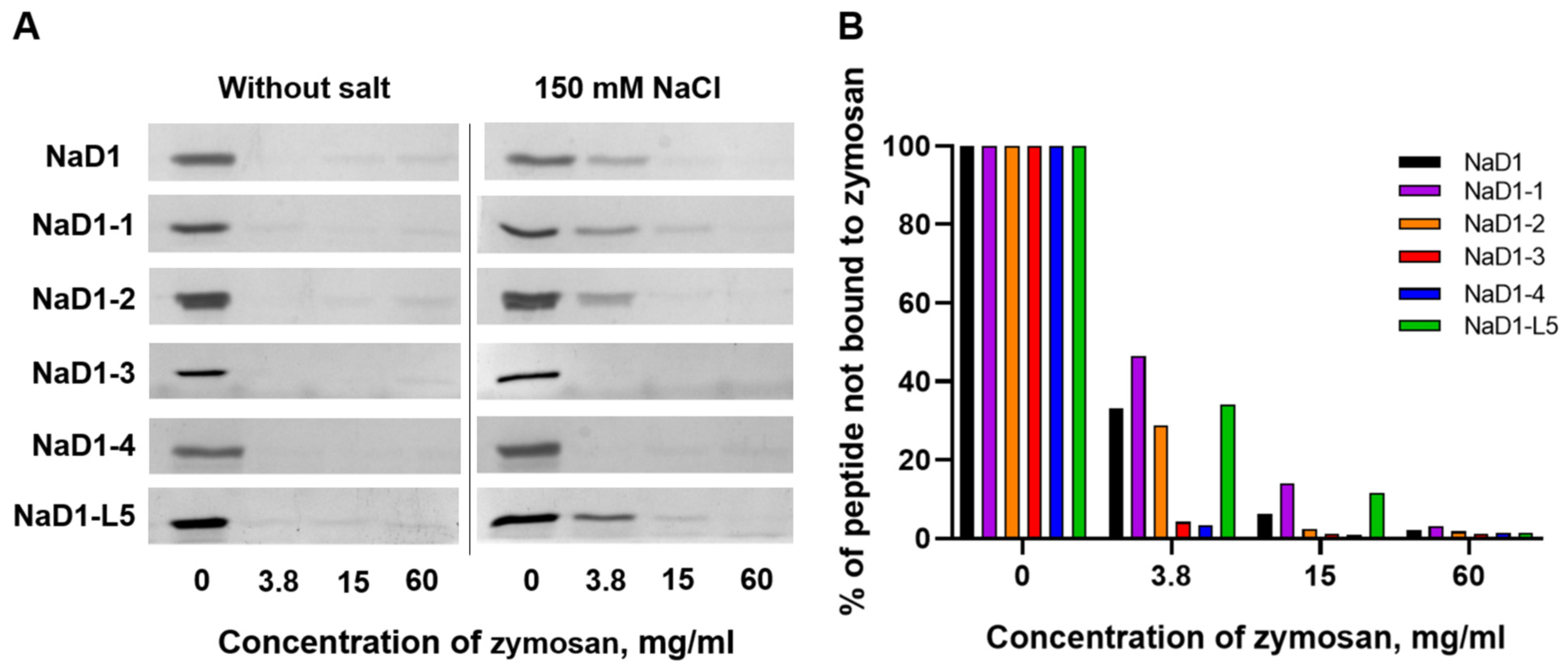
Here we compared the ability of NaD1 and its modified analogues to bind β-glucan in the presence or absence of 150 mM NaCl. Zymosan prepared from yeast cell walls and mainly consisting of β-glucan, and to a lesser extent of mannan, was used in these experiments. The disappearance or weakening of peptide bands on SDS-PAGE suggested efficient peptide binding to insoluble zymosan. We showed that in 10 mM HEPES, pH 7.5, NaD1 and all modified analogues exhibited strong binding capacity to zymosan, as evidenced by the absence of peptide bands after their incubation with insoluble polysaccharides at all concentrations (
Figure 7A). In the presence of 150 mM NaCl, the binding efficiency of NaD1, NaD1-1, and NaD1-2, as well as NaD1-L5, was reduced, as indicated by the stronger residual peptide bands at low zymosan concentrations compared to the salt-free condition (
Figure 7A,B). At the same time, NaD1-3 and NaD1-4 retained relatively strong binding even under saline conditions, indicating a degree of salt tolerance in their interaction with zymosan (
Figure 7A,B). These results for NaD1-3 correlated well with its ability to maintain higher antifungal activity in the presence of NaCl than NaD1 and other modified analogues (
Table 3). We proposed that the presence of Arg36 and Arg38, protruding in different directions on the surface of the peptide, determined the salt-tolerant properties of NaD1-3 (
Figure 2D). The observed salt tolerance of NaD1-4 upon zymosan binding assay is likely due to its more pronounced cationic properties compared to NaD1 and other modified analogues (pI 9.0 and +6.4 charge at pH 7.4). However, these properties alone probably were not sufficient to confer salt-tolerant antifungal activity.
Thus, we showed that NaD1 and all modified analogues bound to zymosan with high affinity under salt-free conditions. However, in the presence of NaCl, salt-induced inhibition of zymosan binding was observed for NaD1 and several analogues, whereas the affinity of NaD1-3 and NaD1-4 remained comparably high. We hypothesized that these properties may contribute to the relative salt-tolerant antifungal activity of NaD1-3.
2.8. All Modified Analogues Except NaD1-1 Are Characterized by the Absence of Hemolytic Activity and Low Cytotoxicity, Unlike NaD1
It is known that tobacco defensin NaD1 exhibits hemolytic and cytotoxic activities [
4]. Previously it has been shown that NaD1-L5 exhibits reduced activity against human monocytic lymphoma U937 cells compared to NaD1, suggesting that binding to PIP4,5 is necessary for the potent cytotoxic effects of tobacco defensin [
14].
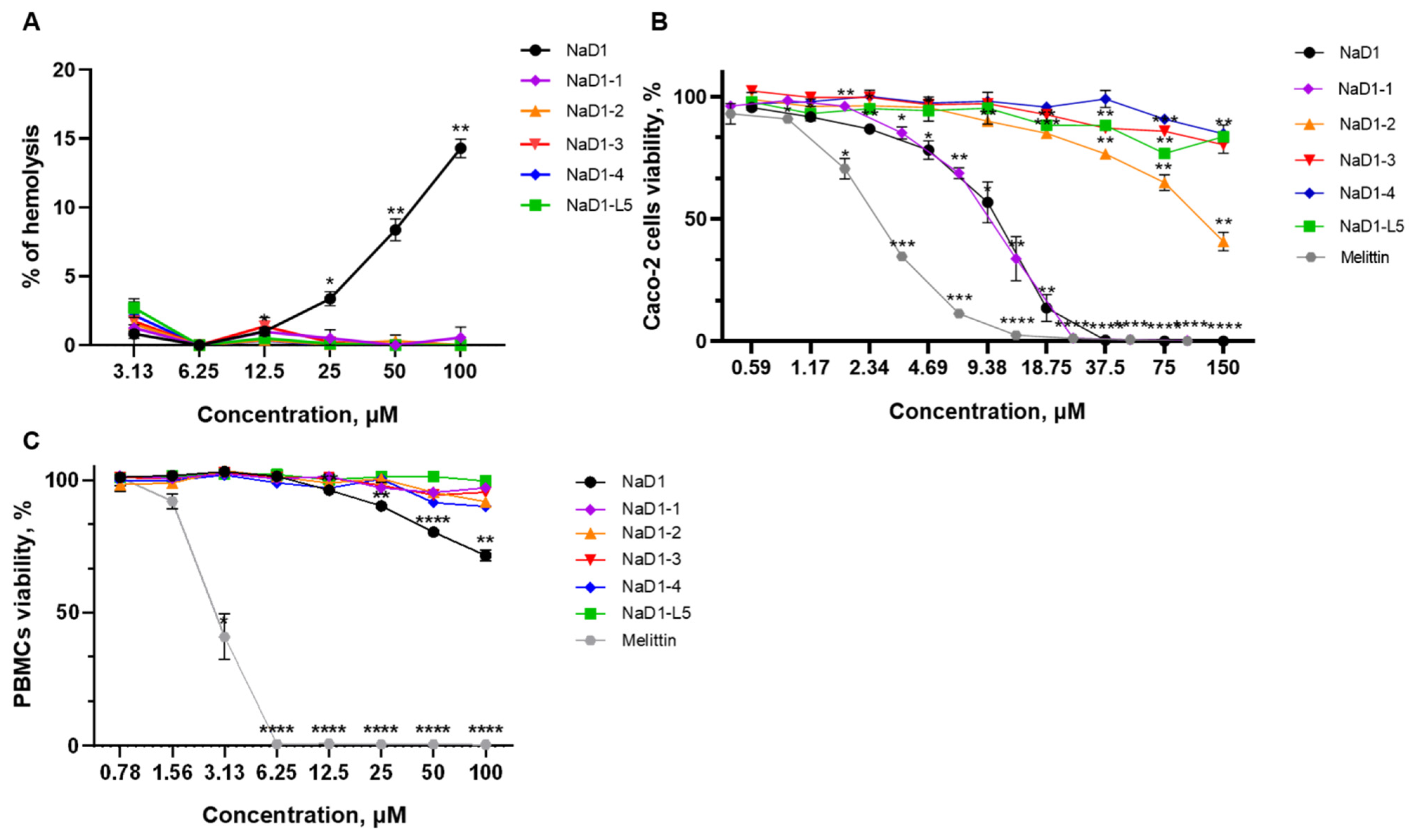
Here we examined the ability of modified analogues of NaD1-(1-4) to cause erythrocyte lysis and their cytotoxic effects toward human epithelial and immune cells. Hemolytic activity of NaD1 and its analogues was determined after peptide incubation with freshly isolated human erythrocytes for 2 h. NaD1 at concentrations of 50 and 100 µM caused lysis of about 10 and 15% of erythrocytes, respectively. It is notable that none of the modified analogues caused lysis of erythrocytes, even at a concentration of 100 μM, which is 16 times higher than their average MIC (
Figure 8A;
Supplementary Materials, Table S2). Under the same conditions, melittin from honeybee venom at 6.25 µM resulted in complete erythrocyte lysis (
Supplementary Materials, Table S2).
Cytotoxic activity of the peptides was investigated against human peripheral blood mononuclear cells (PBMCs) and the Caco-2 epithelial monolayer as an in vitro model of the intestinal barrier using a RPMI-1640 medium and resazurin-test. NaD1 demonstrated high toxicity against the Caco-2 monolayer and cell viability was 50 and 0% at its concentrations of 10.5 and 37.5 µM, respectively (
Figure 8B;
Supplementary Materials, Table S2). These results differed from our previous data where the less pronounced cytotoxic effects of NaD1 against a Caco-2 cell monolayer were demonstrated in DMEM/F12 medium [
2]. We showed that NaD1-1 exhibited the same or even slightly more pronounced cytotoxic effect than NaD1 and 50 and 0% cell viabilities were observed at 8 and 25 µM, respectively (
Figure 8B;
Supplementary Materials, Table S2). It is important to note that three other analogues had much less pronounced cytotoxicity than NaD1. Fifty percent cell viability of Caco-2 cells was observed for NaD1-2 at a high concentration of 120 µM, whereas practically no cytotoxic effects were registered for NaD1-3, NaD1-4, and for NaD-L5 even at a concentration of 150 µM (
Figure 8B;
Supplementary Materials, Table S2). For comparison, melittin induced approximately 50% and 100% cell death at 2.5 and 12.5 µM, respectively (
Figure 8B;
Supplementary Materials, Table S2). All modified analogues did not exhibit cytotoxic effects against PBMCs even at a concentration of 100 μM, and only NaD1 inhibited cell viability at high concentrations (cell viability was approximately 72% at a concentration of 100 μM) (
Figure 8C;
Supplementary Materials, Table S2). The absence of hemolytic activity and cytotoxic effect of NaD1-1 on PBMCs correlated well with its less pronounced ability to disrupt the integrity of PIP4,5-containing liposomes and oligomerize in the presence of this lipid. At the same time, NaD1-1 high toxicity against Caco-2 cells may have been associated with the differences in the lipid composition of human cell membranes and various PIP4,5 content.
Thus, we demonstrated that all modified analogues did not exhibit hemolytic activity unlike NaD1. In addition, all modified analogues except NaD1-1, having no amino acid substitution in the L5 loop, exhibited much less pronounced cytotoxicity than tobacco defensin towards both PBMCs and the Caco-2 epithelial monolayer. It is important to note that an almost-complete absence of cytotoxic effects was observed not only for NaD1-L5, but also for NaD1-3 and NaD1-4, which caused calcein leakage from PIP4,5-containing liposomes.
Based on all the data obtained, we can draw the following conclusions regarding the modified analogues of the tobacco defensin NaD1. The modified analogue NaD1-1, which contains Arg44 and Arg45 in the C-terminal region of the peptide, does not appear promising, as it exhibits anticandidal activity comparable to NaD1, along with low salt tolerance and high cytotoxicity to some human cells. Three other modified analogues of NaD1 contained substitutions with arginine of some amino acid in the L5 loop responsible for the “cationic grip” and binding to PIP4,5 differ from each other, but are all, in our view, promising candidates for further study. NaD1-2, NaD1-3, and NaD1-4 effectively inhibit the growth of susceptible and resistant strains of C. albicans and are characterized by the absence of hemolytic activity and much less pronounced cytotoxicity compared to NaD1. In our opinion, an interesting fact is that the antifungal activity of NaD1-3, which contains K36R and L38R substitutions in the L5 loop, is much less affected by the presence of sodium chloride compared to NaD1 and other modified analogues. NaD1-2, NaD1-3, and Nad1-4 bind to PIP4,5 less effectively than NaD1. We suggest that the decreased affinity for the target lipid PIP4,5 leads to a reduction in the fungicidal and cytotoxic activities of these modified analogues of NaD1 but does not critically affect their ability to inhibit fungal growth. The presence of additional arginine residues in their structure may also contribute to this. The observed differences in salt sensitivity, oligomerization ability, zymosan binding, and combined effects with other antifungals among NaD1-2, NaD1-3, and NaD1-4 suggest that their modes of action may involve diverse strategies. Further studies on the effects of these modified analogues on potential intracellular targets and oxidative damage to fungal cells may shed light on this issue.