Antibiotic Use, Healthcare-Associated Infections, and Antimicrobial Resistance in Intensive Care Unit of a Serbian Tertiary University Hospital, 2018–2024: An Ecological Analysis
Abstract
1. Introduction
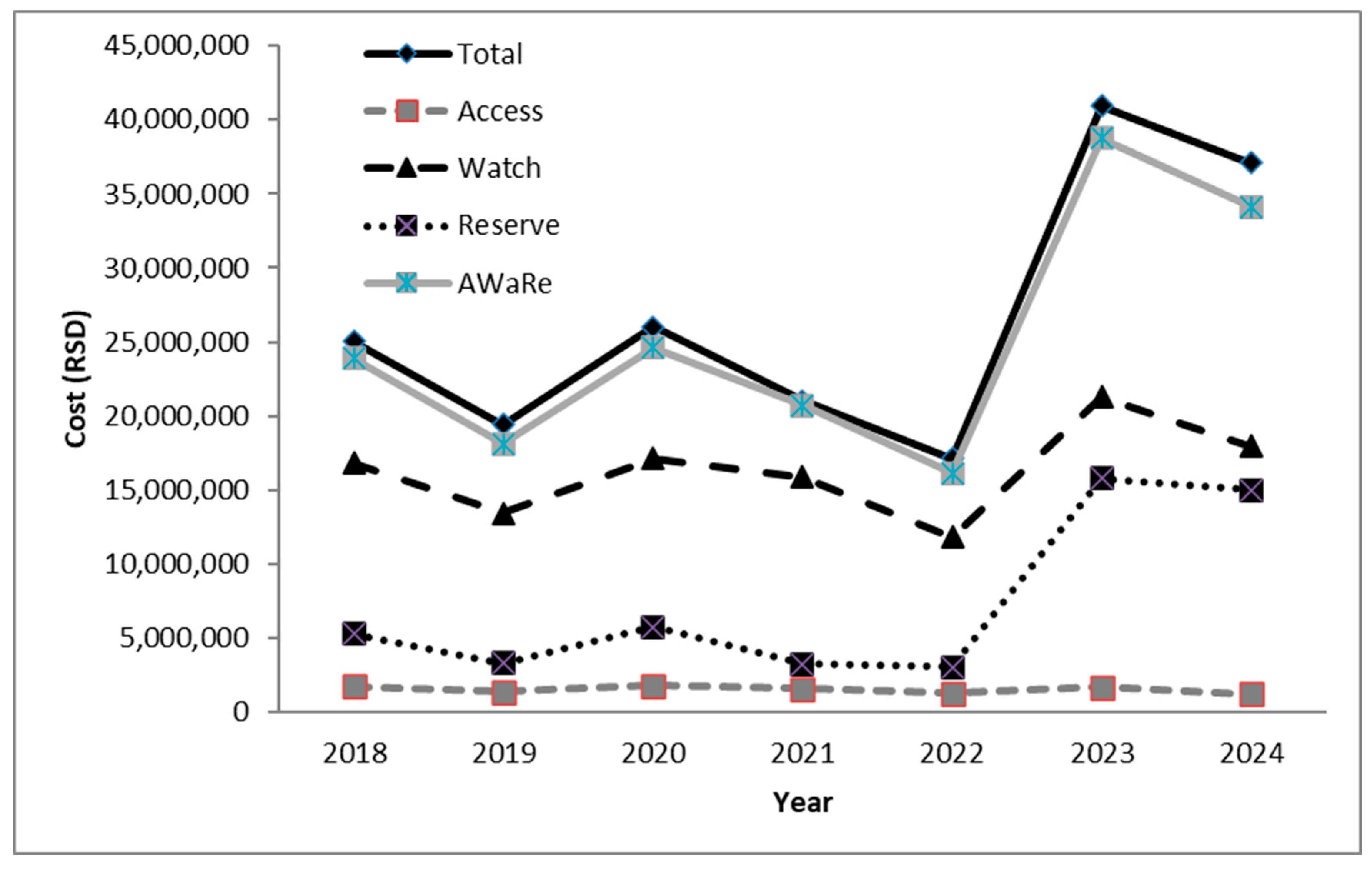
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HAI | Healthcare-associated infections |
| AMR | Antimicrobial resistance |
| AU | Antibiotic use |
| HA-CDI | Healthcare-associated Clostridioides difficile infection |
| ICU | Intensive care units |
| ASP | Antibiotic stewardship program |
| ID | Incidence density |
| BSI | Bloodstream infection |
| CVC | Central venous catheter |
| UTI | Urinary tract infection |
| UC | Urinary catheter |
| WHO | World Health Organization |
| AwaRe | “Access,” “Watch,” or “Reserve” |
| DOT | days of therapy’ |
| ATC | Anatomical therapeutic chemical |
| RSD | Serbian dinars |
| MMA | Military Medical Academy |
| MDR | Multidrug-resistant |
| ECDC | European Centre for Disease Prevention and Control |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| VRE | Vancomycin-resistant Enterococcus spp. |
| EU | European union |
| EEA | European Economic Area |
| UK | United Kingdom |
| MIC | Minimum inhibitory concentrations |
| OXA | Oxacillin |
| GLY | Glycopeptides |
| CTX | Cefotaxime |
| CRO | Ceftriaxone |
| CAZ | Ceftazidime |
| CAR | Carbapenems |
| IPM | Imipenem |
| MEM | Meropenem |
| ETP | Ertapenem |
| GDH | Glutamate dehydrogenase |
| SD | Standard deviation |
References
- Healthcare-Associated Infections Acquired in Intensive Care Units—Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-acquired-intensive-care-units-annual-0 (accessed on 23 September 2025).
- Blot, S.; Ruppé, E.; Harbarth, S.; Asehnoune, K.; Poulakou, G.; Luyt, C.E.; Rello, J.; Klompas, M.; Depuydt, P.; Eckmann, C.; et al. Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensiv. Crit. Care Nurs. 2022, 70, 103227. [Google Scholar] [CrossRef]
- Li, R.J.; Wu, Y.L.; Huang, K.; Hu, X.Q.; Zhang, J.J.; Yang, L.Q.; Yang, X.Y. A prospective surveillance study of healthcare-associated infections in an intensive care unit from a tertiary care teaching hospital from 2012–2019. Medicine 2023, 102, e34469. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Yin, R.; Nercelles, P.; Rivera-Molina, S.E.; Jyoti, S.; Dongol, R.; Aguilar-De-Moros, D.; Tumu, N.; Alarcon-Rua, J.; Stagnaro, J.P.; et al. International Nosocomial Infection Control Consortium (INICC) report of health care associated infections, data summary of 45 countries for 2015 to 2020, adult and pediatric units, device-associated module. Am. J. Infect. Control 2024, 52, 1002–1011. [Google Scholar] [CrossRef]
- Masoudifar, M.; Gouya, M.M.; Pezeshki, Z.; Eshrati, B.; Afhami, S.; Farzami, M.R.; Seifi, A. Health care-associated infections, including device-associated infections, and antimicrobial resistance in Iran: The national update for 2018. J. Prev. Med. Hyg. 2022, 62, E943–E949. [Google Scholar] [CrossRef]
- Baek, J.E.; Choi, I.H.; Cho, Y.W.; Kim, J.; Lee, Y.J.; Kim, M.C.; Kim, K.O.; Cho, Y.S. Clinical characteristics and outcomes of Clostridioides difficile infection in the intensive care unit: A KASID multi-centre study. J. Hosp. Infect. 2023, 139, 106–112. [Google Scholar] [CrossRef]
- Šuljagić, V.; Miljković, I.; Starčević, S.; Stepić, N.; Kostić, Z.; Jovanović, D.; Brusić-Renaud, J.; Mijović, B.; Šipetić-Grujičić, S. Risk factors for Clostridium difficile infection in surgical patients hospitalized in a tertiary hospital in Belgrade, Serbia: A case-control study. Antimicrob. Resist. Infect. Control 2017, 6, 31. [Google Scholar] [CrossRef]
- Popović, R.; Tomić, Z.; Tomas, A.; Anđelić, N.; Vicković, S.; Jovanović, G.; Bukumirić, D.; Horvat, O.; Sabo, A. Five-year surveillance and correlation of antibiotic consumption and resistance of Gram-negative bacteria at an intensive care unit in Serbia. J. Chemother. 2020, 32, 294–303. [Google Scholar] [CrossRef]
- Remschmidt, C.; Schneider, S.; Meyer, E.; Schroeren-Boersch, B.; Gastmeier, P.; Schwab, F. Surveillance of antibiotic use and resistance in intensive care units (SARI)—A 15-year cohort study. Dtsch. Arztebl. Int. 2017, 114, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Okeke, I.N.; de Kraker, M.E.; Van Boeckel, T.P.; Kumar, C.K.; Schmitt, H.; Gales, A.C.; Bertagnolio, S.; Sharland, M.; Laxminarayan, R. The scope of the antimicrobial resistance challenge. Lancet 2024, 403, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Reyes, L.F.; Nseir, S.; Ranzani, O.; Povoa, P.; Diaz, E.; Schultz, M.J.; Rodríguez, A.H.; Serrano-Mayorga, C.C.; De Pascale, G.; et al. European Network for ICU-Related Respiratory Infections (ENIRRIs): A multinational, prospective, cohort study of nosocomial LRTI. Intensiv. Care Med. 2023, 49, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zha, Z.; Li, L.; Tan, H.; Pi, J.; You, C.; Liu, B. Healthcare-associated carbapenem-resistant Klebsiella pneumoniae infections are associated with higher mortality compared to carbapenem-susceptible K. pneumoniae infections in the intensive care unit: A retrospective cohort study. J. Hosp. Infect. 2024, 148, 30–38. [Google Scholar] [CrossRef]
- Fukushige, M.; Ngo, N.H.; Lukmanto, D.; Fukuda, S.; Ohneda, O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front. Public Health 2022, 10, 946077. [Google Scholar] [CrossRef]
- Rabbi, F.; Banfield, L.; Munir, M.; Chagla, Z.; Mayhew, A.; de Souza, R.J. Overprescription of antibiotics for treating hospitalized COVID-19 patients: A systematic review and meta-analysis. Heliyon 2023, 9, e20563. [Google Scholar] [CrossRef] [PubMed]
- van Daalen, F.V.; Opmeer, B.C.; Prins, J.M.; Geerlings, S.E.; Hulscher, M.E.J.L. The economic evaluation of an antibiotic checklist as antimicrobial stewardship intervention. J. Antimicrob. Chemother. 2017, 72, 3213–3221. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. The Cost Dimensions of Antimicrobial Resistance. In Understanding the Economics of Microbial Threats: Proceedings of a Workshop; National Academies Press (US): Washington, DC, USA, 2018. [Google Scholar]
- Huser, J.; Dörr, T.; Berger, A.; Kohler, P.; Kuster, S.P. Economic evaluations of antibiotic stewardship programmes 2015–2024: A systematic review. Swiss Med. Wkly. 2025, 155, 4217. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe. Antimicrobial Medicines Consumption (AMC) Network: AMC Data 2014–2018; WHO: Copenhagen, Denmark, 2021; Available online: https://iris.who.int/server/api/core/bitstreams/c256a0e7-304a-4ab1-a22d-e325a9e17e46/content (accessed on 29 October 2025).
- Medić, D.; Božić Cvijan, B.; Bajčetić, M. Impact of antibiotic consumption on antimicrobial resistance to invasive hospital pathogens. Antibiotics 2023, 12, 259. [Google Scholar] [CrossRef]
- Veličković-Radovanović, R.; Stefanović, N.; Damnjanović, I.; Kocić, B.; Mladenović-Antić, S.; Dinić, M.; Petrović, J.; Mitić, R.; Catić-Đorđević, A. Antibiotic utility and susceptibility changes of multidrug-resistant Escherichia coli and Klebsiella spp.: 5-year experience in a tertiary healthcare centre. Eur. J. Hosp. Pharm. 2022, 29, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Perić, A.; Rančić, N.; Dragojević-Simić, V.; Milenković, B.; Ljubenović, N.; Rakonjac, B.; Begović-Kuprešanin, V.; Šuljagić, V. Association between antibiotic use and hospital-onset Clostridioides difficile infection in university tertiary hospital in Serbia, 2011–2021: An ecological analysis. Antibiotics 2022, 11, 1178. [Google Scholar] [CrossRef] [PubMed]
- Perić, A.; Dragojević-Simić, V.; Milenković, B.; Vezmar Kovačević, S.; Šuljagić, V. Antibiotic consumption and healthcare-associated infections in a tertiary hospital in Belgrade, Serbia from 2011 to 2016. J. Infect. Dev. Ctries. 2018, 12, 855–863. [Google Scholar] [CrossRef]
- De Bus, L.; Gadeyne, B.; Steen, J.; Boelens, J.; Claeys, G.; Benoit, D.; De Waele, J.; Decruyenaere, J.; Depuydt, P. A complete and multifaceted overview of antibiotic use and infection diagnosis in the intensive care unit: Results from a prospective four-year registration. Crit. Care 2018, 22, 241. [Google Scholar] [CrossRef]
- Winroth, A.; Andersson, M.; Fjällström, P.; Johansson, A.F.; Lind, A. Automated surveillance of antimicrobial consumption in intensive care, northern Sweden: An observational case study. Antimicrob. Resist. Infect. Control 2024, 13, 67. [Google Scholar] [CrossRef]
- Macheda, G.; Dyar, O.J.; Luc, A.; Beovic, B.; Béraud, G.; Castan, B.; Gauzit, R.; Lesprit, P.; Tattevin, P.; Thilly, N.; et al. Are infection specialists recommending short antibiotic treatment durations? An ESCMID international cross-sectional survey. J. Antimicrob. Chemother. 2018, 73, 1084–1090. [Google Scholar] [CrossRef]
- Hanretty, A.M.; Gallagher, J.C. Shortened courses of antibiotics for bacterial infections: A systematic review of randomized controlled trials. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 674–687. [Google Scholar] [CrossRef]
- Spellberg, B. The new antibiotic mantra—“shorter is better”. JAMA Intern. Med. 2016, 176, 1254–1255. [Google Scholar] [CrossRef]
- Spellberg, B. The maturing antibiotic mantra: “shorter is still better”. J. Hosp. Med. 2018, 13, 361–362. [Google Scholar] [CrossRef]
- Royer, S.; DeMerle, K.M.; Dickson, R.P.; Prescott, H.C. Shorter versus longer courses of antibiotics for infection in hospitalized patients: A systematic review and meta-analysis. J. Hosp. Med. 2018, 13, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Axente, C.; Licker, M.; Moldovan, R.; Hogea, E.; Muntean, D.; Horhat, F.; Bedreag, O.; Sandesc, D.; Papurica, M.; Dugaesescu, D.; et al. Antimicrobial consumption, costs and resistance patterns: A two-year prospective study in a Romanian intensive care unit. BMC Infect. Dis. 2017, 17, 358. [Google Scholar] [CrossRef]
- Taušan, Đ.; Rančić, N.; Kostić, Z.; Ljubenović, N.; Rakonjac, B.; Šuljagić, V. An assessment of burden of hospital-acquired pneumonia among abdominal surgical patients in a tertiary university hospital in Serbia: A matched nested case-control study. Front. Med. 2022, 9, 1040654. [Google Scholar] [CrossRef] [PubMed]
- Healthcare-Associated Infections Acquired in Intensive Care Units—Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-intensive-care-units-2019 (accessed on 23 September 2025).
- Healthcare-Associated Infections Acquired in Intensive Care Units—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-acquired-intensive-care-units-annual (accessed on 23 September 2025).
- Balkhy, H.H.; El-Saed, A.; El-Metwally, A.; Arabi, Y.M.; Aljohany, S.M.; Al Zaibag, M.; Baharoon, S.; Alothman, A.F. Antimicrobial consumption in five adult intensive care units: A 33-month surveillance study. Antimicrob. Resist. Infect. Control 2018, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Katchanov, J.; Kreuels, B.; Maurer, F.P.; Wöstmann, K.; Jochum, J.; König, C.; Seoudy, K.; Rohde, H.; Lohse, A.W.; Wichmann, D.; et al. Risk factors for excessively prolonged meropenem use in the intensive care setting: A case-control study. BMC Infect. Dis. 2017, 17, 131. [Google Scholar] [CrossRef]
- Hillyer, M.M.; Jaggi, P.; Chanani, N.K.; Fernandez, A.J.; Zaki, H.; Fundora, M.P. Antimicrobial stewardship and improved antibiotic utilization in the pediatric cardiac intensive care unit. Pediatr. Qual. Saf. 2024, 9, e710. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, P.J.; Rohailla, S.; Taggart, L.R.; Lightfoot, D.; Havey, T.; Daneman, N.; Lowe, C.; Muller, M.P. Antimicrobial stewardship and intensive care unit mortality: A systematic review. Clin. Infect. Dis. 2019, 68, 748–756. [Google Scholar] [CrossRef]
- Pogue, J.M.; Tam, V.H. Toxicity in patients. Adv. Exp. Med. Biol. 2019, 1145, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.M.; Ly, N.; Anderson, D.; Yang, J.C.; Macander, L.; Jarkowski, A., III; Forrest, A.; Bulitta, J.B.; Tsuji, B.T. Resurgence of colistin: A review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 2010, 30, 1279–1291. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Lucenteforte, E.; Pea, F.; Soriano, A.; Tavoschi, L.; Steele, V.R.; Henriksen, A.S.; Longshaw, C.; Manissero, D.; Pecini, R.; et al. Systematic review on estimated rates of nephrotoxicity and neurotoxicity in patients treated with polymyxins. Clin. Microbiol. Infect. 2021, 27, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xiang, B.X.; Song, X.L.; Que, R.M.; Zuo, X.C.; Xie, Y.L. Prevalence of polymyxin-induced nephrotoxicity and its predictors in critically ill adult patients: A meta-analysis. World J. Clin. Cases 2022, 10, 11466–11485. [Google Scholar] [CrossRef]
- Tirlapur, N.; Puthucheary, Z.A.; Cooper, J.A.; Sanders, J.; Coen, P.G.; Moonesinghe, S.R.; Wilson, A.P.; Mythen, M.G.; Montgomery, H.E. Diarrhoea in the critically ill is common, associated with poor outcome and rarely due to Clostridium difficile. Sci. Rep. 2016, 6, 24691. [Google Scholar] [CrossRef]
- Akorful, R.A.A.; Odoom, A.; Awere-Duodu, A.; Donkor, E.S. The global burden of Clostridioides difficile infections, 2016–2024: A systematic review and meta-analysis. Infect. Dis. Rep. 2025, 17, 31. [Google Scholar] [CrossRef]
- Cohen, C.C.; Azhar, G.; Muggy, L. When is an outbreak an outbreak? Using literature and discharge data to define Clostridioides difficile incidence changes referred to as outbreaks. J. Hosp. Infect. 2020, 105, 225–231. [Google Scholar] [CrossRef]
- UK Government. Increase in Clostridioides Difficile Infections: Technical Report. Available online: https://www.gov.uk/government/publications/increase-in-clostridioides-difficile-infections-technical-report/increase-in-clostridioides-difficile-infections-cdi-current-epidemiology-data-and-investigations-technical-report (accessed on 19 September 2025).
- Chen, Y.; Xie, X.; Ge, Q.; He, X.; Sun, Z.; Li, Y.; Guo, Y.; Geng, C.; Li, X.; Wang, C. The global burden and trend of Clostridioides difficile and its association with world antibiotic consumption, 1990–2019. J. Glob. Health 2024, 14, 04135. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, V.D.; Yin, R.; Lu, Y.; Rodrigues, C.; Myatra, S.N.; Kharbanda, M.; Valderrama-Beltran, S.L.; Mehta, Y.; Daboor, M.A.; Todi, S.K.; et al. The impact of healthcare-associated infections on mortality in ICU: A prospective study in Asia, Africa, Eastern Europe, Latin America, and the Middle East. Am. J. Infect. Control 2023, 51, 675–682. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 19 September 2025).
- Benkő, R.; Matuz, M.; Pető, Z.; Weist, K.; Heuer, O.; Vlahović-Palčevski, V.; Monnet, D.L.; Galistiani, G.F.; Blix, H.S.; Soós, G.; et al. Trends in the hospital-sector consumption of the WHO AWaRe Reserve group antibiotics in EU/EEA countries and the United Kingdom, 2010 to 2018. Euro. Surveill. 2022, 27, 2101058. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Surveillance in Europe 2023-2021 Data. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2023-2021-data (accessed on 23 September 2025).
- Brkić, S.; Ćirković, I. Carbapenem-resistant Enterobacterales in the Western Balkans. Antibiotics 2024, 13, 895. [Google Scholar] [CrossRef]
- Uzairue, L.I.; Rabaan, A.A.; Adewumi, F.A.; Okolie, O.J.; Folorunso, J.B.; Bakhrebah, M.A.; Garout, M.; Alfouzan, W.A.; Halwani, M.A.; Alamri, A.A.; et al. Global prevalence of colistin resistance in Klebsiella pneumoniae from bloodstream infection: A systematic review and meta-analysis. Pathogens 2022, 11, 1092. [Google Scholar] [CrossRef]
- Rakonjac, B.; Djurić, M.; Djurić-Petković, D.; Dabić, J.; Simonović, M.; Milić, M.; Arsović, A. Evaluation of the NG-Test CARBA 5 for Rapid Detection of Carbapenemases in Clinical Isolates of Klebsiella pneumoniae. Antibiotics 2025, 14, 989. [Google Scholar] [CrossRef]
- Karkhane, M.; Pourhosiengholi, M.A.; Torkabad, M.R.A.; Kimiia, Z.; Mortazavi, S.M.; Aghdam, S.K.H.; Marzban, A.; Zali, M.R. Annual antibiotic-related economic burden of healthcare-associated infections: A cross-sectional population-based study. Iran. J. Pharm. Res. 2016, 15, 605–610. [Google Scholar]
- Liu, X.; Shrestha, R.; Koju, P.; Maharjan, B.; Shah, P.; Thapa, P.; Li, H. The direct medical economic burden of healthcare-associated infections and antimicrobial resistance: A preliminary study in a teaching hospital of Nepal. J. Glob. Antimicrob. Resist. 2022, 29, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hodoșan, V.; Zaha, D.C.; Daina, L.G.; Tîrb, A.M.; Mărcuț, L.F.; Mohan, A.G.; Cotrău, P.; Daina, C.M. Financial evaluation and pattern of antibiotic consumption in intensive care units of a university hospital. Pharmacophore 2023, 14, 25–32. [Google Scholar] [CrossRef]
- Karanika, S.; Paudel, S.; Grigoras, C.; Kalbasi, A.; Mylonakis, E. Systematic Review and Meta-analysis of Clinical and Economic Outcomes from the Implementation of Hospital-Based Antimicrobial Stewardship Programs. Antimicrob. Agents Chemother. 2016, 60, 4840–4852. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.; Gladstone, B.P.; Burkert, F.; Carrara, E.; Foschi, F.; Döbele, S.; Tacconelli, E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book. Available online: https://www.who.int/publications/i/item/9789240062382 (accessed on 23 September 2025).
- European Centre for Disease Prevention and Control. Surveillance of Healthcare-Associated Infections and Prevention Indicators in European Intensive Care Units: HAI Net ICU Protocol, Version 2.2; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2017. [Google Scholar]
- European Surveillance of Clostridium Difficile Infections—Surveillance Protocol Version 2.3. Available online: https://www.ecdc.europa.eu/en/publications-data/european-surveillance-clostridium-difficile-infections-surveillance-protocol-1 (accessed on 23 September 2025).
- European Surveillance of Clostridioides (Clostridium) Difficile Infections—Surveillance Protocol Version 2.4. Available online: https://www.ecdc.europa.eu/en/publications-data/european-surveillance-clostridium-difficile-infections-surveillance-protocol-2 (accessed on 23 September 2025).

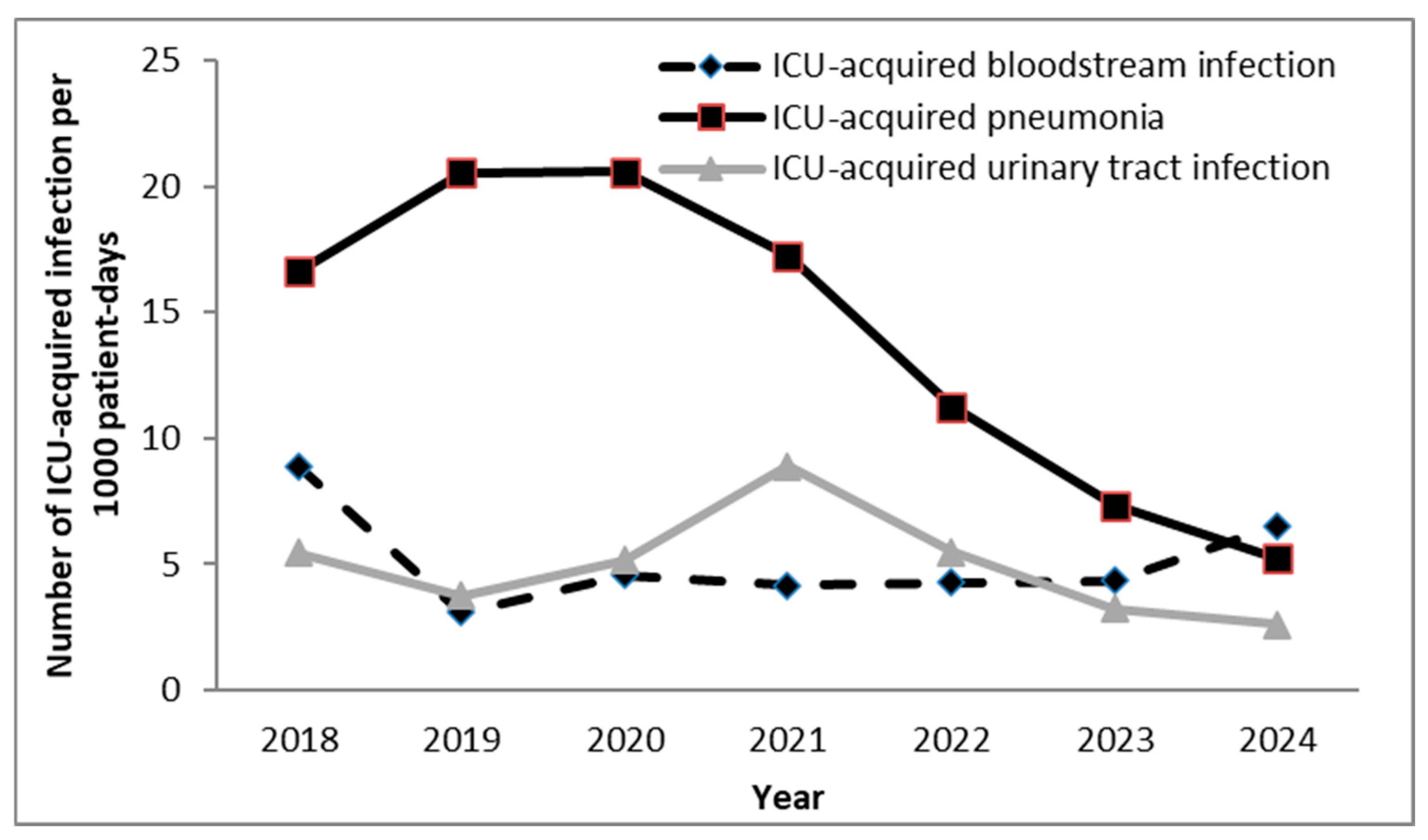
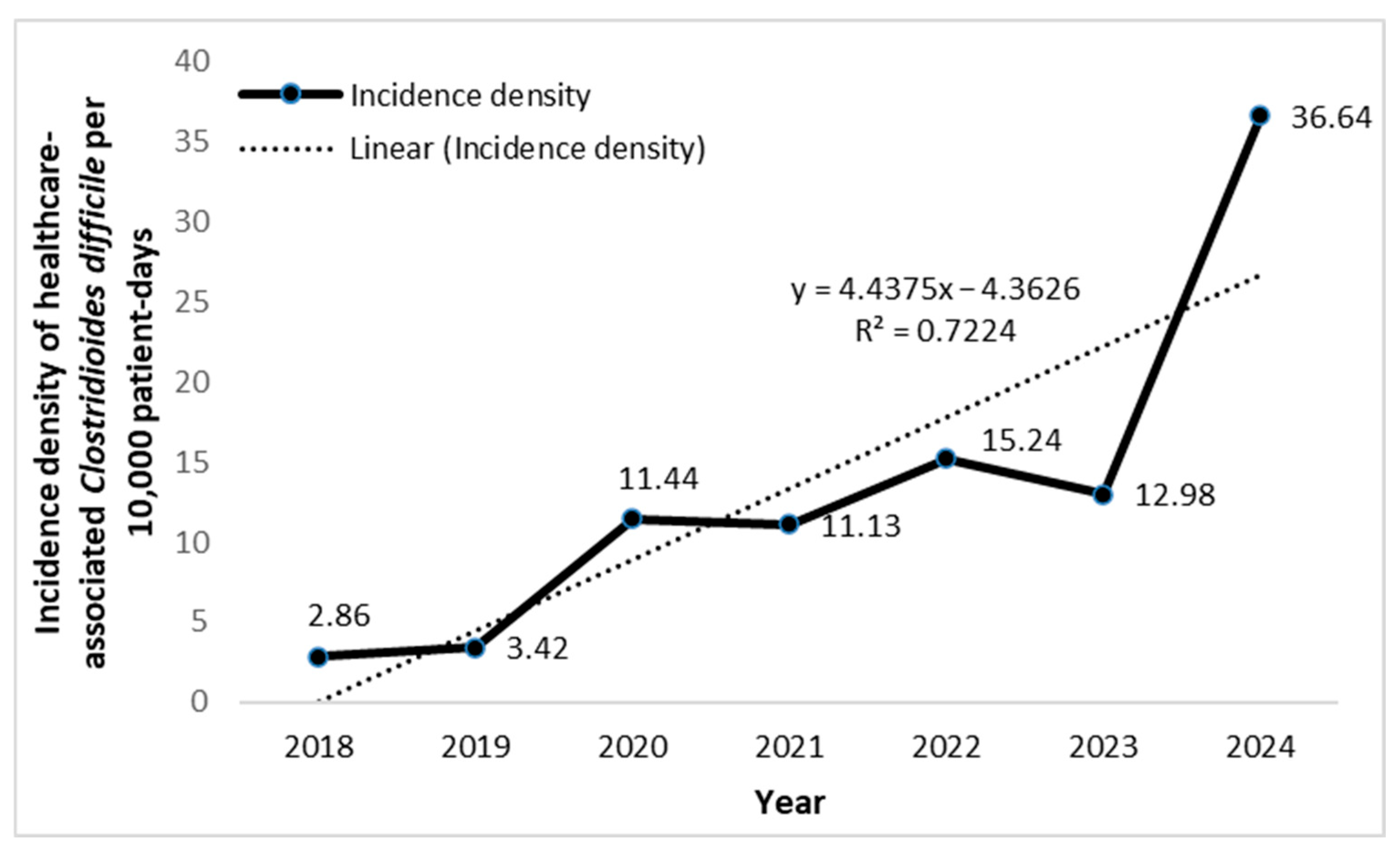
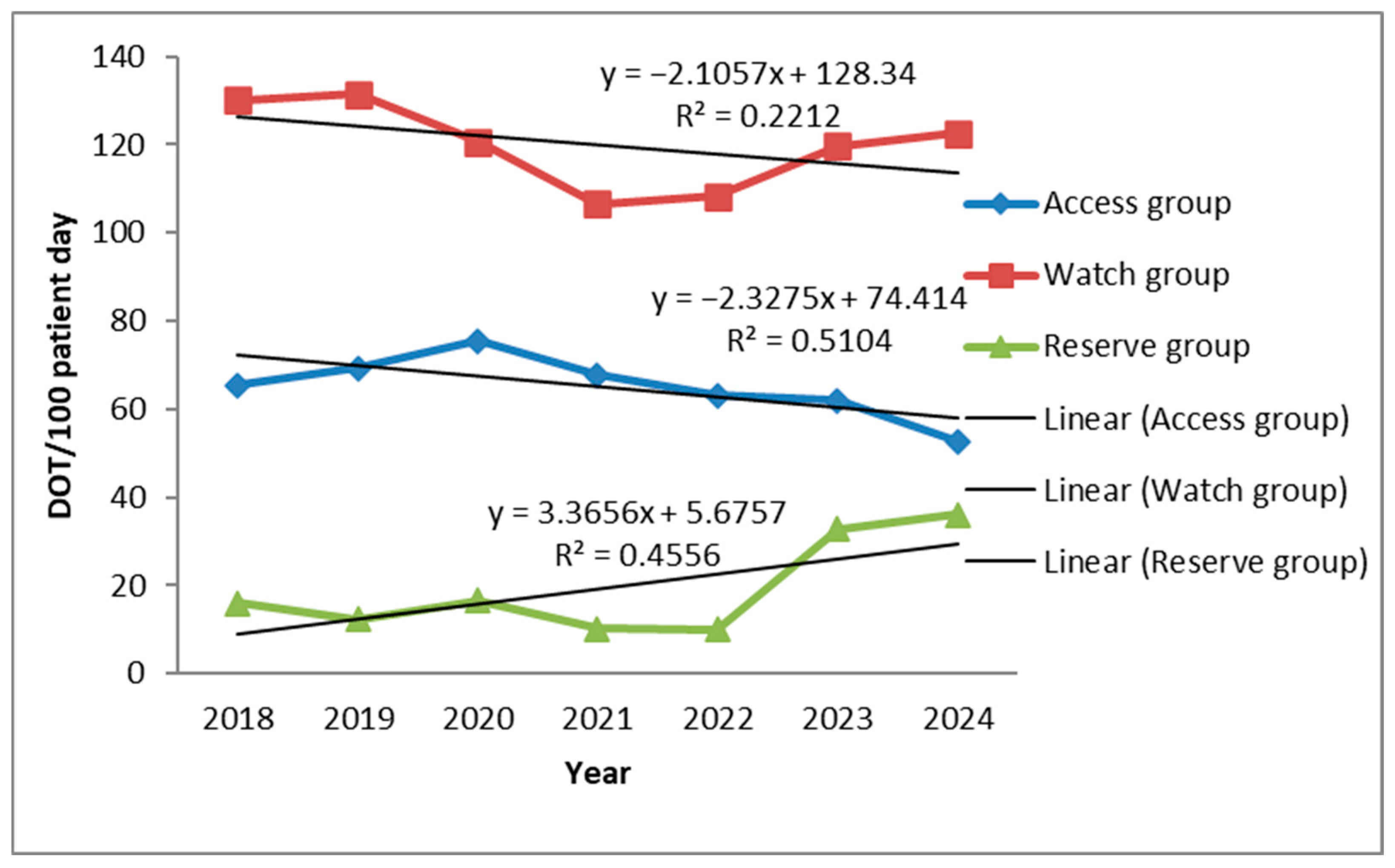
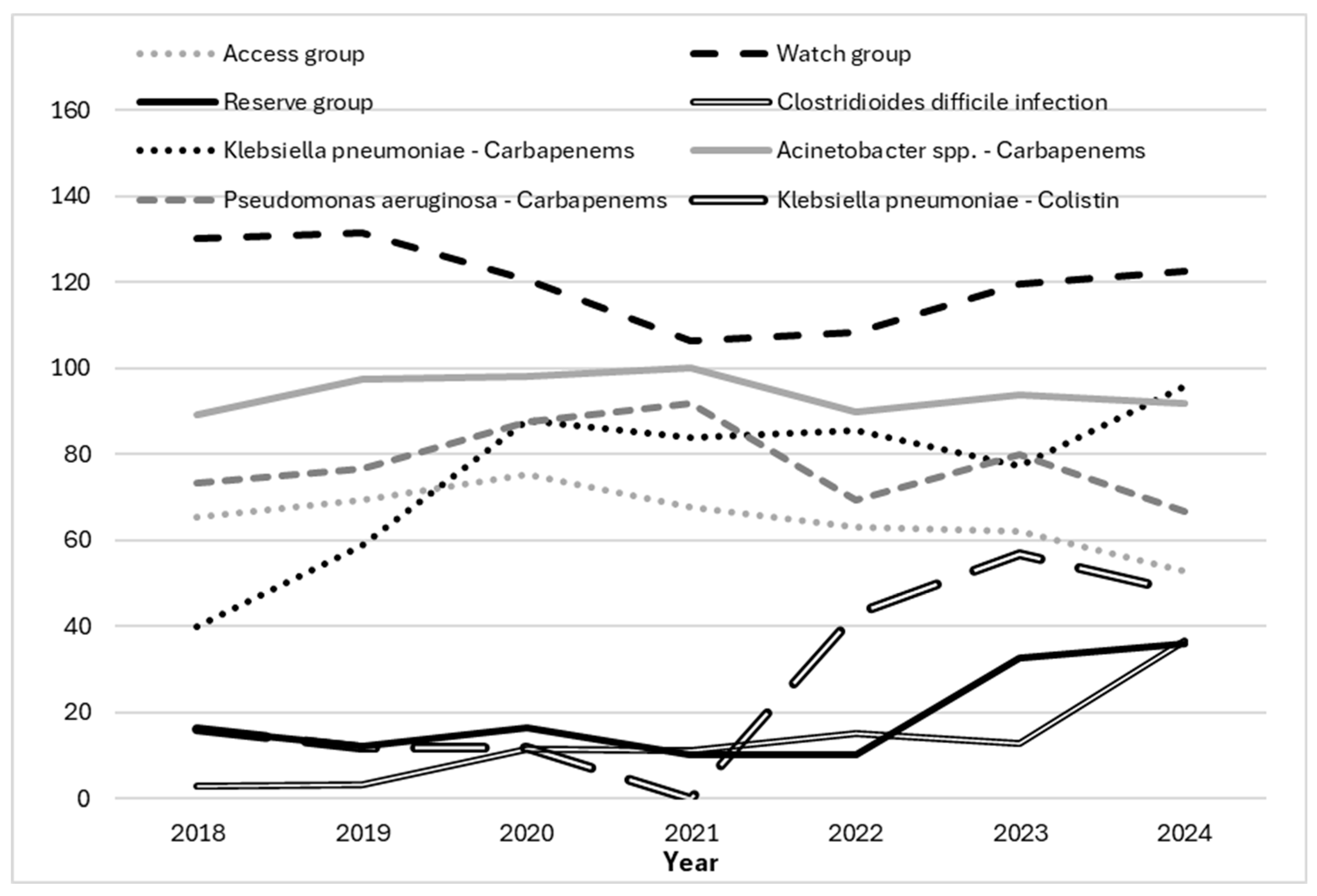
| Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics; N (%) | 2018 N = 238 | 2019 N = 236 | 2020 N = 298 | 2021 N = 298 | 2022 N = 279 | 2023 N = 367 | 2024 N = 339 | Total N = 2055 | p |
| Male sex | 159 66.8 | 163 69.1 | 179 60.1 | 181 65.6 | 183 65.6 | 216 58.9 | 209 61.7 | 1290 62.8 | 0.081 |
| Age | 62.38 ± 17.272 | 63.10 ± 16.536 | 65.20 ± 15.941 | 64.48 ± 16.422 | 63.91 ± 16.601 | 65.68 ± 116.145 | 64.06 ± 16.090 | 64.25 ± 16.397 | 0.128 |
| Age ≥ 65 | 116 48.7 | 130 55.1 | 178 59.7 | 173 58.1 | 154 55.2 | 220 60.1 | 191 56.3 | 1162 56.6 | 0.128 |
| Surgery | 136 57.1 | 157 66.5 | 189 63.4 | 187 62.8 | 190 68.1 | 263 71.7 | 234 69.0 | 1356 66.0 | 0.023 |
| Transfer from other hospital | 13 5.5 | 15 6.3 | 52 17.4 | 50 16.8 | 42 15.1 | 25 6.8 | 19 5.6 | 220 10.7 | 1.000 |
| Infection on admission to ICU | 40 16.8 | 62 26.3 | 71 23.8 | 70 23.5 | 72 25.8 | 107 29.2 | 66 19.8 | 453 22.0 | 0.704 |
| Central vascular catheter | 222 93.3 | 227 96.2 | 275 92.3 | 276 92.6 | 256 91.8 | 357 97.3 | 317 93.5 | 1930 93.9 | 0.254 |
| Urinary catheter | 196 82.4 | 233 98.7 | 293 98.3 | 296 99.3 | 276 98.9 | 366 97.8 | 337 99.4 | 1997 97.2 | 0.128 |
| Mechanical ventilation | 217 91.2 | 196 83.1 | 237 79.5 | 230 77.2 | 223 79.9 | 296 80.7 | 270 79.6 | 1669 81.2 | 0.610 |
| Length of ICU stay (in days) (M-IQR) | 8 (5−16) | 7 (5−13.7) | 7 (4−14) | 7 (4−14) | 8 (4−14) | 8 (5−15) | 8 (5−13) | / | 0.180 |
| In hospital mortality | 110 55.9 | 108 45.8 | 130 43.6 | 149 50.0 | 143 51.3 | 146 39.8 | 154 45.4 | 940 45.7 | 0.704 |
| In hospital mortality with HAI (%) | 47 56.9 | 36 49.3 | 56 62.9 | 48 59.3 | 41 71.9 | 40 54.8 | 39 72.2 | 307 60.1 | 0.128 |
| In hospital mortality without HAI (%) | 63 41.2 | 72 44.2 | 74 35.4 | 101 46.5 | 102 45.9 | 106 36.1 | 115 40.4 | 633 41.0 | 1.000 |
| ATC Code | DOT Per 100 Patient-Days | |||||||
|---|---|---|---|---|---|---|---|---|
| Year | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | p * |
| Total consumption of antibiotics | 240.39 | 236.77 | 224.10 | 190.60 | 190.28 | 242.39 | 242.03 | 1.000 |
| J01GB06 Amikacin | 0.000 | 6.197 | 8.862 | 11.043 | 9.052 | 8.916 | 7.223 | 0.134 |
| J01CR02 Amoxicilline Clavulanic acid | 0.057 | 0.000 | 0.000 | 0.334 | 0.000 | 0.000 | 0.000 | / |
| J01CA01 Ampicillin | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | / |
| J01CR01 Ampicillin Sulbactam | 0.000 | 0.240 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | / |
| J01CE09 Procaine benzylpenicillin | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | / |
| J01CE01 PenicillinGsodium | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | / |
| J01DB04 Cefazolin | 1.318 | 0.205 | 1.801 | 1.725 | 0.000 | 0.628 | 1.649 | 1.000 |
| J01BA01 Chloramphenicol | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | / |
| J01FF01 Clindamycin | 1.060 | 1.917 | 0.114 | 0.501 | 1.128 | 3.636 | 4.030 | 0.057 |
| J01GB03 Gentamicin | 1.031 | 1.609 | 0.829 | 0.779 | 0.518 | 2.402 | 0.366 | 0.128 |
| J01XD01 Metronidazole | 59.353 | 57.994 | 62.550 | 50.320 | 48.766 | 42.783 | 37.163 | 0.002 |
| J01EE01 Sulfa.trimeto | 2.635 | 1.198 | 1.229 | 3.032 | 3.596 | 3.614 | 2.329 | 0.128 |
| J01FA10 Azithromycin | 0.086 | 0.000 | 1.744 | 0.334 | 0.000 | 0.000 | 0.000 | / |
| J01DC02 Cefuroxime | 12.117 | 6.231 | 16.810 | 12.656 | 14.904 | 9.089 | 10.364 | 1.000 |
| J01DD01 Cefotaxime | 0.286 | 0.000 | 0.000 | 0.000 | 0.000 | 0.974 | 0.183 | / |
| J01DD09 Ceftazidime | 1.604 | 0.890 | 0.343 | 0.834 | 0.640 | 0.281 | 0.262 | 0.002 |
| J01DD04 Ceftriaxone | 7.992 | 24.444 | 8.262 | 12.295 | 15.087 | 16.447 | 19.969 | 0.057 |
| J01DE01 Cefepime | 3.552 | 2.396 | 1.429 | 0.890 | 2.073 | 2.986 | 0.968 | 0.254 |
| J01MA02 Ciprofloxacin | 0.201 | 0.205 | 0.343 | 1.558 | 0.000 | 0.173 | 1.361 | 0.617 |
| J01FA01 Erythromycin | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | / |
| J01DH03 Ertapenem | 4.555 | 3.458 | 9.920 | 9.930 | 8.412 | 4.977 | 3.559 | 1.000 |
| J01DH51 Imipenem Cilastatin | 20.682 | 14.071 | 6.575 | 13.046 | 12.923 | 11.534 | 11.515 | 0.023 |
| J01MA12 Levofloxacin | 5.586 | 5.272 | 3.316 | 3.310 | 4.115 | 4.090 | 2.329 | 0.023 |
| J01DH02 Meropenem | 28.588 | 27.764 | 33.533 | 22.142 | 19.994 | 32.417 | 34.284 | 0.704 |
| J01MA14 Moxifloxacin | 0.000 | 0.000 | 0.000 | 0.195 | 0.000 | 0.000 | 0.000 | / |
| J01CR05 Piperacillin Tazobactam | 8.021 | 8.456 | 3.087 | 0.612 | 1.372 | 5.908 | 5.234 | 0.447 |
| J01XA02 Teicoplanin | 3.351 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | / |
| J01XA01 Vancomycin | 33.543 | 38.206 | 35.363 | 28.734 | 28.741 | 30.859 | 32.478 | 0.447 |
| J01XB01 Colistimethate sodium | 9.940 | 8.216 | 12.579 | 7.121 | 7.802 | 18.589 | 20.073 | 0.254 |
| J01XX08 Linezolid | 3.753 | 3.184 | 1.630 | 2.253 | 1.402 | 10.755 | 12.248 | 0.704 |
| J01AA12 Tigecycline | 2.378 | 0.924 | 2.373 | 0.751 | 0.884 | 1.060 | 0.497 | 0.057 |
| J01DD52 Ceftazidime–avibactam | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 2.337 | 3.219 | / |
| J02AC01 Flukanazol | 28.702 | 22.937 | 10.978 | 6.092 | 7.802 | 27.440 | 28.710 | 1.000 |
| J02AC03 Vorikonazol | 0.000 | 0.342 | 0.000 | 0.111 | 0.488 | 0.000 | 0.000 | / |
| J02AC04 Posakonazol | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | / |
| J02AX04 Kaspofungin | 0.000 | 0.308 | 0.429 | 0.000 | 0.579 | 0.195 | 1.910 | / |
| A07AA02 Nystatin | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.105 | / |
| J02AX05 Mikafungin | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.303 | 0.000 | / |
| J04AB02 Rifampicin | 0.000 | 0.103 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | / |
| ACCESS GROUP | 65.454 | 69.360 | 75.385 | 67.734 | 63.060 | 61.979 | 52.760 | 0.023 |
| WATCH GROUP | 130.164 | 131.496 | 120.725 | 106.536 | 108.261 | 119.735 | 122.506 | 0.447 |
| RESERVE GROUP | 16.071 | 12.324 | 16.582 | 10.125 | 10.088 | 32.741 | 36.037 | 0.447 |
| Antifungal medicines | 28.702 | 23.587 | 11.407 | 6.203 | 8.869 | 27.938 | 30.725 | 1.000 |
| ATC Code | Antibiotics | Total ICU ID of Clostridioides difficile * |
|---|---|---|
| J01MA02 | Ciprofloxacin | r = −0.071 p = 0.879 |
| J01DD04 | Ceftriaxone | r = 0.393 p = 0.383 |
| J01XD01 | Metronidazole | r = −0.75 p = 0.052 |
| J01XA01 | Vancomycin | r = −0.393 p = 0.383 |
| J01MA12 | Levofloxacin | r = −0.643 p = 0.119 |
| J01DH02 | Meropenem | r = 0.286 p = 0.535 |
| J01XB01 | Colistimethate Na | r = 0.429 p = 0.337 |
| J01MA14 | Moxifloxacin | r = −0.204 p = 0.661 |
| J01GB03 | Gentamicin | r = −0.571 p = 0.180 |
| J01DE01 | Cefepime | r = −0.429 p = 0.337 |
| J01CR01 | Ampicillin + sulbactam | r = −0.408 p = 0.363 |
| J01DH03 | Ertapenem | r = 0.071 p = 0.879 |
| J01AA12 | Tigecycline | r = −0.607 p = 0.148 |
| J01FA10 | Azithromycin | r = −0.433 p = 0.331 |
| J01CR05 | Piperacillin + tazobactam | r = −0.429 p = 0.337 |
| J01FF01 | Clindamycin | r = 0.500 p = 0.253 |
| J01DB04 | Cefazolin | r = −0.071 p = 0.879 |
| J01XX08 | Linezolid | r = 0.107 p = 0.819 |
| J01DC03 | Cefuroxime | r = 0.143 p = 0.760 |
| J01EE01 | Trimethoprim + Sulfamethoxazole | r = 0.321 p = 0.482 |
| J01DH51 | Imipenem + cilastatin | r = −0.750 p = 0.052 |
| J01XA02 | Teicoplanin | r = −0.612 p = 0.144 |
| J01DD09 | Ceftazidime | r = −0.893 p = 0.007 |
| J01GB06 | Amikacin | r = 0.429 p = 0.337 |
| J01DD01 | Cefotaxime | r = 0.059 p = 0.900 |
| J01CR02 | Amoxicilline + clavulanic acid | r = −0.579 p = 0.173 |
| Resistance (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Microorganism | Antibiotic | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 |
| Klebsiella pneumoniae | 3G | 21/25 84.0% | 18/17 94.01% | 25/25 100.0% | 23/25 92.0% | 26/28 92.9% | 40/44 90.9% | 23/23 100% |
| Carbapenems | 10/25 40.0% | 10/17 58.8% | 22/25 88.0% | 21/25 84.0% | 24/28 85.7% | 34/44 77.27% | 22/23 95.65% | |
| Colistin | 4/25 16.0% | 2/17 11.76% | 3/25 12.0% | 0/25 0% | 12/28 42.86% | 25/44 56.82% | 11/23 47.83% | |
| Acinetobacter spp. | Carbapenems | 41/46 89.1% | 40/41 97.56% | 51/52 98.07% | 45/45 100.0% | 27/30 90.0% | 15/16 93.75% | 11/12 91.67% |
| Colistin | 14/46 30.43% | 1/41 2.44% | 0/52 0% | 0/45 0% | 0/30 0% | 2/16 12.5% | 0/12 0% | |
| Pseudomonas aeruginosa | Carbapenems | 11/15 73.33% | 13/17 76.47% | 28/32 87.5% | 11/12 91.67% | 9/13 69.23% | 12/15 80.0% | 6/9 66.67% |
| Colistin | 2/15 13.33% | 0/17 0% | 1/32 3.12% | 0/12 0% | 2/13 15.38% | 2/15 13.33% | 0/9 0% | |
| Providentia stuartii | 3G | 3/5 60.0% | 3/3 100.0% | 1/1 100.0% | 4/4 100.0% | 2/2 100.0% | 1/1 100.0% | 5/7 71.43% |
| Carbapenems | 0/5 0.0% | 1/3 33.3 | 0/1 0.0 | 3/4 75.0% | 1/2 50.0% | 1/1 100.0% | 5/7 71.43 | |
| Colistin | 0/5 0% | 0/3 0% | 0/1 0% | 0/4 0% | 1/2 50.0% | 0/1 0% | 0/7 0% | |
| Staphylococcus aureus | Oxacylin | 10/11 90.9% | 6/9 66.67% | 2/4 50.0% | 6/6 100.0% | 6/7 85.71% | 0/2 0% | 0/0 0% |
| Enterococcus spp. | Vancomycin | 4/7 57.1% | 1/4 25.0% | 5/8 62.5% | 3/8 37.5% | 0/3 0% | 4/8 50.0% | 4/10 40.0% |
| Antimicrobial Resistance | Antibiotic Consumption | Correlations ** | ||||
|---|---|---|---|---|---|---|
| Microorganism | Resistance | Trend * | Antibiotic | Trend * | Coefficient | p Value |
| Klebsiella spp. | 3rd Cephalosporin | 0.254 | 3rd Ceph | 0.128 | −0.214 | 0.645 |
| Carbapenems | 0.057 | Carbapenems | 0.447 | −0.107 | 0.819 | |
| Colistin | 0.046 | Colistin | 0.254 | 0.714 | 0.071 | |
| Acinetobacter spp. | Carbapenems | 1.000 | Carbapenems | 0.447 | −0.250 | 0.589 |
| Colistin | / | Colistin | 0.254 | 0.177 | 0.704 | |
| Pseudomonas aeruginosa | Carbapenems | 0.704 | Carbapenems | 0.447 | −0.071 | 0.879 |
| Colistin | / | Colistin | 0.254 | −0.037 | 0.937 | |
| Providentia stuartii | 3G | 1.000 | 3rd Ceph | 0.128 | 0.089 | 0.849 |
| Carbapenems | / | Carbapenems | 0.447 | −0.523 | 0.229 | |
| Colistin | / | Colistin | 0.254 | −0.408 | 0.363 | |
| Staphylococcus aureus | Oxacylin | / | Vancomycin | 0.447 | −0.306 | 0.504 |
| Enterococcus spp. | Vancomycin | 0.617 | Vancomycin | 0.447 | 0.357 | 0.432 |
| Infection Type | Present Study (Serbia, 2018–2024) | ECDC Mean (2019–2021) [1,33,34] | INICC (2015–2020) [4] |
|---|---|---|---|
| Pneumonia/VAP | 14.1/20.0 per 1000 patient-/ventilator-days | 6.8 per 1000 patient-days | 11.96 per 1000 ventilator-days |
| Bloodstream Infection (BSI) | 5.1 per 1000 patient-days | 5.1 per 1000 patient-days | — |
| CVC-related BSI (CLABSI) | 2.3 per 1000 CVC-days | — | 4.55 per 1000 central line-days |
| Urinary Tract Infection (UTI/CAUTI) | 4.9/5.1 per 1000 patient-/catheter-days | 2.9 per 1000 patient-days | 2.91 per 1000 catheter-days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuljagić, V.; Nešković, V.; Maksimović, D.; Udovičić, I.; Đurić-Petković, D.; Perišić, N.; Leković, I.; Taušan, Đ.; Rakonjac, B.; Vasiljević, K.; et al. Antibiotic Use, Healthcare-Associated Infections, and Antimicrobial Resistance in Intensive Care Unit of a Serbian Tertiary University Hospital, 2018–2024: An Ecological Analysis. Antibiotics 2025, 14, 1110. https://doi.org/10.3390/antibiotics14111110
Šuljagić V, Nešković V, Maksimović D, Udovičić I, Đurić-Petković D, Perišić N, Leković I, Taušan Đ, Rakonjac B, Vasiljević K, et al. Antibiotic Use, Healthcare-Associated Infections, and Antimicrobial Resistance in Intensive Care Unit of a Serbian Tertiary University Hospital, 2018–2024: An Ecological Analysis. Antibiotics. 2025; 14(11):1110. https://doi.org/10.3390/antibiotics14111110
Chicago/Turabian StyleŠuljagić, Vesna, Vojislava Nešković, Duško Maksimović, Ivo Udovičić, Danijela Đurić-Petković, Nenad Perišić, Ivan Leković, Đorđe Taušan, Bojan Rakonjac, Katarina Vasiljević, and et al. 2025. "Antibiotic Use, Healthcare-Associated Infections, and Antimicrobial Resistance in Intensive Care Unit of a Serbian Tertiary University Hospital, 2018–2024: An Ecological Analysis" Antibiotics 14, no. 11: 1110. https://doi.org/10.3390/antibiotics14111110
APA StyleŠuljagić, V., Nešković, V., Maksimović, D., Udovičić, I., Đurić-Petković, D., Perišić, N., Leković, I., Taušan, Đ., Rakonjac, B., Vasiljević, K., & Rančić, N. (2025). Antibiotic Use, Healthcare-Associated Infections, and Antimicrobial Resistance in Intensive Care Unit of a Serbian Tertiary University Hospital, 2018–2024: An Ecological Analysis. Antibiotics, 14(11), 1110. https://doi.org/10.3390/antibiotics14111110








